Prophylactic Intra-Arterial Eptifibatide in Stent-Assisted Coiling and Flow Diverter Treatment of Cerebral Aneurysms: A Single-Center Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.2.1. Endovascular Treatment
2.2.2. Anticoagulation/Antiplatelet Protocol
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briganti, F.; Leone, G.; Marseglia, M.; Mariniello, G.; Caranci, F.; Brunetti, A.; Maiuri, F. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol. J. 2015, 28, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Adamou, A.; Alexandrou, M.; Roth, C.; Chatziioannou, A.; Papanagiotou, P. Endovascular treatment of intracranial aneurysms. Life 2021, 11, 335. [Google Scholar] [CrossRef]
- Becske, T.; Kallmes, D.F.; Saatci, I.; McDougall, C.G.; Szikora, I.; Lanzino, G.; Moran, C.J.; Woo, H.H.; Lopes, D.K.; Berez, A.L.; et al. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology 2013, 267, 858–868. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Hanel, R.A.; Sauvageau, E.A.; Aghaebrahim, A.; Lin, E.; Jadhav, A.P.; Jovin, T.G.; Khaldi, A.; Gupta, R.G.; Johnson, A.; et al. Pivotal trial of the Neuroform Atlas stent for treatment of anterior circulation aneurysms: One-year outcomes. Stroke 2020, 51, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.S.; Hwang, G.; Park, J.C.; Lee, C.-Y.; Chung, J.; Lee, S.-W.; Kwon, H.-J.; Kim, S.-R.; Kang, D.-H.; Kwon, S.C.; et al. Tailored antiplatelet therapy in stent assisted coiling for unruptured aneurysms: A nationwide registry study. J. Neurointerv. Surg. 2023, 15, 1095–1104. [Google Scholar] [CrossRef]
- Jesser, J.; Alberalar, N.D.; Kizilkilic, O.; Saatci, I.; Baltacioglu, F.; Özlük, E.; Killer-Oberpfalzer, M.; Vollherbst, D.F.; Islak, C.; Cekirge, S.H.; et al. Safety and efficacy of the FRED Jr flow re-direction endoluminal device for intracranial aneurysms: Retrospective multicenter experience with emphasis on midterm results. Front. Neurol. 2021, 12, 722183. [Google Scholar] [CrossRef]
