Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock: A Narrative Review in Light of Recent Evidence
Abstract
1. Introduction
2. Intra-Aortic Balloon Pump
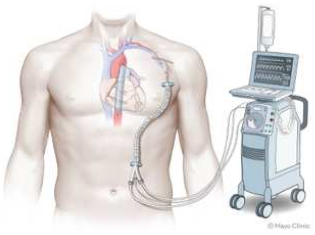
3. Microaxial Flow Pump
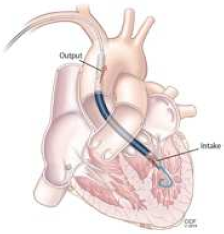
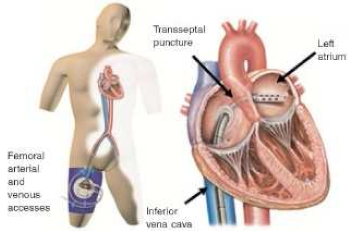
4. Tandem Heart
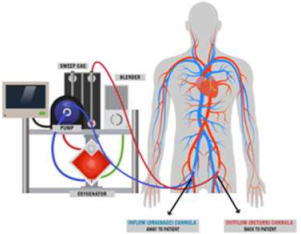
5. VA-ECMO
6. Comparative Analysis
7. Combined Strategy
8. Gaps and Future Directions
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| AKI | Acute kidney injury |
| AMI | Acute myocardial infarction |
| CS | Cardiogenic shock |
| HF | Heart failure |
| IABP | Intra-aortic balloon pump |
| LV | Left ventricle/left ventricular |
| MAP | Mean arterial pressure |
| MCS | Mechanical circulatory support |
| MODS | Multiorgan dysfunction syndrome |
| mAFP | Microaxial flow pump |
| OR | Odds ratio |
| PAD | Peripheral arterial disease |
| pMCS | Percutaneous mechanical circulatory support |
| PCI | Percutaneous coronary intervention |
| RV | Right ventricle/right ventricular |
| STEMI | ST-elevation myocardial infarction |
| VA-ECMO | Veno-arterial extracorporeal membrane oxygenation |
References
- Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Pahuja, M.; van Diepen, S.; Proudfoot, A.G.; Morrow, D.; Spitzer, E.; Nichol, G.; Weisfeldt, M.L.; Moscucci, M.; Lawler, P.R.; et al. Standardized Definitions for Cardiogenic Shock Research and Mechanical Circulatory Support Devices: Scientific Expert Panel from the Shock Academic Research Consortium (SHARC). Circulation 2023, 148, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, M.; Morici, N.; Frea, S.; Garatti, L.; Briani, M.; Sorini, C.; Villanova, L.; Corrada, E.; Sacco, A.; Moltrasio, M.; et al. Differences between cardiogenic shock related to acute decompensated heart failure and acute myocardial infarction. ESC Heart Fail. 2023, 10, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- Morici, N.; Frea, S.; Bertaina, M.; Sacco, A.; Corrada, E.; Dini, C.S.; Briani, M.; Tedeschi, M.; Saia, F.; Colombo, C.; et al. SCAI stage reclassification at 24 h predicts outcome of cardiogenic shock: Insights from the Altshock-2 registry. Catheter. Cardiovasc. Interv. 2023, 101, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Patnaik, S.; Patel, B.; Ram, P.; Garg, L.; Agarwal, M.; Agrawal, S.; Arora, S.; Patel, N.; Wald, J.; et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin. Res. Cardiol. 2018, 107, 287–303. [Google Scholar] [CrossRef]
- Harjola, V.; Lassus, J.; Sionis, A.; Køber, L.; Tarvasmäki, T.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur. J. Heart Fail. 2015, 17, 501–509. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef]
- Henry, T.D.; Tomey, M.I.; Tamis-Holland, J.E.; Thiele, H.; Rao, S.V.; Menon, V.; Klein, D.G.; Naka, Y.; Piña, I.L.; Kapur, N.K.; et al. Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2021, 143, E815–E829. [Google Scholar] [CrossRef]
- Webb, J.G.; Sleeper, L.A.; Buller, C.E.; Boland, J.; Palazzo, A.; Buller, E.; White, H.D.; Hochman, J.S. Implications of the timing of onset of cardiogenic shock after acute myocardial infarction: A report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 2000, 36, 1084–1090. [Google Scholar] [CrossRef]
