Pulmo–Cardio–Renal Continuum in Chronic Lung Diseases: A 3-Year Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
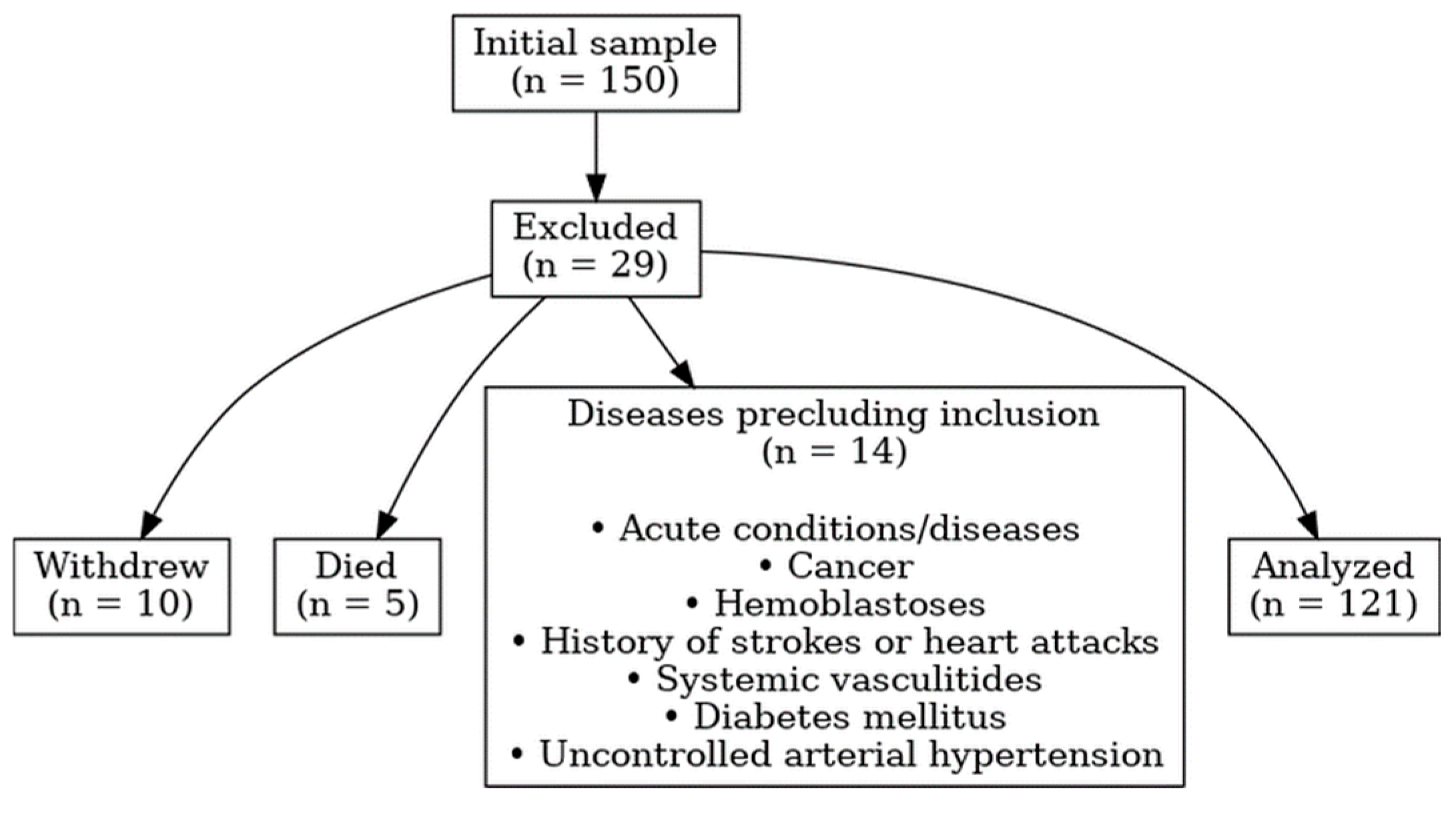
2.1. Study Design and Population
2.2. Baseline Characteristics
2.3. Clinical Assessment
2.4. Statistical Analysis
2.5. Profile and Sub-Profile Formation
3. Results
3.1. Dynamics of Profile Changes in Patients with SSc-ILD and COPD
3.1.1. Pulmonary Profile Indicators
3.1.2. Cardiac Profile Parameters
3.1.3. Renal Profile Indicators
3.2. Integrated Profile Metrics
3.2.1. Dynamics of Changes Across Profiles and Subprofiles
3.2.2. Interrelationships Between Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSc-ILD | Systemic Sclerosis-associated Interstitial Lung Disease |
| COPD | Chronic Obstructive Pulmonary Disease |
| 6MWT | Six-Minute Walk Test |
| 6MWD | Six-Minute Walk Distance |
| SpO2 | Peripheral Oxygen Saturation |
| SaO2 | Arterial Oxygen Saturation |
| pO2 | Partial Pressure of Oxygen |
| pCO2 | Partial Pressure of Carbon Dioxide |
| sPAP | Systolic Pulmonary Artery Pressure |
| LVEF | Left Ventricular Ejection Fraction |
| NT-proBNP | N-terminal pro–B-type Natriuretic Peptide |
| MR-proANP | Mid-Regional pro–Atrial Natriuretic Peptide |
| hsTnT | High-Sensitivity Troponin T |
| hs-cTnT | High-Sensitivity Cardiac Troponin T |
| eGFR | Estimated Glomerular Filtration Rate |
| ACR | Albumin-to-Creatinine Ratio |
| HRV | Heart Rate Variability |
| HF | High Frequency (HRV component) |
| LF | Low Frequency (HRV component) |
| VLF | Very Low Frequency (HRV component) |
| TP | Total Power (HRV component) |
| CT | Computed Tomography |
| PCA | Principal Component Analysis |
| FDR | False Discovery Rate |
| δ (Cliff’s delta) | Cliff’s Delta Effect Size |
| log2FC | Log2 Fold Change |
| KMO | Kaiser–Meyer–Olkin Test |
| EFA | Exploratory Factor Analysis |
| ATS | American Thoracic Society |
| ASE | American Society of Echocardiography |
| EACVI | European Association of Cardiovascular Imaging |
| EULAR | European Alliance of Associations for Rheumatology |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
References
- Matthay, M. Resolution of pulmonary edema. Thirty years of progress. Am. J. Respir. Crit. Care Med. 2014, 189, 1301–1308. [Google Scholar] [CrossRef]