- Brinjikji, W.; Lanzino, G.; Cloft, H.; Siddiqui, A.; Boccardi, E.; Cekirge, S.; Fiorella, D.; Hanel, R.; Jabbour, P.; Levy, E.; et al. Risk factors for ischemic complications following pipeline embolization device treatment of intracranial aneurysms: Results from the IntrePED study. Am. J. Neuroradiol. 2016, 37, 1673–1678. [Google Scholar] [CrossRef]
- Kim, J.; Han, H.; Lee, W.; Park, S.; Chung, J.; Kim, Y.; Park, K. Safety and efficacy of stent-assisted coiling of unruptured intracranial aneurysms using low-profile stents in small parent arteries. Am. J. Neuroradiol. 2021, 42, 1621–1626. [Google Scholar] [CrossRef]
- Caroff, J.; Aubert, L.; Lavenu-Bombled, C.; Figueiredo, S.; Habchi, K.; Cortese, J.; Eugene, F.; Ognard, J.; Tahon, F.; Forestier, G.; et al. Antithrombotic therapies for neurointerventional surgery: A 2021 French comprehensive national survey. J. Neurointerv. Surg. 2023, 15, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, C.M.; Bulsara, K.R.; Al-Mufti, F.; Haranhalli, N.; Thibault, L.; Hetts, S.W. Antiplatelets and antithrombotics in neurointerventional procedures: Guideline update. J. Neurointerv. Surg. 2023, 15, 1155–1162. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, Q.; Tang, H.; Xue, G.; Yang, P.; Zhao, R.; Li, Q.; Fang, Y.; Xu, Y.; Hong, B.; et al. Stent assisted coiling versus non-stent assisted coiling for the management of ruptured intracranial aneurysms: A meta-analysis and systematic review. J. Neurointerv. Surg. 2019, 11, 489–496. [Google Scholar] [CrossRef]
- Arias, E.J.; Patel, B.; Cross, D.T.; Moran, C.J.; Dacey, R.G.; Zipfel, G.J.; Derdeyn, C.P. Timing and nature of in-house postoperative events following uncomplicated elective endovascular aneurysm treatment. J. Neurosurg. 2014, 121, 1063–1070. [Google Scholar] [CrossRef]
- Larco, J.; Abbasi, M.; Liu, Y.; Dai, D.; Lanzino, G.; Savastano, L.; Cloft, H.; Kallmes, D.; Kadirvel, R.; Brinjikji, W. Postprocedural thrombosis following endovascular treatment of intracranial aneurysm with flow diverters or coiling: A histologic study. Am. J. Neuroradiol. 2022, 43, 258–264. [Google Scholar] [CrossRef]