- Hunziker, L.; Radovanovic, D.; Jeger, R.; Pedrazzini, G.; Cuculi, F.; Urban, P.; Erne, P.; Rickli, H.; Pilgrim, T.; the AMIS Plus Registry Investigators. Twenty-year trends in the incidence and outcome of cardiogenic shock in AMIS plus registry. Circ. Cardiovasc. Interv. 2019, 12, e007293. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; De Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Dzavik, V.; Buller, C.E.; Aylward, P.; Col, J.; White, H.D.; for the SHOCK Investigators. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA 2006, 295, 2511–2515. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2019, 74, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; Cleland, J.G.F.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Byrne, R.; Coughlan, J.J.; Rossello, X.; Ibanez, B.; Members of the Task Force for the 2023 ESC Guidelines for the Management of Acute Coronary Syndromes; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Werdan, K.; Gielen, S.; Ebelt, H.; Hochman, J.S. Mechanical circulatory support in cardiogenic shock. Eur. Heart J. 2014, 35, 156–167. [Google Scholar] [CrossRef]
- Baldetti, L.; Gallone, G.; Filiberti, G.; Pescarmona, L.; Cesari, A.; Rizza, V.; Roagna, E.; Gurrieri, D.; Peveri, B.; Nocera, L.; et al. Mixed Shock Complicating Cardiogenic Shock: Frequency, Predictors, and Clinical Outcomes. Circ. Heart Fail. 2024, 17, e011404. [Google Scholar] [CrossRef]
- Giannini, F.; Toselli, M.; Palmisano, A.; Cereda, A.; Vignale, D.; Leone, R.; Nicoletti, V.; Gnasso, C.; Monello, A.; Manfrini, M.; et al. Coronary and total thoracic calcium scores predict mortality and provides pathophysiologic insights in COVID-19 patients. J. Cardiovasc. Comput. Tomogr. 2021, 15, 421–430. [Google Scholar] [CrossRef]
- Kantrowitz, A.; Tjønneland, S.; Freed, P.S.; Phillips, S.J.; Butner, A.N.; Sherman, J.L. Initial Clinical Experience with Intraaortic Balloon Pumping in Cardiogenic Shock. JAMA 1968, 203, 113–118. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Lin, Y. Perspectives and Considerations of IABP in the Era of ECMO for Cardiogenic Shock. Adv. Ther. 2023, 40, 4151–4165. [Google Scholar] [CrossRef]
- Fuchs, R.M.; Brin, K.P.; Brinker, J.A.; Guzman, P.A.; Heuser, R.R.; Yin, F.C. Augmentation of regional coronary blood flow by intra-aortic balloon counterpulsation in patients with unstable angina. Circulation 1983, 68, 117–123. [Google Scholar] [CrossRef]
- Estep, J.D.; Cordero-Reyes, A.M.; Bhimaraj, A.; Trachtenberg, B.; Khalil, N.; Loebe, M.; Bruckner, B.; Orrego, C.M.; Bismuth, J.; Kleiman, N.S.; et al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail. 2013, 1, 382–388. [Google Scholar] [CrossRef]
- Barron, H.V.; Every, N.R.; Parsons, L.S.; Angeja, B.; Goldberg, R.J.; Gore, J.M.; Chou, T.M. The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: Data from the national registry of myocardial infarction 2. Am. Heart J. 2001, 141, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Thelemann, N.; Neumann, F.J.; Hausleiter, J.; Abdel-Wahab, M.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Hambrecht, R.; et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation 2019, 139, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 85, 22. [Google Scholar]
- Scoccia, A.; Gallone, G.; Cereda, A.; Palmisano, A.; Vignale, D.; Leone, R.; Nicoletti, V.; Gnasso, C.; Monello, A.; Khokhar, A.; et al. Impact of clinical and subclinical coronary artery disease as assessed by coronary artery calcium in COVID-19. Atherosclerosis 2021, 328, 136–143. [Google Scholar] [CrossRef]
- Malick, W.; Fried, J.A.; Masoumi, A.; Nair, A.; Zuver, A.; Huang, A.; Haythe, J.; Farr, M.; Rabbani, L.; Karmpaliotis, D.; et al. Comparison of the Hemodynamic Response to Intra-Aortic Balloon Counterpulsation in Patients with Cardiogenic Shock Resulting from Acute Myocardial Infarction Versus Acute Decompensated Heart Failure. Am. J. Cardiol. 2019, 124, 1947–1953. [Google Scholar] [CrossRef]
- Brown, M.A.; Sheikh, F.H.; Ahmed, S.; Najjar, S.S.; Molina, E.J. Intra-aortic balloon pump as a bridge to durable left ventricular assist device. J. Am. Heart Assoc. 2021, 10, e019376. [Google Scholar] [CrossRef]
