Biofilms and Chronic Wounds: Pathogenesis and Treatment Options
Abstract
1. Introduction
2. Methods
3. Chronic Wounds
4. Diabetic Foot Ulcers
5. Burn Wounds
6. Treatment and Strategies
7. Cross-Wound Comparisons and Shared Mechanisms
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three faces of biofilms: A microbial lifestyle, a nascent multicellular organism, and an incubator for diversity. Npj Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Attinger, C.; Wolcott, R. Clinically Addressing Biofilm in Chronic Wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S.; Mathew-Steiner, S.S.; Gordillo, G.M. Biofilm Management in Wound Care. Plast. Reconstr. Surg. 2021, 148, 275e–288e. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef]
- A Mendoza, R.; Hsieh, J.C.; D Galiano, R. The Impact of Biofilm Formation on Wound Healing. In Wound Healing—Current Perspectives; Dogan, H.K., Ed.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/wound-healing-current-perspectives/the-impact-of-biofilm-formation-on-wound-healing (accessed on 8 September 2025).
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D.; et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef]
- Liu, Y.; Long, S.; Wang, H.; Wang, Y. Biofilm therapy for chronic wounds. Int. Wound J. 2024, 21, e14667. [Google Scholar] [CrossRef]
- Cho, K.H.; Caparon, M.G. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol. Microbiol. 2005, 57, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Stoodley, P.; Kathju, S.; Høiby, N.; Moser, C.; William Costerton, J.; Moter, A.; Bjarnsholt, T. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol. Med. Microbiol. 2012, 65, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Haesler, E.; Swanson, T.; Ousey, K.; Carville, K. Clinical indicators of wound infection and biofilm: Reaching international consensus. J. Wound Care 2019, 28, s4–s12. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020, 21 (Suppl. S1), 36–43. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rumbaugh, K.P.; James, G.; Schultz, G.; Phillips, P.; Yang, Q.; Watters, C.; Stewart, P.S.; Dowd, S.E. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J. Wound Care 2010, 19, 320–328. [Google Scholar] [CrossRef]
- Stewart, P.S.; Grab, L.; Diemer, J.A. Analysis of biocide transport limitation in an artificial biofilm system. J. Appl. Microbiol. 1998, 85, 495–500. [Google Scholar] [CrossRef]
- Schaber, J.A.; Triffo, W.J.; Suh, S.J.; Oliver, J.W.; Hastert, M.C.; Griswold, J.A.; Auer, M.; Hamood, A.N.; Rumbaugh, K.P. Pseudomonas aeruginosa Forms Biofilms in Acute Infection Independent of Cell-to-Cell Signaling. Infect. Immun. 2007, 75, 3715–3721. [Google Scholar] [CrossRef]
- Davis, S.C.; Ricotti, C.; Cazzaniga, A.; Welsh, E.; Eaglstein, W.H.; Mertz, P.M. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen. 2008, 16, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Cox, S. More effective cell-based therapy through biofilm suppression. J. Wound Care 2013, 22, 26–31. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates. Adv. Wound Care 2023, 12, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Thaarup, I.C.; Iversen, A.K.S.; Lichtenberg, M.; Bjarnsholt, T.; Jakobsen, T.H. Biofilm Survival Strategies in Chronic Wounds. Microorganisms 2022, 10, 775. [Google Scholar] [CrossRef]
- Versey, Z.; da Cruz Nizer, W.S.; Russell, E.; Zigic, S.; DeZeeuw, K.G.; Marek, J.E.; Overhage, J.; Cassol, E. Biofilm-Innate Immune Interface: Contribution to Chronic Wound Formation. Front. Immunol. 2021, 12, 648554. [Google Scholar] [CrossRef]
- Darvishi, S.; Tavakoli, S.; Kharaziha, M.; Girault, H.H.; Kaminski, C.F.; Mela, I. Advances in the Sensing and Treatment of Wound Biofilms. Angew. Chem. Int. Ed. 2022, 61, e202112218. [Google Scholar] [CrossRef]
- Thaarup, I.C.; Bjarnsholt, T. Biofilms and Impaired Wound Healing: How Do We Detect the Presence of Biofilms in Chronic Wounds Non-invasively. In Chronic Wound Management; Mani, R., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 195–228. Available online: https://link.springer.com/10.1007/978-3-031-26110-7_10 (accessed on 8 September 2025).