- Meyer, B.M.; Campos, J.K.; de Beaufort, J.C.C.; Chen, I.; Khan, M.W.; Amin, G.; Zarrin, D.A.; Lien, B.V.; Coon, A.L. Trends in dual antiplatelet therapy use for neurointerventional procedures for the management of intracranial aneurysms. Biomedicines 2023, 11, 2234. [Google Scholar] [CrossRef]
- Bilgin, C.; Ghozy, S.; Shehata, M.; Ibrahim, M.; Jabal, M.S.; Kobeissi, H.; Gerberi, D.J.; Kadirvel, R.; Kallmes, D.F. The prophylactic use of glycoprotein 2B/3A inhibitors in the endovascular treatment of intracranial aneurysms: A systematic review and meta-analysis. World Neurosurg. 2022, 168, e50–e66. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, L.; Ladenhauf, V.; Rass, V.; Treichl, S.A.; Beer, R.; Freyschlag, C.F.; Gizewski, E.R.; Grams, A.E. Abciximab in rescue therapy of thromboembolic events during endovascular treatment of cerebral aneurysms: Single center experience of 49 cases. Front. Neurol. 2025, 16, 1573247. [Google Scholar] [CrossRef]
- Bürkle, F.; Weyland, C.S.; Hasan, D.; Yousefi, F.; Ridwan, H.; Nikoubashman, O.; Wiesmann, M. Propensity score-adjusted analysis on early tirofiban administration to prevent thromboembolic complications during stand-alone coil embolization of ruptured aneurysms. Sci. Rep. 2024, 14, 26350. [Google Scholar] [CrossRef] [PubMed]
- Sedat, J.; Chau, Y.; Mondot, L.; Chemla, R.; Lonjon, M.; Padovani, B. Is eptifibatide a safe and effective rescue therapy in thromboembolic events complicating cerebral aneurysm coil embolization? Single-center experience in 42 cases and review of the literature. Neuroradiology 2014, 56, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhao, H.; Ding, C.; Chen, H.; Wang, D.; Liu, A. The prophylactic use of tirofiban versus oral antiplatelet medications in stent-assisted coiling of intracranial aneurysms: A meta-analysis. Am. J. Neuroradiol. 2021, 42, 713–719. [Google Scholar] [CrossRef]
- Altenburg, A.; Haage, P. Antiplatelet and anticoagulant drugs in interventional radiology. Cardiovasc. Interv. Radiol. 2012, 35, 30–42. [Google Scholar] [CrossRef]
