Bacterial Isolates from Bronchoalveolar Lavage in Pediatric Patients with Protracted Bacterial Bronchitis or Bronchiectasis: A Retrospective Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
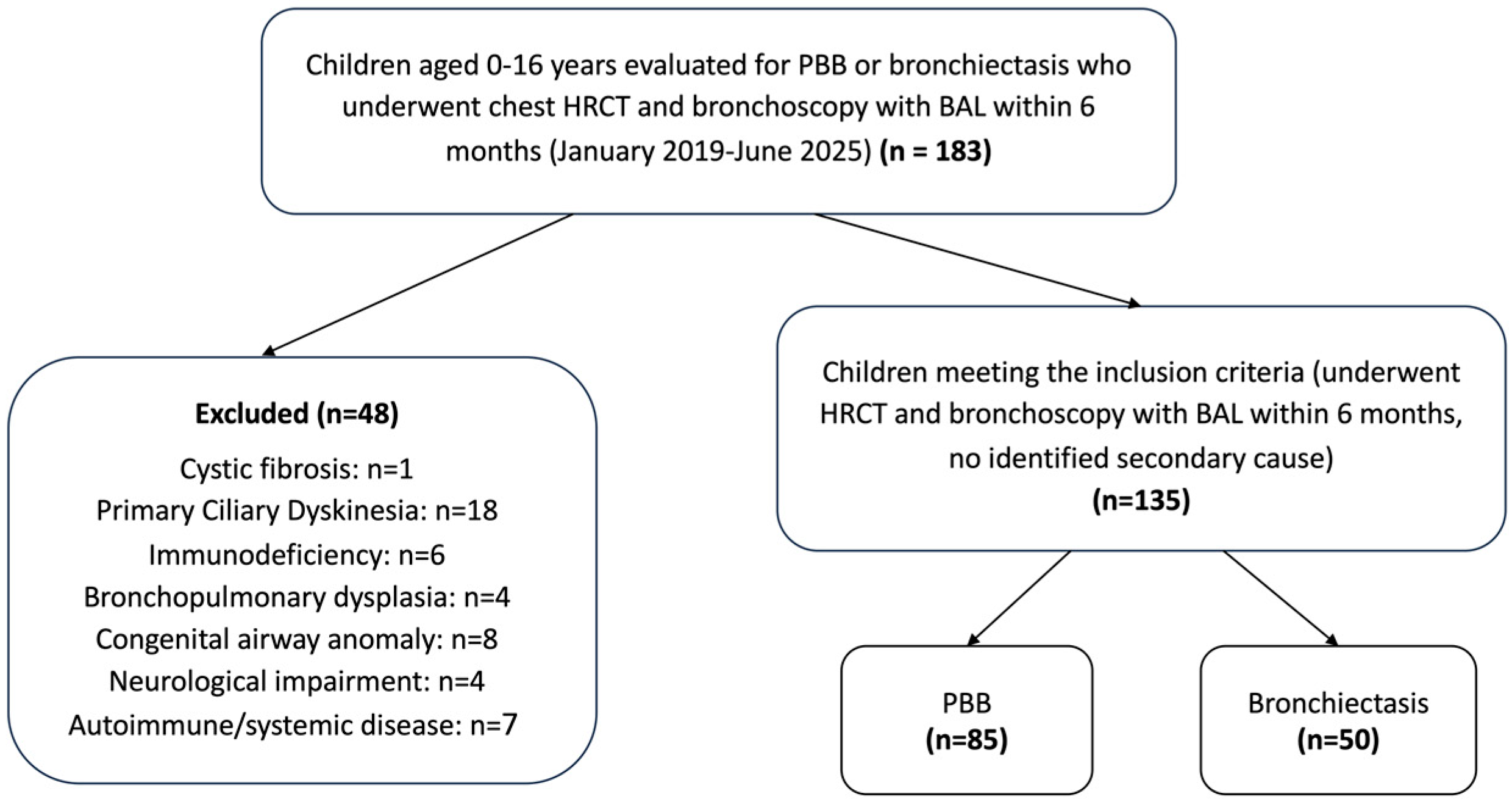
2.2. Study Population
2.3. Data Collection
2.4. Outcomes and Definitions
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. BAL Microbiology
3.3. Logistic Regression Analyses
3.4. Pathogen-Specific Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBB | Protracted bacterial bronchitis |
| BAL | Bronchoalveolar lavage |
| HRCT | High-resolution computed tomography |
| BAR | Broncho-arterial ratio |
| CSLD | Chronic suppurative lung disease |
| PCD | Primary ciliary dyskinesia |
| CF | Cystic fibrosis |
| ERS | European Respiratory Society |
| LMA | Laryngeal mask airway |
| CFU | Colony-forming units |
| IQR/IQRs | Interquartile ranges |
| VIFs | Variance inflation factors |
| OR/ORs | Odds ratios |
| CI/CIs | Confidence intervals |
| PCR | Polymerase chain reaction |
References
- Chang, A.B.; Upham, J.W.; Masters, I.B.; Redding, G.R.; Gibson, P.G.; Marchant, J.M.; Grimwood, K. Protracted bacterial bronchitis: The last decade and the road ahead. Pediatr. Pulmonol. 2016, 51, 225–242. [Google Scholar] [CrossRef]
- Kapur, N.; Masel, J.P.; Watson, D.; Masters, I.B.; Chang, A.B. Bronchoarterial ratio on high-resolution CT scan of the chest in children without pulmonary pathology: Need to redefine bronchial dilatation. Chest 2011, 139, 1445–1450. [Google Scholar] [CrossRef]
- Wurzel, D.F.; Marchant, J.M.; Yerkovich, S.T.; Upham, J.W.; Petsky, H.L.; Smith-Vaughan, H.; Masters, B.; Buntain, H.; Chang, A.B. Protracted Bacterial Bronchitis in Children: Natural History and Risk Factors for Bronchiectasis. Chest 2016, 150, 1101–1108. [Google Scholar] [CrossRef]
- Ruffles, T.J.C.; Marchant, J.M.; Masters, I.B.; Yerkovich, S.T.; Wurzel, D.F.; Gibson, P.G.; Busch, G.; Baines, K.J.; Simpson, J.L.; Smith-Vaughan, H.C.; et al. Outcomes of protracted bacterial bronchitis in children: A 5-year prospective cohort study. Respirology 2021, 26, 241–248. [Google Scholar] [CrossRef]
- Cole, P.J. Inflammation: A two-edged sword--the model of bronchiectasis. Eur. J. Respir. Dis. Suppl. 1986, 147, 6–15. [Google Scholar]
- Marsh, R.L.; Binks, M.J.; Smith-Vaughan, H.C.; Janka, M.; Clark, S.; Richmond, P.; Chang, A.B.; Thornton, R.B. Prevalence and subtyping of biofilms present in bronchoalveolar lavage from children with protracted bacterial bronchitis or non-cystic fibrosis bronchiectasis: A cross-sectional study. Lancet Microbe 2022, 3, e215–e223. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Biofilm Production and Its Implications in Pediatrics. Microorganisms 2024, 12, 1522. [Google Scholar] [CrossRef] [PubMed]