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Low. Extrem. Wounds 2022, 21, 18–30. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Chong, D.L.; Trinder, S.; Labelle, M.; Rodriguez-Justo, M.; Hughes, S.; Holmes, A.M.; Scotton, C.J.; Porter, J.C. Platelet-derived transforming growth factor-β1 promotes keratinocyte proliferation in cutaneous wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Gajula, B.; Munnamgi, S.; Basu, S. How bacterial biofilms affect chronic wound healing: A narrative review. Int. J. Surg. Glob. Health 2020, 3, e16. [Google Scholar] [CrossRef]
- Karched, M.; Guleri Bhardwaj, R.; Abu Al-Melh, M.; Qudeimat, M.A. Cytokine release and NLRP3 inflammasome activation induced by low-abundance oral bacterial biofilms. J. Oral Microbiol. 2025, 17, 2552167. [Google Scholar] [CrossRef]
- Barker, J.C.; Khansa, I.; Gordillo, G.M. A Formidable Foe Is Sabotaging Your Results: What You Should Know about Biofilms and Wound Healing. Plast. Reconstr. Surg. 2017, 139, 1184e–1194e. [Google Scholar] [CrossRef]
- Hossain, M.d.J.; Al-Mamun Md Islam, M.d.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62. [Google Scholar] [CrossRef]
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Erasmus, A.; Schwarzer, S.; Lau, N.S.; Ahmad, M.; Dickson, H.G. Utilisation of the 2019 IWGDF diabetic foot infection guidelines to benchmark practice and improve the delivery of care in persons with diabetic foot infections. J. Foot Ankle Res. 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Subrata, S.A.; Phuphaibul, R. Diabetic foot ulcer care: A concept analysis of the term integrated into nursing practice. Scand. J. Caring Sci. 2019, 33, 298–310. [Google Scholar] [CrossRef]
- Bus, S.A.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Raspovic, A.; Sacco, I.C.; van Netten, J.J.; International Working Group on the Diabetic Foot. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res Rev. 2020, 36, e3269. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546. [Google Scholar] [CrossRef] [PubMed]
- Raymakers, J.T.; Houben, A.J.; Heyden, J.J.V.; Tordoir, J.H.; Kitslaar, P.J.; Schaper, N.C. The effect of diabetes and severe ischaemia on the penetration of ceftazidime into tissues of the limb. Diabet. Med. 2001, 18, 229–234. [Google Scholar] [CrossRef]