- Walsh, R.D.; Barrett, K.M.; Aguilar, M.I.; Lanzino, G.; Hanel, R.A.; Miller, D.A.; Chong, B.W.; Freeman, W.D. Intracranial hemorrhage following neuroendovascular procedures with abciximab is associated with high mortality: A multicenter series. Neurocritical Care 2011, 15, 85–95. [Google Scholar] [CrossRef]
- Dumont, T.M.; Kan, P.; Snyder, K.V.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I. Adjunctive use of eptifibatide for complication management during elective neuroendovascular procedures. J. Neurointerv. Surg. 2013, 5, 226–230. [Google Scholar] [CrossRef]
- Hasanpour, M.; Maleki, S.; Rezaee, H.; Aminzadeh, B.; Shaye, Z.A.; Keykhosravi, E. Glycoprotein IIb/IIIa inhibitors in the treatment of thromboembolic events related to endovascular treatment of cerebral aneurysms-systematic review and meta-analysis. Neuroradiol. J. 2024, 37, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Sedat, J.; Chau, Y.; Gaudard, J.; Suissa, L.; Lachaud, S.; Lonjon, M. Administration of eptifibatide during endovascular treatment of ruptured cerebral aneurysms reduces the rate of thromboembolic events. Neuroradiology 2015, 57, 197–203. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Yoo, A.J.; Rabinov, J.D.; Ogilvy, C.S.; Hirsch, J.A.; Nogueira, R.G. Intra-arterial eptifibatide in the management of thromboembolism during endovascular treatment of intracranial aneurysms: Case series and a review of the literature. Interv. Neurol. 2013, 2, 19–29. [Google Scholar] [CrossRef]
- Jeong, H.; Jin, S.-C. Intra-arterial infusion of a glycoprotein IIb/IIIa antagonist for the treatment of thromboembolism during coil embolization of intracranial aneurysm: A comparison of abciximab and tirofiban. Am. J. Neuroradiol. 2013, 34, 1621–1625. [Google Scholar] [CrossRef]
- Katsaridis, V.; Papagiannaki, C.; Skoulios, N.; Achoulias, I.; Peios, D. Local intra-arterial eptifibatide for intraoperative vessel thrombosis during aneurysm coiling. Am. J. Neuroradiol. 2008, 29, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Horev, A.; Zlotnik, Y.; Borodetsky, V.; Biederko, R.; Star, M.; Zvenigorodsky, V.; Shelef, I.; Ifergane, G. Adjunctive treatment with low dose intra-arterial eptifibatide and intravenous aspirin during carotid stenting: A case series. J. Clin. Neurosci. 2021, 84, 29–32. [Google Scholar] [CrossRef] [PubMed]