- Uil, C.A.D.; Van Mieghem, N.M.; Bastos, M.B.; Jewbali, L.S.; Lenzen, M.J.; Engstrom, A.E.; Bunge, J.J.; Brugts, J.J.; Manintveld, O.C.; Daemen, J.; et al. Primary intra-aortic balloon support versus inotropes for decompensated heart failure and low output: A randomised trial. EuroIntervention 2019, 15, 586–593. [Google Scholar] [CrossRef]
- Morici, N.; Sacco, A.; Frea, S.; Rota, M.; Villanova, L.; Gravinese, C.; Dini, C.S.; D’Ettore, N.; Maj, G.; De Lio, G.; et al. Early Intra-Aortic Balloon Support for Heart Failure-Related Cardiogenic Shock: A Randomized Clinical Trial. J. Am. Coll. Cardiol. 2025, 85, 1587–1597. [Google Scholar] [CrossRef]
- Burzotta, F.; Trani, C.; Doshi, S.N.; Townend, J.; van Geuns, R.J.; Hunziker, P.; Schieffer, B.; Karatolios, K.; Møller, J.E.; Ribichini, F.L.; et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int. J. Cardiol. 2015, 201, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Wayangankar, S.A.; Bangalore, S.; McCoy, L.A.; Jneid, H.; Latif, F.; Karrowni, W.; Charitakis, K.; Feldman, D.N.; Dakik, H.A.; Mauri, L.; et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: A report from the cathpci registry. JACC Cardiovasc. Interv. 2016, 9, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Uriel, N.; Carnicelli, A.; John, K.; Li, S.; Kong, C.; Zweck, E.; Sinha, S.S.; Ton, V.K.; Garan, A.R.; et al. Outcomes of patients supported on Impella 5.5 for more than 14 days: A Cardiogenic Shock Working Group registry analysis. J. Heart Lung Transplant. 2025, 44, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ako, J.; Toda, K.; Hirayama, A.; Kinugawa, K.; Kobayashi, Y.; Ono, M.; Nishimura, T.; Sato, N.; Shindo, T.; et al. Short-Term Outcomes of Impella Support in Japanese Patients with Cardiogenic Shock Due to Acute Myocardial Infarction―Japanese Registry for Percutaneous Ventricular Assist Device (J-PVAD). Circ. J. 2023, 87, 588–597. [Google Scholar] [CrossRef]
- Pieri, M.; Iannaccone, M.; Burzotta, F.; Botti, G.; Aurigemma, C.; Trani, C.; Ajello, S.; Altizio, S.; Sanna, T.; Romagnoli, E.; et al. Can a mechanical circulatory support comprehensive approach to cardiogenic shock at referral centers reduce 30-day mortality? Front. Cardiovasc. Med. 2024, 11, 1509162. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Schreiber, T.; Wohns, D.H.W.; Rihal, C.; Naidu, S.S.; Civitello, A.B.; Dixon, S.R.; Massaro, J.M.; Maini, B.; Ohman, E.M. The current use of impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: Results from the USpella Registry. J. Interv. Cardiol. 2014, 27, 1–11. [Google Scholar] [CrossRef]
- Iannaccone, M.; Albani, S.; Giannini, F.; Colangelo, S.; Boccuzzi, G.G.; Garbo, R.; Brilakis, E.S.; D’AScenzo, F.; de Ferrari, G.M.; Colombo, A. Short term outcomes of Impella in cardiogenic shock: A review and meta-analysis of observational studies. Int. J. Cardiol. 2021, 324, 44–51. [Google Scholar] [CrossRef]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734–745. [Google Scholar] [CrossRef]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella support for acute myocardial infarction complicated by cardiogenic shock: Matched-pair iabp-shock II trial 30-day mortality analysis. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Tarvasmäki, T.; Haapio, M.; Mebazaa, A.; Sionis, A.; Silva-Cardoso, J.; Tolppanen, H.; Lindholm, M.G.; Pulkki, K.; Parissis, J.; Harjola, V.; et al. Acute kidney injury in cardiogenic shock: Definitions, incidence, haemodynamic alterations, and mortality. Eur. J. Heart Fail. 2018, 20, 572–581. [Google Scholar] [CrossRef]