- Lecube, A.; Pachón, G.; Petriz, J.; Hernández, C.; Simó, R. Phagocytic Activity Is Impaired in Type 2 Diabetes Mellitus and Increases after Metabolic Improvement. Sesti G, editor. PLoS ONE 2011, 6, e23366. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef]
- Yang, S.; Hu, L.; Han, R.; Yang, Y. Neuropeptides, Inflammation, Biofilms, and diabetic Foot Ulcers. Exp. Clin. Endocrinol. Diabetes 2022, 130, 439–446. [Google Scholar] [CrossRef]
- Sahu, A.; Ruhal, R. Immune system dynamics in response to Pseudomonas aeruginosa biofilms. npj Biofilms Microbiomes 2025, 11, 104. [Google Scholar] [CrossRef]
- Nouvong, A.; Ambrus, A.M.; Zhang, E.R.; Hultman, L.; Coller, H.A. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol. Genom. 2016, 48, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and Reversing Chronic Wounds in Diabetic Mice by Manipulating Wound Redox Parameters. J. Diabetes Res. 2014, 2014, 562625. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Ge Zhao, A.; Usui, M.; Underwood, R.A.; Nguyen, H.; Beyenal, H.; deLancey Pulcini, E.; Agostinho Hunt, A.; Bernstein, H.C.; Fleckman, P.; et al. Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen. 2016, 24, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, Q. Current research on fungi in chronic wounds. Front. Mol. Biosci. 2023, 9, 1057766. [Google Scholar] [CrossRef] [PubMed]
- Short, B.; Bakri, A.; Baz, A.; Williams, C.; Brown, J.; Ramage, G. There Is More to Wounds than Bacteria: Fungal Biofilms in Chronic Wounds. Curr. Clin. Microbiol. Rep. 2023, 10, 9–16. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef]
- Wei, D.; Zhu, X.M.; Chen, Y.Y.; Li, X.Y.; Chen, Y.P.; Liu, H.Y.; Zhang, M. Chronic wound biofilms: Diagnosis and therapeutic strategies. Chin. Med. J. 2019, 132, 2737–2744. [Google Scholar] [CrossRef]
- Sen, C.K.; Mathew-Steiner, S.S.; Das, A.; Sundaresan, V.B.; Roy, S. Electroceutical Management of Bacterial Biofilms and Surgical Infection. Antioxid. Redox Signal 2020, 33, 713–724. [Google Scholar] [CrossRef]
- Ramage, G.; Williams, C. The Clinical Importance of Fungal Biofilms. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 27–83. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780124076730000023 (accessed on 8 September 2025).