- FDA. Integrilin (Eptifibatide) [Prescribing Information]; Revised 2013; Millennium Pharmaceuticals, Inc.: Cambridge, MA, USA, 2013. [Google Scholar]
- Simonato, D.; Borchert, R.J.; Labeyrie, M.-A.; Fuschi, M.; Thibault, L.; Henkes, H.; Fiorella, D.; Tan, B.Y.; Yeo, L.L.; Makalanda, H.L.; et al. Glycoprotein IIb/IIIa inhibitors for the neurointerventionalist. Interv. Neuroradiol. 2022, 28, 84–91. [Google Scholar] [CrossRef]
- Boisseau, W.; Darsaut, T.; Fahed, R.; Drake, B.; Lesiuk, H.; Rempel, J.; Gentric, J.-C.; Ognard, J.; Nico, L.; Iancu, D.; et al. Stent-assisted coiling in the treatment of unruptured intracranial aneurysms: A randomized clinical trial. Am. J. Neuroradiol. 2023, 44, 381–389. [Google Scholar] [CrossRef]
- Jee, T.K.; Yeon, J.Y.; Kim, K.H.; Kim, J.-S.; Jeon, P. Flow Diversion for Cerebral Aneurysms: A Decade-Long Experience with Improved Outcomes and Predictors of Success. Brain Sci. 2024, 14, 847. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhu, Y.-Q.; Su, M.; Gao, K.-D.; Li, M.-H. Flow-diverting devices versus coil embolization for intracranial aneurysms: A systematic literature review and meta-analysis. World Neurosurg. 2016, 88, 640–645. [Google Scholar] [CrossRef]
- Nishido, H.; Piotin, M.; Bartolini, B.; Pistocchi, S.; Redjem, H.; Blanc, R. Analysis of complications and recurrences of aneurysm coiling with special emphasis on the stent-assisted technique. Am. J. Neuroradiol. 2014, 35, 339–344. [Google Scholar] [CrossRef]
- Yi, H.; Gupta, R.; Jovin, T.; Tayal, A.; Genevro, J.; Gologorsky, Y.; Horowitz, M. Initial experience with the use of intravenous eptifibatide bolus during endovascular treatment of intracranial aneurysms. Am. J. Neuroradiol. 2006, 27, 1856–1860. [Google Scholar]