- Zweck, E.; Hassager, C.; Beske, R.P.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; et al. Microaxial Flow Pump Use and Renal Outcomes in Infarct-Related Cardiogenic Shock: A Secondary Analysis of the DanGer Shock Trial. Circulation 2024, 150, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Basir, M.B.; Schreiber, T.L.; Grines, C.L.; Dixon, S.R.; Moses, J.W.; Maini, B.S.; Khandelwal, A.K.; Ohman, E.M.; O’Neill, W.W. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am. J. Cardiol. 2017, 119, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Swain, L.; Reyelt, L.; Bhave, S.; Qiao, X.; Thomas, C.J.; Zweck, E.; Crowley, P.; Boggins, C.; Esposito, M.; Chin, M.; et al. Transvalvular Ventricular Unloading Before Reperfusion in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Udesen, N.L.J.; Beske, R.P.; Hassager, C.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; et al. Microaxial Flow Pump Hemodynamic and Metabolic Effects in Infarct-Related Cardiogenic Shock: A Substudy of the DanGer Shock Randomized Clinical Trial. JAMA Cardiol. 2024, 10, 9–16. [Google Scholar] [CrossRef]
- Basir, M.B.; Lemor, A.; Gorgis, S.; Patel, K.C.; Kolski, B.C.; Bharadwaj, A.S.; Todd, J.W.; Tehrani, B.N.; Truesdell, A.G.; Lasorda, D.M.; et al. Early Utilization of Mechanical Circulatory Support in Acute Myocardial Infarction Complicated by Cardiogenic Shock: The National Cardiogenic Shock Initiative. J. Am. Heart Assoc. 2023, 12, e031401. [Google Scholar] [CrossRef]
- Iannaccone, M.; Franchin, L.; Hanson, I.D.; Boccuzzi, G.; Basir, M.B.; Truesdell, A.G.; O’Neill, W. Timing of impella placement in PCI for acute myocardial infarction complicated by cardiogenic shock: An updated meta-analysis. Int. J. Cardiol. 2022, 362, 47–54. [Google Scholar] [CrossRef]
- Møller, J.E.; Beske, R.P.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; et al. Long-Term Outcomes of the DanGer Shock Trial. N. Engl. J. Med. 2025, 393, 1037–1038. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kim, R.J.; Moses, J.W.; Stone, G.W.; Udelson, J.E.; Ben-Yehuda, O.; Redfors, B.; Issever, M.O.; Josephy, N.; Polak, S.J.; et al. Primary left ventricular unloading with delayed reperfusion in patients with anterior ST-elevation myocardial infarction: Rationale and design of the STEMI-DTU randomized pivotal trial. Am. Heart J. 2022, 254, 122–132. [Google Scholar] [CrossRef]
- Jain, P.; Thayer, K.L.; Abraham, J.; Everett, K.D.; Pahuja, M.; Whitehead, E.H.; Schwartz, B.P.; Lala, A.; Sinha, S.S.; Kanwar, M.K.; et al. Right Ventricular Dysfunction Is Common and Identifies Patients at Risk of Dying in Cardiogenic Shock. J. Card. Fail. 2021, 27, 1061–1072. [Google Scholar] [CrossRef]
- Engström, A.E.; Vis, M.M.; Bouma, B.J.; Van Den Brink, R.B.A.; Baan, J.; Claessen, B.E.P.M.; Kikkert, W.J.; Sjauw, K.D.; Meuwissen, M.; Koch, K.T.; et al. Right ventricular dysfunction is an independent predictor for mortality in ST-elevation myocardial infarction patients presenting with cardiogenic shock on admission. Eur. J. Heart Fail. 2010, 12, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, M.; Galluzzo, A.; Morici, N.; Sacco, A.; Oliva, F.; Valente, S.; D’aScenzo, F.; Frea, S.; Sbarra, P.; Petitti, E.; et al. Pulmonary Artery Catheter Monitoring in Patients with Cardiogenic Shock: Time for a Reappraisal? Card. Fail. Rev. 2022, 8, e15. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.A.; Brittain, E.L.; Tedford, R.J. Right Ventricular Failure. N. Engl. J. Med. 2023, 388, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Platz, E.; Cullen, L.; Tavazzi, G.; Christ, M.; Cowie, M.R.; Maisel, A.S.; Masip, J.; Miro, O.; McMurray, J.J.; et al. Expert consensus document: Echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat. Rev. Cardiol. 2017, 14, 427–440. [Google Scholar] [CrossRef]