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal Susceptibility of Candida Biofilms: Unique Efficacy of Amphotericin B Lipid Formulations and Echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef]
- Maslova, E.; Eisaiankhongi, L.; Sjöberg, F.; McCarthy, R.R. Burns and biofilms: Priority pathogens and in vivo models. Npj Biofilms Microbiomes 2021, 7, 73. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Ducret, V.; Leoni, S.; Fleuchot, B.; Jafari, P.; Raffoul, W.; Applegate, L.A.; Que, Y.A.; Perron, K. Transcriptome Analysis of Pseudomonas aeruginosa Cultured in Human Burn Wound Exudates. Front. Cell. Infect. Microbiol. 2018, 8, 39. [Google Scholar] [CrossRef]
- Banin, E.; Vasil, M.L.; Greenberg, E.P. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA 2005, 102, 11076–11081. [Google Scholar] [CrossRef] [PubMed]
- Yali, G.; Jing, C.; Chunjiang, L.; Cheng, Z.; Xiaoqiang, L.; Yizhi, P. Comparison of pathogens and antibiotic resistance of burn patients in the burn ICU or in the common burn ward. Burns 2014, 40, 402–407. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Berends, E.T.; Chan, R.; Schwab, E.; Roy, S.; Sen, C.K.; Torres, V.J.; Wozniak, D.J. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc. Natl. Acad. Sci. USA 2018, 115, 7416–7421. [Google Scholar] [CrossRef]
- Branski, L.K.; Al-Mousawi, A.; Rivero, H.; Jeschke, M.G.; Sanford, A.P.; Herndon, D.N. Emerging Infections in Burns. Surg. Infect. 2009, 10, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Thomas, B.C. Reducing Biofilm Infections in Burn Patients’ Wounds and Biofilms on Surfaces in Hospitals, Medical Facilities and Medical Equipment to Improve Burn Care: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 13195. [Google Scholar] [CrossRef]
- Jaroš, P.; Vrublevskaya, M.; Lokočová, K.; Michailidu, J.; Kolouchová, I.; Demnerová, K. Boswellia serrata Extract as an Antibiofilm Agent against Candida spp. Microorganisms 2022, 10, 171. [Google Scholar] [PubMed]
- Senevirathne, S.; Ekanayake, G.; Samarathunge, D.; Basnayke, O. The Use of Polyhexanide and Betaine Combined Preparation in Adult Burn Care in Sri Lanka. Cureus 2024, 16, e67274. [Google Scholar] [CrossRef]
- Roy, S.; Mukherjee, P.; Kundu, S.; Majumder, D.; Raychaudhuri, V.; Choudhury, L. Microbial infections in burn patients. Acute Crit. Care 2024, 39, 214–225. [Google Scholar] [CrossRef]