- Grams, M.; Rabb, H. The distant organ effects of acute kidney injury. Kidney Int. 2012, 81, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Virzì, G.; Day, S.; de Cal, M.; Vescovo, G.; Ronco, C. Heart-kidney crosstalk and role of humoral signaling in critical illness. Crit. Care 2014, 18, 201. [Google Scholar] [CrossRef]
- Husain-Syed, F.; McCullough, P.A.; Birk, H.W.; Renker, M.; Brocca, A.; Seeger, W.; Ronco, C. Cardio-pulmonary-renal interactions. J. Am. Coll. Cardiol. 2015, 65, 2433–2448. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Sanchez, M.A.G.; Kumar, R.K.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. 25), D34–D41. [Google Scholar] [CrossRef]
- Azarbar, S.; Dupuis, J. Lung capillary injury and repair in left heart disease: A new target for therapy? Clin. Sci. 2014, 127, 65–76. [Google Scholar] [CrossRef]
- Hemlin, M.; Ljungman, S.; Carlson, J.; Maljukanovic, S.; Mobini, R.; Bech-Hanssen, O.; Skoogh, B. The effects of hypoxia and hypercapnia on renal and heart function, haemodynamics and plasma hormone levels in stable COPD patients. Clin. Respir. J. 2007, 1, 80–90. [Google Scholar] [CrossRef]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef]
- Bollenbecker, S.; Czaya, B.; Gutiérrez, O.M.; Krick, S. Lung–kidney interactions and their role in chronic kidney disease-associated pulmonary diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 322, L625–L640. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.S.; Silva, P.L.; Robba, C.; Battaglini, D.; Lopes-Pacheco, M.; Caruso-Neves, C.; Rocco, P.R.M. Advancements in understanding the mechanisms of lung–kidney crosstalk. Intensive Care Med. Exp. 2024, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Asano, Y. The Evolving Landscape of Systemic Sclerosis Pathogenesis: From Foundational Mechanisms to Organ-Specific Modifiers. Sclerosis 2025, 3, 20. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Kim, Y.; Cho, S.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; Joo, K.W.; et al. Kidney function and obstructive lung disease: A bidirectional Mendelian randomisation study. Eur. Respir. J. 2021, 58, 2100848. [Google Scholar] [CrossRef]
- De Rosa, S.; Lassola, S.; Taccone, F.S.; Battaglini, D. Chronic lung diseases and kidney disease: Pathophysiology and management. Nephrol. Dial. Transplant. 2025, gfaf077. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- ISO 15189:2015; Medical Laboratories—Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2015.