- Pizzutto, S.J.; Grimwood, K.; Bauert, P.; Schutz, K.L.; Yerkovich, S.T.; Upham, J.W.; Chang, A.B. Bronchoscopy contributes to the clinical management of indigenous children newly diagnosed with bronchiectasis. Pediatr. Pulmonol. 2013, 48, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kantar, A.; Chang, A.B.; Shields, M.D.; Marchant, J.M.; Grimwood, K.; Grigg, J.; Priftis, K.N.; Cutrera, R.; Midulla, F.; Brand, P.L.P.; et al. ERS statement on protracted bacterial bronchitis in children. Eur. Respir. J. 2017, 50, 1602139. [Google Scholar] [CrossRef]
- Pritchard, M.G.; Lenney, W.; Gilchrist, F.J. Outcomes in children with protracted bacterial bronchitis confirmed by bronchoscopy. Arch. Dis. Child. 2015, 100, 112. [Google Scholar] [CrossRef]
- Kapur, N.; Grimwood, K.; Masters, I.B.; Morris, P.S.; Chang, A.B. Lower airway microbiology and cellularity in children with newly diagnosed non-CF bronchiectasis. Pediatr. Pulmonol. 2012, 47, 300–307. [Google Scholar] [CrossRef]
- Priftis, K.N.; Litt, D.; Manglani, S.; Anthracopoulos, M.B.; Thickett, K.; Tzanakaki, G.; Fenton, P.; Syrogiannopoulos, G.A.; Vogiatzi, A.; Douros, K.; et al. Bacterial bronchitis caused by Streptococcus pneumoniae and nontypable Haemophilus influenzae in children: The impact of vaccination. Chest 2013, 143, 152–157. [Google Scholar] [CrossRef]
- de Vries, J.J.V.; Chang, A.B.; Marchant, J.M. Comparison of bronchoscopy and bronchoalveolar lavage findings in three types of suppurative lung disease. Pediatr. Pulmonol. 2018, 53, 467–474. [Google Scholar] [CrossRef]
- de Blic, J.; Midulla, F.; Barbato, A.; Clement, A.; Dab, I.; Eber, E.; Green, C.; Grigg, J.; Kotecha, S.; Kurland, G.; et al. Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. European Respiratory Society. Eur. Respir. J. 2000, 15, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Faro, A.; Wood, R.E.; Schechter, M.S.; Leong, A.B.; Wittkugel, E.; Abode, K.; Chmiel, J.F.; Daines, C.; Davis, S.; Eber, E.; et al. Official American Thoracic Society technical standards: Flexible airway endoscopy in children. Am. J. Respir. Crit. Care Med. 2015, 191, 1066–1080. [Google Scholar] [CrossRef]
- Radhakrishnan, D.; Yamashita, C.; Gillio-Meina, C.; Fraser, D.D. Translational research in pediatrics III: Bronchoalveolar lavage. Pediatrics 2014, 134, 135–154. [Google Scholar] [CrossRef]
- Gaillard, E.A.; Kuehni, C.E.; Turner, S.; Goutaki, M.; Holden, K.A.; de Jong, C.C.M.; Lex, C.; Lo, D.K.H.; Lucas, J.S.; Midulla, F.; et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5-16 years. Eur. Respir. J. 2021, 58, 2004173. [Google Scholar] [CrossRef]