- Domingo, R.A.; Tripathi, S.; Perez-Vega, C.; Vivas-Buitrago, T.; Lu, V.M.; Todnem, N.D.; Quinones-Hinojosa, A.; Tawk, R.G. Treatment of posterior circulation non-saccular aneurysms with flow diversion versus stent-assisted coiling: A systematic review and meta-analysis. J. Neurointerv. Surg. 2021, 13, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Yang, P.; Liu, J.; Xu, Y.; Hong, B.; Zhao, W.; Chen, Q.; Huang, Q.-H. Comparison of the flow diverter and stent-assisted coiling in large and giant aneurysms: Safety and efficacy based on a propensity score-matched analysis. Eur. Radiol. 2016, 26, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, J.-F.; Li, C.-H.; Wang, J.-W.; Li, H.; Gao, B.-L. Effects of stent-assisted coiling in comparison with flow diversion on intracranial aneurysms. Front. Neurol. 2022, 13, 937536. [Google Scholar] [CrossRef]
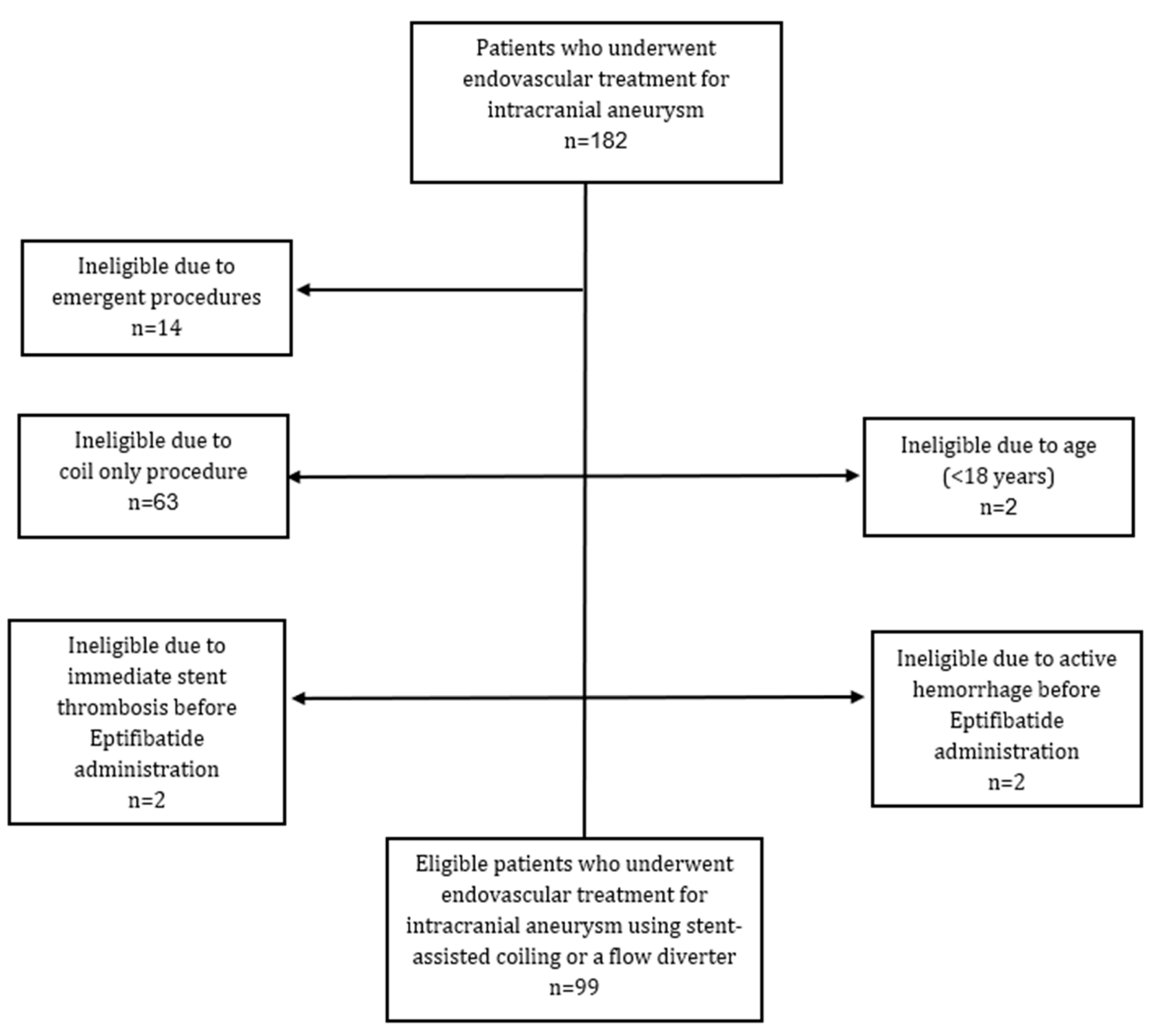
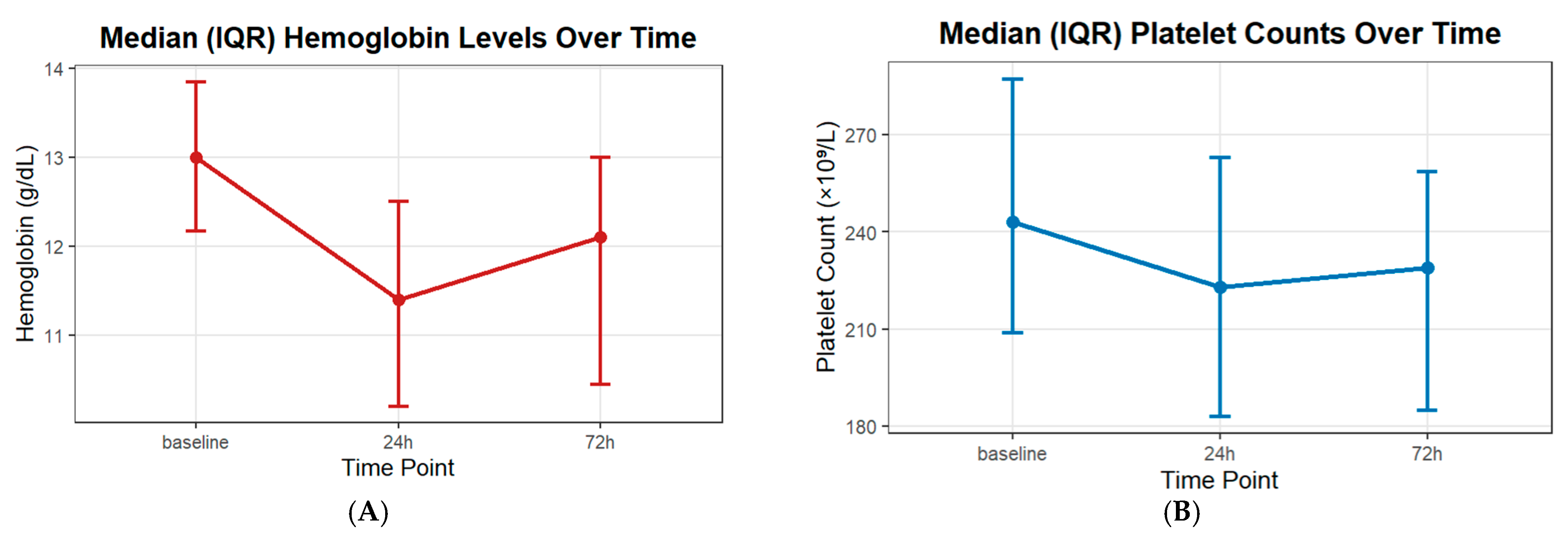
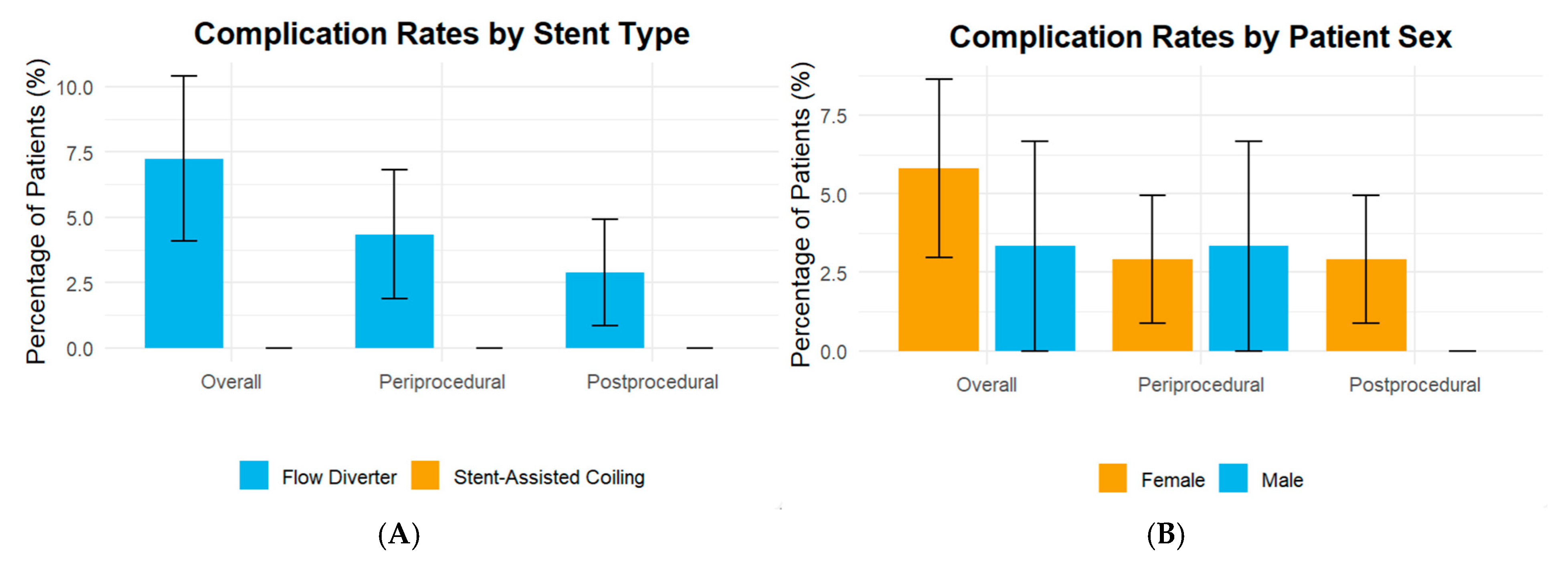
| Variable | N = 99 |
|---|---|
| Age, Mean ± SD | 57.0 ± 10.8 |
| Sex, n (%) | |
| Female | 69 (69.7) |
| Male | 30 (30.3) |
| Smoking, n (%) | |
| Current | 33 (33.3) |
| Past | 8 (8.1) |
| Hypertension, n (%) | 51 (51.5) |
| Diabetes mellitus, n (%) | 17 (17.2) |
| Dyslipidemia, n (%) | 28 (28.3) |
| Chronic migraines, n (%) | 5 (5.1) |
| Stent type, n (%) | |
| Flow diverter | 69 (69.7) |
| FRED | 59 (85.5) |
| PIPELINE | 8 (11.6) |
| ACCERO | 2 (2.9) |
| Stent-assisted coiling | 30 (30.3) |
| LVIS Jr. | 21 (70.0) |
| LEO | 1 (3.3) |
| ENTERPRISE | 1 (3.3) |
| DERIVO | 1 (3.3) |
| LVIS EVO | 4 (13.3) |
| BARREL | 1 (3.3) |
| SOLITAIRE AB | 1 (3.3) |
| Additional aneurysms observed, n (%) | 31 (31.3) |
| The current procedure is a correction of a previous procedure, n (%) | 19 (19.2) |
| Variable | Stent-Assisted Coiling N = 30 | Flow Diverter N = 69 | p-Value |
|---|---|---|---|
| Aneurysm type, n (%) | 0.749 | ||
| Saccular | 27 (90.0) | 59 (85.5) | |
| Dissecting | 3 (10.0) | 10 (14.5) | |
| Aneurysm location, n (%) | 0.634 | ||
| Anterior circulation | 5 (16.7) | 9 (13.0) | |
| Posterior circulation | 25 (83.3) | 60 (87.0) | |
| Coiled, n (%) | 24 (80.0) | 45 (65.2) | 0.141 |
| Pre-procedure medication in addition to Aspirin, n (%) | 0.190 | ||
| Clopidogrel | 16 (53.3) | 27 (39.1) | |
| Prasugrel | 14 (46.7) | 42 (60.9) |