- Gramegna, M.; Vandenbriele, C.; Tavazzi, G.; Basir, M.B.; Bleakley, C.; Iannaccone, M.; Kretzschmar, D.; Maisano, F.; Scandroglio, A.M.; Schrage, B.; et al. Percutaneous mechanical circulatory support for acute right heart failure: A practical approach. ESC Heart Fail. 2025, 12, 2652–2668. [Google Scholar] [CrossRef]
- Anderson, M.B.; Goldstein, J.; Milano, C.; Morris, L.D.; Kormos, R.L.; Bhama, J.; Kapur, N.K.; Bansal, A.; Garcia, J.; Baker, J.N.; et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J. Heart Lung Transplant. 2015, 34, 1549–1560. [Google Scholar] [CrossRef]
- Kuchibhotla, S.; Esposito, M.L.; Breton, C.; Pedicini, R.; Mullin, A.; O’KElly, R.; Anderson, M.; Morris, D.L.; Batsides, G.; Ramzy, D.; et al. Acute biventricular mechanical circulatory support for cardiogenic shock. J. Am. Heart Assoc. 2017, 6, e006670. [Google Scholar] [CrossRef]
- Megaly, M.; Gandolfo, C.; Zakhour, S.; Jiang, M.; Burgess, K.; Chetcuti, S.; Ragosta, M.; Adler, E.; Coletti, A.; O’NEill, B.; et al. Utilization of TandemHeart in cardiogenic shock: Insights from the THEME registry. Catheter. Cardiovasc. Interv. 2023, 101, 756–763. [Google Scholar] [CrossRef]
- Burkhoff, D.; Cohen, H.; Brunckhorst, C.; O’Neill, W.W.; TandemHeart Investigators Group. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am. Heart J. 2006, 152, 469.e1–469.e8. [Google Scholar] [CrossRef]
- Kar, B.; Gregoric, I.D.; Basra, S.S.; Idelchik, G.M.; Loyalka, P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J. Am. Coll. Cardiol. 2011, 57, 688–696. [Google Scholar] [CrossRef]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for Adults. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef]
- Telukuntla, K.S.; Estep, J.D. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist DeBakey Cardiovasc. J. 2020, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nakata, J.; Yamamoto, T.; Saku, K.; Ikeda, Y.; Unoki, T.; Asai, K. Mechanical circulatory support in cardiogenic shock. J. Intensive Care 2023, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Fadel, R.A.; Jabri, A.; Cowger, J.; O’Neill, B.; Basir, M.B.; Gonzalez, P.E.; Frisoli, T.; Lee, J.; Généreux, P.; et al. Left Atrial Veno-Arterial Extracorporeal Membrane Oxygenation in Valvular Cardiogenic Shock. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102615. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1866 adult patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Zeymer, U.; Bueno, H.; Granger, C.B.; Hochman, J.; Huber, K.; Lettino, M.; Price, S.; Schiele, F.; Tubaro, M.; Vranckx, P.; et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: A document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 183–197. [Google Scholar]
- Banning, A.S.; Sabaté, M.; Orban, M.; Gracey, J.; López-Sobrino, T.; Massberg, S.; Kastrati, A.; Bogaerts, K.; Adriaenssens, T.; Berry, C.; et al. Venoarterial extracorporeal membrane oxygenation or standard care in patients with cardiogenic shock complicating acute myocardial infarction: The multicentre, randomised EURO SHOCK trial. EuroIntervention 2023, 19, 482–492. [Google Scholar] [CrossRef]
- Ostadal, P.; Rokyta, R.; Karasek, J.; Kruger, A.; Vondrakova, D.; Janotka, M.; Naar, J.; Smalcova, J.; Hubatova, M.; Hromadka, M.; et al. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation 2023, 147, 454–464. [Google Scholar] [CrossRef]
- Thiele, H.; Desch, S.; Freund, A.; Zeymer, U. Why VA-ECMO should not be used routinely in AMI-Cardiogenic Shock. J. Heart Lung Transplant. 2024, 43, 695–699. [Google Scholar] [CrossRef]