- Makrinioti, H.; Fainardi, V.; Bonnelykke, K.; Custovic, A.; Cicutto, L.; Coleman, C.; Eiwegger, T.; Kuehni, C.; Moeller, A.; Pedersen, E.; et al. European Respiratory Society statement on preschool wheezing disorders: Updated definitions, knowledge gaps and proposed future research directions. Eur. Respir. J. 2024, 64, 2400624. [Google Scholar] [CrossRef]
- Wallis, C.; Alexopoulou, E.; Antón-Pacheco, J.L.; Bhatt, J.M.; Bush, A.; Chang, A.B.; Charatsi, A.M.; Coleman, C.; Depiazzi, J.; Douros, K.; et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur. Respir. J. 2019, 54, 1900382. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, M.; Turcios, N.; Aponte, V.; Jenkins, M.; Leitman, B.S.; McCauley, D.I.; Naidich, D.P. Cystic fibrosis: Scoring system with thin-section CT. Radiology 1991, 179, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.F.; Ibiapina Cda, C.; Alvim, C.G.; Camargos, P.A.; Figueiredo, R.; Pedrosa, J.F. Correlation between Bhalla score and spirometry in children and adolescents with cystic fibrosis. Rev. Assoc. Med. Bras. 2014, 60, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Wiltingh, H.; Marchant, J.M.; Goyal, V. Cough in Protracted Bacterial Bronchitis and Bronchiectasis. J. Clin. Med. 2024, 13, 3305. [Google Scholar] [CrossRef]
- Marrella, V.; Nicchiotti, F.; Cassani, B. Microbiota and Immunity during Respiratory Infections: Lung and Gut Affair. Int. J. Mol. Sci. 2024, 25, 4051. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Montalvo, A.; Gohy, S.; Rombaux, P.; Pilette, C.; Hox, V. The Role of IgA in Chronic Upper Airway Disease: Friend or Foe? Front. Allergy 2022, 3, 852546. [Google Scholar] [CrossRef]
- Saliu, F.; Rizzo, G.; Bragonzi, A.; Cariani, L.; Cirillo, D.M.; Colombo, C.; Daccò, V.; Girelli, D.; Rizzetto, S.; Sipione, B.; et al. Chronic infection by nontypeable Haemophilus influenzae fuels airway inflammation. ERJ Open Res. 2021, 7, 00614-2020. [Google Scholar] [CrossRef] [PubMed]
- Jurcisek, J.A.; Bakaletz, L.O. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J. Bacteriol. 2007, 189, 3868–3875. [Google Scholar] [CrossRef]
- Chang, A.B.; Fortescue, R.; Grimwood, K.; Alexopoulou, E.; Bell, L.; Boyd, J.; Bush, A.; Chalmers, J.D.; Hill, A.T.; Karadag, B.; et al. European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur. Respir. J. 2021, 58, 2002990. [Google Scholar] [CrossRef]
- Chang, A.B.; Oppenheimer, J.J.; Irwin, R.S. Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report. Chest 2020, 158, 303–329. [Google Scholar] [CrossRef]
- Goyal, V.; Grimwood, K.; Marchant, J.; Masters, I.B.; Chang, A.B. Does failed chronic wet cough response to antibiotics predict bronchiectasis? Arch. Dis. Child. 2014, 99, 522–525. [Google Scholar] [CrossRef]