- Robson, M.C.; Payne, W.G.; Ko, F.; Mentis, M.; Donati, G.; Shafii, S.M.; Culverhouse, S.; Wang, L.; Khosrovi, B.; Najafi, R.; et al. Hypochlorous Acid as a Potential Wound Care Agent: Part II. Stabilized Hypochlorous Acid: Its Role in Decreasing Tissue Bacterial Bioburden and Overcoming the Inhibition of Infection on Wound Healing. J. Burns Wounds 2007, 6, e6. [Google Scholar]
- Cambiaso-Daniel, J.; Boukovalas, S.; Bitz, G.H.; Branski, L.K.; Herndon, D.N.; Culnan, D.M. Topical Antimicrobials in Burn Care: Part 1-Topical Antiseptics. Ann. Plast. Surg. 2018, 80, 239–249. [Google Scholar] [CrossRef]
- Diaz-Guerrero, M.; López-Jácome, L.E.; Franco-Cendejas, R.; Coria-Jiménez, R.; Martínez-Zavaleta, M.G.; González-Pedrajo, B.; Huelgas-Méndez, D.; García-Contreras, R. Curcumin inhibits type III secretion of Pseudomonas aeruginosa. PeerJ 2025, 13, e19725. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cokcetin, N.N.; Burke, C.M.; Turnbull, L.; Liu, M.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 18160. [Google Scholar] [CrossRef]
- Al-Madboly, L.A.; Aboulmagd, A.; El-Salam, M.A.; Kushkevych, I.; El-Morsi, R.M. Microbial enzymes as powerful natural anti-biofilm candidates. Microb. Cell Factories 2024, 23, 343. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.K.; et al. Nanotechnology in combating biofilm: A smart and promising therapeutic strategy. Front. Microbiol. 2022, 13, 1028086. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Muzammil, S.; Rasool, M.H.; Nisar, Z.; Hussain, S.Z.; Sabri, A.N.; Jamil, S. In vitro antibiofilm and anti-adhesion effects of magnesium oxide nanoparticles against antibiotic resistant bacteria. Microbiol. Immunol. 2018, 62, 211–220. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Agarwala, M.; Choudhury, B.; Yadav, R.N.S. Comparative Study of Antibiofilm Activity of Copper Oxide and Iron Oxide Nanoparticles Against Multidrug Resistant Biofilm Forming Uropathogens. Indian J. Microbiol. 2014, 54, 365–368. [Google Scholar] [CrossRef]
- Burkhart, C.G. Assessment of Cutibacterium acnes: Acne Biofilm, Comedones, and Future Treatments for Acne. Open Dermatol. J. 2024, 18, e18743722279314. [Google Scholar] [CrossRef]