- Zeymer, U.; Freund, A.; Hochadel, M.; Ostadal, P.; Belohlavek, J.; Rokyta, R.; Massberg, S.; Brunner, S.; Lüsebrink, E.; Flather, M.; et al. Venoarterial extracorporeal membrane oxygenation in patients with infarct-related cardiogenic shock: An individual patient data meta-analysis of randomised trials. Lancet 2023, 402, 1338–1346. [Google Scholar] [CrossRef]
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Fröhlich, G.; Bott-Flügel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schömig, A. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588. [Google Scholar] [CrossRef]
- Cheng, J.M.; Uil, C.A.D.; Hoeks, S.E.; van der Ent, M.; Jewbali, L.S.; van Domburg, R.T.; Serruys, P.W. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: A meta-analysis of controlled trials. Eur. Heart J. 2009, 30, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.P.; Spertus, J.A.; Curtis, J.P.; Desai, N.; Masoudi, F.A.; Bach, R.G.; McNeely, C.; Al-Badarin, F.; House, J.A.; Kulkarni, H.; et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention with Mechanical Circulatory Support. Circulation 2020, 141, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Imori, Y.; D’Ascenzo, F.; Gori, T.; Münzel, T.; Fabrizio, U.; Campo, G.; Cerrato, E.; Napp, L.C.; Iannaccone, M.; Ghadri, J.R.; et al. Impact of postdilatation on performance of bioresorbable vascular scaffolds in patients with acute coronary syndrome compared with everolimus-eluting stents: A propensity score-matched analysis from a multicenter “real-world” registry. Cardiol. J. 2016, 23, 374–383. [Google Scholar] [CrossRef]
- Desch, S.; Zeymer, U.; Akin, I.; Behnes, M.; Duerschmied, D.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; et al. Routine Extracorporeal Life Support in Infarct-Related Cardiogenic Shock: 1-Year Results of the ECLS-SHOCK Trial. Eur. Heart J. 2024, 45, 4200–4203. [Google Scholar] [CrossRef]
- Tarantini, G.; Panza, A.; Lorenzoni, G.; Gregori, D.; Masiero, G. Breaking Down Cardiogenic Shock: An Analytical Reflection on the DanGer-SHOCK and ECLS-SHOCK Trials. Am. J. Cardiol. 2025, 236, 30–33. [Google Scholar] [CrossRef]
- Bhatia, K.; Jain, V.; Hendrickson, M.J.; Aggarwal, D.; Aguilar-Gallardo, J.S.; Lopez, P.D.; Narasimhan, B.; Wu, L.; Arora, S.; Joshi, A.; et al. Meta-Analysis Comparing Venoarterial Extracorporeal Membrane Oxygenation with or Without Impella in Patients with Cardiogenic Shock. Am. J. Cardiol. 2022, 181, 94–101. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; O’Horo, J.C.; Antharam, P.; Ananthaneni, S.; Vallabhajosyula, S.; Stulak, J.M.; Eleid, M.F.; Dunlay, S.M.; Gersh, B.J.; Rihal, C.S.; et al. Concomitant intra-aortic balloon pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. Circ. Cardiovasc. Interv. 2018, 11, e006930. [Google Scholar] [CrossRef]
- Thiele, H.; Henriques, J.P.S.; Bogerd, M.; Seyfarth, M.; Burkhoff, D.; Ostadal, P.; Rokyta, R.; Belohlavek, J.; Massberg, S.; Flather, M.; et al. Temporary mechanical circulatory support in infarct-related cardiogenic shock: An individual patient data meta-analysis of randomised trials with 6-month follow-up. Lancet 2024, 404, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Deseda, G.C.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated with Lower Mortality in Patients with Cardiogenic Shock Treated with Venoarterial Extracorporeal Membrane Oxygenation Results from an International, Multicenter Cohort Study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Lipinski, J.; Al-Kindi, S.G.; Patel, T.; Saric, P.; Li, J.; Nadeem, F.; Ladas, T.; Alaiti, A.; Phillips, A.; et al. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with impella is associated with improved outcomes in refractory cardiogenic shock. Asaio J. 2019, 65, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Sundermeyer, J.; Blankenberg, S.; Colson, P.; Eckner, D.; Eden, M.; Eitel, I.; Frank, D.; Frey, N.; Graf, T.; et al. Timing of Active Left Ventricular Unloading in Patients on Venoarterial Extracorporeal Membrane Oxygenation Therapy. JACC: Heart Fail. 2023, 11, 321–330. [Google Scholar] [CrossRef]