- Zemanick, E.T.; Wagner, B.D.; Robertson, C.E.; Ahrens, R.C.; Chmiel, J.F.; Clancy, J.P.; Gibson, R.L.; Harris, W.T.; Kurland, G.; Laguna, T.A.; et al. Airway microbiota across age and disease spectrum in cystic fibrosis. Eur. Respir. J. 2017, 50, 1700832. [Google Scholar] [CrossRef]
- Atto, B.; Anteneh, Y.; Bialasiewicz, S.; Binks, M.J.; Hashemi, M.; Hill, J.; Thornton, R.B.; Westaway, J.; Marsh, R.L. The Respiratory Microbiome in Paediatric Chronic Wet Cough: What Is Known and Future Directions. J. Clin. Med. 2023, 13, 171. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Secretory IgA: Designed for Anti-Microbial Defense. Front. Immunol. 2013, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Bosch, A.A.; Biesbroek, G.; Trzcinski, K.; Sanders, E.A.; Bogaert, D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013, 9, e1003057. [Google Scholar] [CrossRef]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Miao, X.Y.; Ji, X.B.; Lu, H.W.; Yang, J.W.; Xu, J.F. Distribution of Major Pathogens from Sputum and Bronchoalveolar Lavage Fluid in Patients with Noncystic Fibrosis Bronchiectasis: A Systematic Review. Chin. Med. J. 2015, 128, 2792–2797. [Google Scholar] [CrossRef]
- Tunney, M.M.; Einarsson, G.G.; Wei, L.; Drain, M.; Klem, E.R.; Cardwell, C.; Ennis, M.; Boucher, R.C.; Wolfgang, M.C.; Elborn, J.S. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am. J. Respir. Crit. Care Med. 2013, 187, 1118–1126. [Google Scholar] [CrossRef]
- Rogers, G.B.; Carroll, M.P.; Serisier, D.J.; Hockey, P.M.; Jones, G.; Bruce, K.D. characterization of bacterial community diversity in cystic fibrosis lung infections by use of 16s ribosomal DNA terminal restriction fragment length polymorphism profiling. J. Clin. Microbiol. 2004, 42, 5176–5183. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Chalmers, J.D. Viruses in bronchiectasis. ERJ Open Res. 2025, 11, 01131-2024. [Google Scholar] [CrossRef] [PubMed]

| PBB (n = 85) | BE (n = 50) | p-Value | |
|---|---|---|---|
| Gender | |||
| Male | 48 (56.5) | 29 (58) | 0.862 |
| Female | 37 (43.5) | 21 (42) | |
| Age | 4.2 (2.2–6.1) | 7.8 (5.7–12.1) | <0.001 |
| Bhalla score * | - | 7 (6–8) | |
| Comorbidities | |||
| Tracheomalacia/bronchomalacia | 35 (41.2) | 21 (42) | 0.925 |
| Asthma | 15 (17.6) | 11 (22) | 0.536 |
| Lower airway infection ** | 69 (81.2) | 37 (74) | 0.327 |
| H. influenzae | 29 (34.1) | 14 (28) | 0.461 |
| S. pneumoniae | 16 (18.8) | 4 (8) | 0.087 |
| M. catarrhalis | 18 (21.2) | 4 (8) | 0.045 |
| H. parainfluenzae | 9 (10.6) | 7 (14) | 0.554 |
| P. aeruginosa | 7 (8.2) | 7 (14) | 0.289 |
| S. aureus | 11 (12.9) | 9 (18) | 0.424 |