- Kravvas, G.; Veitch, D.; Al-Niaimi, F. The increasing relevance of biofilms in common dermatological conditions. J. Dermatol. Treat. 2018, 29, 202–207. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Sucher, A.J.; Chahine, E.B.; Balcer, H.E. Echinocandins: The Newest Class of Antifungals. Ann. Pharmacother. 2009, 43, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Iñigo, M.; Del Pozo, J.L. Fungal biofilms: From bench to bedside. Rev Esp. Quim. Publ. Soc Esp. Quim. 2018, 31 (Suppl. S1), 35. [Google Scholar]
- Highmore, C.J.; Melaugh, G.; Morris, R.J.; Parker, J.; Direito, S.O.L.; Romero, M.; Soukarieh, F.; Robertson, S.N.; Bamford, N.C. Translational challenges and opportunities in biofilm science: A BRIEF for the future. Npj Biofilms Microbiomes 2022, 8, 68. [Google Scholar] [CrossRef]
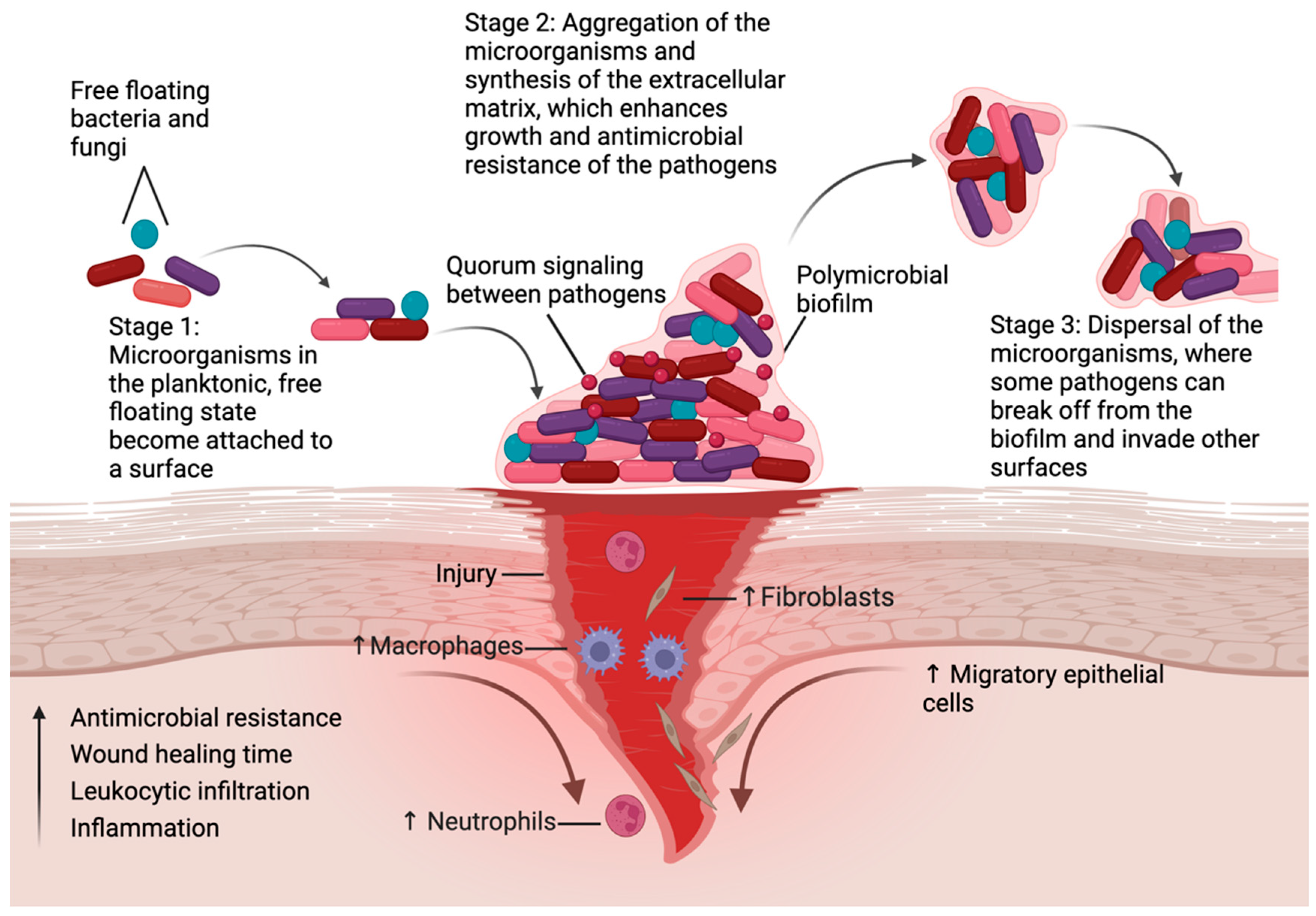
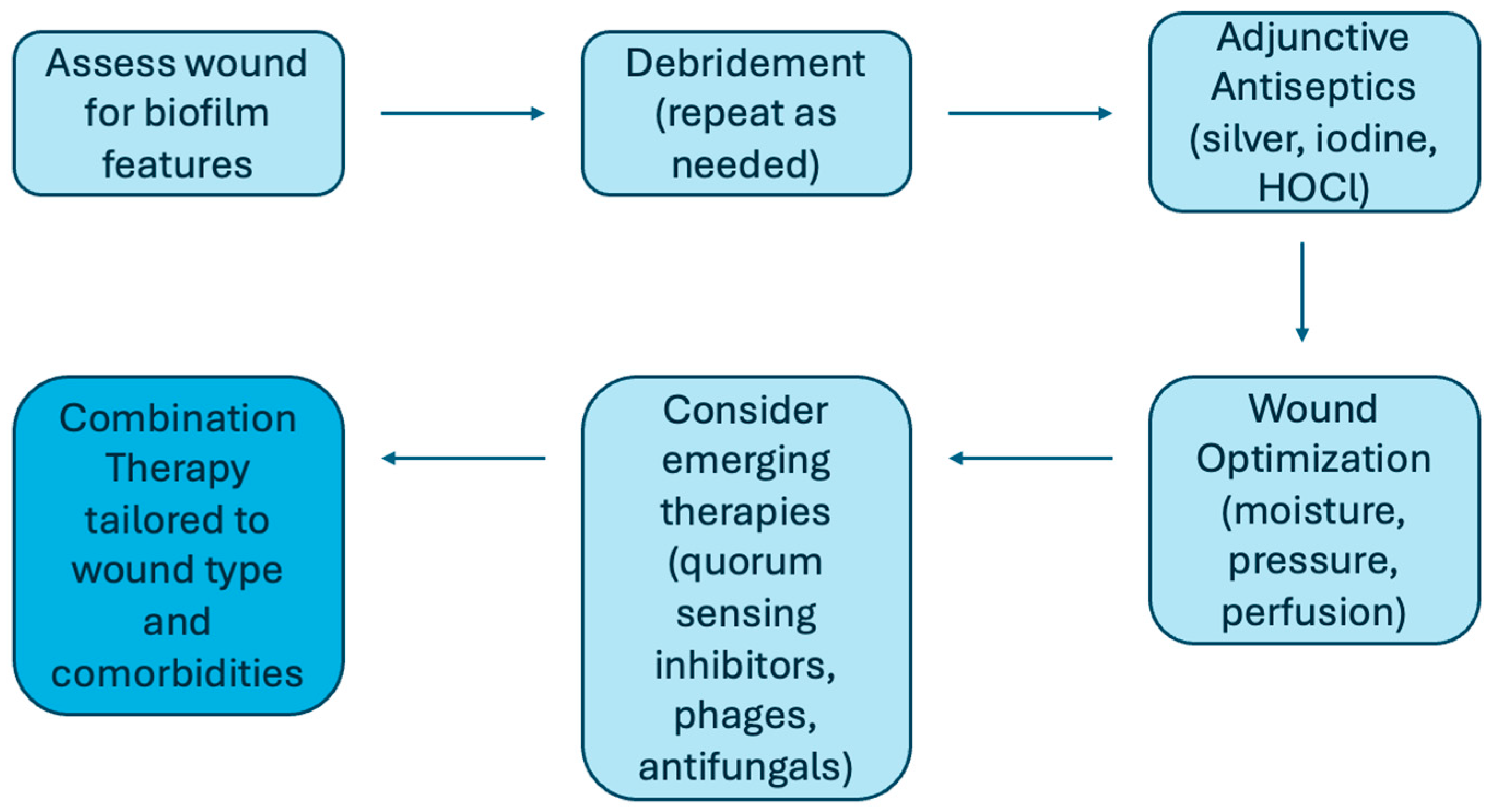
| Treatment | Examples | Action | Evidence Level | Translational Readiness |
| Debridement | Physical debridement, ultrasound therapy | Removes necrotic tissue and biofilm from wound surface | Clinical | Widely established in practice |
| Antiseptics | Silver, iodine | Antimicrobials, some evidence of reducing or preventing biofilm formation | Clinical | Routinely used; strain-specific efficacy |
| Hydrogels | Prontosan, Carrasyn, Curagel, Nu-Gel, Purilon, Restore, SAF-gel, XCell | Anti-biofilm activity, particularly against S. aureus and P. aeruginosa | Clinical | FDA-approved wound dressings |
| Amphotericin B | Liposomal AMB and AMB lipid complex | Binds to ergosterol, leading to formation of pores in the fungal cell membrane, ion leakage and cell death | Clinical | Moderate adoption; limited DFU data |
| Echinocandins | Caspofungin, micafungin | Inhibit beta-(1,3)-D-glucan synthase, an enzyme required for fungal cell wall synthesis. This results in decreased cell wall stability, which dysregulates osmotic pressure, leading to cell lysis | Clinical | Moderate adoption; limited DFU data |
| D-Amino acids | d-Leu, d-Met, d-Trp, d-Tyr | Enhances antibiotic activity against biofilms, particularly S. aureus and P. aeruginosa | Preclinical | In vitro and animal data |
| Light | Antimicrobial blue light | Anti-biofilm activity against Acinetobacter baumannii and P. aeruginosa | Preclinical/Early Clinical | Pilot trials; emerging adjunctive therapy |