- Iannaccone, M.; Venuti, G.; di Simone, E.; De Filippo, O.; Bertaina, M.; Colangelo, S.; Boccuzzi, G.; de Piero, M.E.; Attisani, M.; Barbero, U.; et al. Comparison of ECMO vs ECpella in Patients with Non-Post-Pericardiotomy Cardiogenic Shock: An Updated Meta-Analysis. Cardiovasc. Revascularization Med. 2022, 40, 134–141. [Google Scholar] [CrossRef]
- Low, C.J.W.; Ling, R.R.; Lau, M.P.X.L.; Liu, N.S.H.; Tan, M.; Tan, C.S.; Lim, S.L.; Rochwerg, B.; Combes, A.; Brodie, D.; et al. Mechanical circulatory support for cardiogenic shock: A network meta-analysis of randomized controlled trials and propensity score-matched studies. Intensive Care Med. 2024, 50, 209–221. [Google Scholar] [CrossRef]
- Senman, B.; Jentzer, J.C.; Barnett, C.F.; Bartos, J.A.; Berg, D.D.; Chih, S.; Drakos, S.G.; Dudzinski, D.M.; Elliott, A.; Gage, A.; et al. Need for a Cardiogenic Shock Team Collaborative—Promoting a Team-Based Model of Care to Improve Outcomes and Identify Best Practices. J. Am. Heart Assoc. 2024, 13, e031979. [Google Scholar] [CrossRef]
| ESC Heart Failure Guidelines 2021 |
|
|
| ACVC position statement 2020 | All of the following:
| Clinical signs/symptoms (at least one of the following):
And elevated left ventricular filling pressures:
|
| Shock Academic Research Consortium (Clinical trial def) | CLINICAL PRACTICE DEFINITION
| Hemodynamic criteria * (optional)
|
 |  |  |  |  | |
| IAPB | IMPELLA CP | IMPELLA RP | TANDEM HEART | VA-ECMO | |
| CO | ↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑↑ |
| CVP | no effect/↓ | no effect/↓ | ↓↓ | ↓↓ | ↓↓ |
| PCWP/LVEDP | no effect/↓ | ↓↓ | no effect/↑ | ↓↓ | ↑ |
| MAP | ↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑↑ |
| TOTAL CPO | no effect/↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑↑ |
| AFTERLOAD | ↓ | no effect | no effect | ↑↑ | ↑↑ |
| SAT (O2) | no effect | no effect | no effect | no effect | ↑↑ |
| SHEAT SIZE | Arterial, 7–8 Fr | Arterial, 14 Fr | Venous | Arterial, 15–19 Fr Venous, 21 Fr | Arterial, 15–17 Fr Venous, 21–23 Fr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paragliola, V.; Gamardella, M.; Franchin, L.; Bertaina, M.; Colombo, F.; Zanini, P.; Colangelo, S.; Sbarra, P.; Boccuzzi, G.; Iannaccone, M. Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock: A Narrative Review in Light of Recent Evidence. J. Clin. Med. 2025, 14, 7731. https://doi.org/10.3390/jcm14217731
Paragliola V, Gamardella M, Franchin L, Bertaina M, Colombo F, Zanini P, Colangelo S, Sbarra P, Boccuzzi G, Iannaccone M. Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock: A Narrative Review in Light of Recent Evidence. Journal of Clinical Medicine. 2025; 14(21):7731. https://doi.org/10.3390/jcm14217731
Chicago/Turabian StyleParagliola, Vincenzo, Marco Gamardella, Luca Franchin, Maurizio Bertaina, Francesco Colombo, Paola Zanini, Salvatore Colangelo, Pierluigi Sbarra, Giacomo Boccuzzi, and Mario Iannaccone. 2025. "Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock: A Narrative Review in Light of Recent Evidence" Journal of Clinical Medicine 14, no. 21: 7731. https://doi.org/10.3390/jcm14217731
APA StyleParagliola, V., Gamardella, M., Franchin, L., Bertaina, M., Colombo, F., Zanini, P., Colangelo, S., Sbarra, P., Boccuzzi, G., & Iannaccone, M. (2025). Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock: A Narrative Review in Light of Recent Evidence. Journal of Clinical Medicine, 14(21), 7731. https://doi.org/10.3390/jcm14217731