| Other Gram-negative | 7 (8.2) | 1 (2) | 0.138 |
| Polymicrobial infection *** | 22 (25.9) | 8 (16) | 0.182 |
| Outcome | Predictor | OR (95% CI) | p-Value |
|---|---|---|---|
| Lower airway infection | Diagnosis (BE vs. PBB) | 0.93 (0.35–2.51) | 0.888 |
| Gender (female vs. male) | 0.98 (0.42–2.32) | 0.971 | |
| Age (years) | 0.92 (0.82–1.04) | 0.182 | |
| Tracheomalacia/bronchomalacia (yes vs. no) | 0.53 (0.23–1.26) | 0.152 | |
| Asthma (yes vs. no) | 0.84 (0.30–2.31) | 0.734 | |
| Polymicrobial infection | Diagnosis (BE vs. PBB) | 1.15 (0.40–3.32) | 0.803 |
| Gender (female vs. male) | 0.96 (0.40–2.26) | 0.916 | |
| Age (years) | 0.81 (0.69–0.95) | 0.009 | |
| Tracheomalacia/bronchomalacia (yes vs. no) | 0.57 (0.23–1.45) | 0.240 | |
| Asthma (yes vs. no) | 1.61 (0.53–4.92) | 0.401 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| H. influenzae | 1.07 | 0.43–2.69 | 0.887 |
| S. pneumoniae | 0.49 | 0.14–1.79 | 0.273 |
| M. catarrhalis | 1.30 | 0.33–5.21 | 0.706 |
| H. parainfluenzae | 1.55 | 0.47–5.16 | 0.474 |
| P. aeruginosa | 1.06 | 0.33–3.44 | 0.917 |
| S. aureus | 1.32 | 0.45–3.83 | 0.613 |
| Other Gram-negative | 0.41 | 0.04–4.19 | 0.449 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriki, D.; Tsouprou, M.; Prountzos, S.; Koumpagioti, D.; Kalogiannis, M.; Alexopoulou, E.; Douros, K. Bacterial Isolates from Bronchoalveolar Lavage in Pediatric Patients with Protracted Bacterial Bronchitis or Bronchiectasis: A Retrospective Comparative Study. J. Clin. Med. 2025, 14, 7653. https://doi.org/10.3390/jcm14217653
Moriki D, Tsouprou M, Prountzos S, Koumpagioti D, Kalogiannis M, Alexopoulou E, Douros K. Bacterial Isolates from Bronchoalveolar Lavage in Pediatric Patients with Protracted Bacterial Bronchitis or Bronchiectasis: A Retrospective Comparative Study. Journal of Clinical Medicine. 2025; 14(21):7653. https://doi.org/10.3390/jcm14217653
Chicago/Turabian StyleMoriki, Dafni, Maria Tsouprou, Spyridon Prountzos, Despoina Koumpagioti, Michalis Kalogiannis, Efthymia Alexopoulou, and Konstantinos Douros. 2025. "Bacterial Isolates from Bronchoalveolar Lavage in Pediatric Patients with Protracted Bacterial Bronchitis or Bronchiectasis: A Retrospective Comparative Study" Journal of Clinical Medicine 14, no. 21: 7653. https://doi.org/10.3390/jcm14217653
APA StyleMoriki, D., Tsouprou, M., Prountzos, S., Koumpagioti, D., Kalogiannis, M., Alexopoulou, E., & Douros, K. (2025). Bacterial Isolates from Bronchoalveolar Lavage in Pediatric Patients with Protracted Bacterial Bronchitis or Bronchiectasis: A Retrospective Comparative Study. Journal of Clinical Medicine, 14(21), 7653. https://doi.org/10.3390/jcm14217653