| Variable | Stent-Assisted Coiling N = 30 | Flow Diverter N = 69 | p-Value |
|---|---|---|---|
| Periprocedural complications | |||
| Intra-procedural intracranial hemorrhage | 0 (-) | 0 (-) | |
| Intra-procedural intracranial thrombosis | 0 (-) | 0 (-) | |
| Ischemic-related neurological deficits | 0 (-) | 3 (4.3) | 0.551 |
| Non-intracranial hemorrhage | 0 (-) | 0 (-) | |
| Post-procedural complications after 24 h | |||
| Ischemic-related neurological deficits | 0 (-) | 2 (2.9) | 1.0 |
| Overall complications | 0 (-) | 5 (7.2) | 0.319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gothelf, I.; Epstein, M.; Shiloh, A.; Lebowitz, Z.; Zlotnik, Y.; Sacho, R.H.; Mechnik Steen, Y.; Ben-Arie, G.; Horev, A. Prophylactic Intra-Arterial Eptifibatide in Stent-Assisted Coiling and Flow Diverter Treatment of Cerebral Aneurysms: A Single-Center Retrospective Analysis. J. Clin. Med. 2025, 14, 7733. https://doi.org/10.3390/jcm14217733
Gothelf I, Epstein M, Shiloh A, Lebowitz Z, Zlotnik Y, Sacho RH, Mechnik Steen Y, Ben-Arie G, Horev A. Prophylactic Intra-Arterial Eptifibatide in Stent-Assisted Coiling and Flow Diverter Treatment of Cerebral Aneurysms: A Single-Center Retrospective Analysis. Journal of Clinical Medicine. 2025; 14(21):7733. https://doi.org/10.3390/jcm14217733
Chicago/Turabian StyleGothelf, Itamar, Maor Epstein, Adi Shiloh, Zachary Lebowitz, Yair Zlotnik, Raphael Hillel Sacho, Yana Mechnik Steen, Gal Ben-Arie, and Anat Horev. 2025. "Prophylactic Intra-Arterial Eptifibatide in Stent-Assisted Coiling and Flow Diverter Treatment of Cerebral Aneurysms: A Single-Center Retrospective Analysis" Journal of Clinical Medicine 14, no. 21: 7733. https://doi.org/10.3390/jcm14217733
APA StyleGothelf, I., Epstein, M., Shiloh, A., Lebowitz, Z., Zlotnik, Y., Sacho, R. H., Mechnik Steen, Y., Ben-Arie, G., & Horev, A. (2025). Prophylactic Intra-Arterial Eptifibatide in Stent-Assisted Coiling and Flow Diverter Treatment of Cerebral Aneurysms: A Single-Center Retrospective Analysis. Journal of Clinical Medicine, 14(21), 7733. https://doi.org/10.3390/jcm14217733