| Bacteriophages | - | Target bacteria with high specificity, secrete enzymes that disrupt biofilm matrix | Preclinical | Expanding clinical trials |
| Small molecules | Mannosidase, trypsin enzymes, platensimycin, salicylidene acylhydrazide | Various mechanisms of anti-biofilm activity, target matrix proteins, microbial membranes and virulence factors | Preclinical | Conceptual; in vitro efficacy |
| Quorum sensing inhibitors | Azithromycin, bergamottin, ellagic acid, quercetin, usnic acid | Inhibit biofilm formation and reduce bacterial virulence | Preclinical | Natural compounds under investigation |
| Matrix degrading enzymes | DNAase I, Dispersin B, a-amylase | Degrade biofilm structures which increases antibiotic penetration | Preclinical | Limited human data |
| Surfactants | Sodium dodecyl sulfate, rhamnolipids, poloxamer-188 | Inhibit microbial adhesion and interfere with EPS matrix formation | Preclinical | Potential wound care additives |
| Antimicrobial peptides | Nisin A, human cathelicidin/LL-37, human beta-defensin 3, manuka honey | Antimicrobial activity, bind to negatively charged molecules on the microbial membrane | Preclinical/Early Clinical | Natural peptides under early evaluation |
| Nanoparticles | CuO, ZnO, AgO | Modification of surface adhesion and generation of reactive oxygen species generation | Preclinical | Proof-of-concept; active materials research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, A.Z.; Taha, M.; Ghannoum, M.; Tyring, S.K. Biofilms and Chronic Wounds: Pathogenesis and Treatment Options. J. Clin. Med. 2025, 14, 7784. https://doi.org/10.3390/jcm14217784
Shen AZ, Taha M, Ghannoum M, Tyring SK. Biofilms and Chronic Wounds: Pathogenesis and Treatment Options. Journal of Clinical Medicine. 2025; 14(21):7784. https://doi.org/10.3390/jcm14217784
Chicago/Turabian StyleShen, Annabel Z., Mohamad Taha, Mahmoud Ghannoum, and Stephen K. Tyring. 2025. "Biofilms and Chronic Wounds: Pathogenesis and Treatment Options" Journal of Clinical Medicine 14, no. 21: 7784. https://doi.org/10.3390/jcm14217784
APA StyleShen, A. Z., Taha, M., Ghannoum, M., & Tyring, S. K. (2025). Biofilms and Chronic Wounds: Pathogenesis and Treatment Options. Journal of Clinical Medicine, 14(21), 7784. https://doi.org/10.3390/jcm14217784