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Ibrayeva, L.; Aubakirova, M.; Bacheva, I.; Alina, A.; Bazarova, N.; Zhanabayeva, A.; Avdiyenko, O.; Borchashvili, S.; Tazhikhanova, S.; Murzabaeyev, A. Features of heart failure with preserved ejection fraction in patients with chronic obstructive pulmonary disease and systemic sclerosis-associated interstitial lung diseases. J. Pers. Med. 2025, 15, 206. [Google Scholar] [CrossRef]
- Ibrayeva, L.; Bacheva, I.; Alina, A.; Klassen, O. Assessing fibrosis progression and endothelial dysfunction in SSc-ILD and COPD: An integrated biomarker and CT densitometry approach. Medicina 2025, 61, 1572. [Google Scholar] [CrossRef] [PubMed]
- Jurevičienė, E.; Burneikaitė, G.; Dambrauskas, L.; Kasiulevičius, V.; Kazėnaitė, E.; Navickas, R.; Puronaitė, R.; Smailytė, G.; Visockienė, Ž.; Danila, E. Epidemiology of chronic obstructive pulmonary disease (COPD) comorbidities in Lithuanian national database: A cluster analysis. Int. J. Environ. Res. Public Health 2022, 19, 970. [Google Scholar] [CrossRef]
- Modak, M.; Rowlands, W.M.; Sleiman, J.; Attaway, A.H.; Bleecker, E.R.; Zein, J. Hospitalization outcomes of patients with asthma, COPD, and asthma-COPD overlap syndrome. Chronic Obstr. Pulm. Dis. 2025, 12, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Kvisvik, B.; Skjørten, I.; Hilde, J.M.; Strand, H.; Omland, T.; Steine, K. High-sensitivity troponin T predicts mortality independently of ventricular dysfunction and pulmonary hypertension in stable chronic obstructive pulmonary disease. Circulation 2019, 140 (Suppl. 1), A17161. [Google Scholar]
- Sá-Sousa, A.; Rodrigues, C.; Jácome, C.; Cardoso, J.; Fortuna, I.; Guimarães, M.; Pinto, P.; Sarmento, P.M.; Baptista, R. Cardiovascular risk in patients with chronic obstructive pulmonary disease: A systematic review. J. Clin. Med. 2024, 13, 5173. [Google Scholar] [CrossRef]
- Knarborg, M.; Hyldgaard, C.; Bendstrup, E.; Davidsen, J.R.; Løkke, A.; Shaker, S.B.; Hilberg, O. Comorbidity and mortality in systemic sclerosis and matched controls: Impact of interstitial lung disease. Chron. Respir. Dis. 2023, 20, 14799731231195041. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Lei, T.; Yu, H.; Zhang, L.; Feng, Z.; Shuai, T.; Guo, H.; Liu, J. NT-proBNP in different patient groups of COPD: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 811–825. [Google Scholar] [CrossRef]
- Ma, K.K.; Ogawa, T.; de Bold, A.J. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J. Mol. Cell Cardiol. 2004, 36, 505–513. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Z.; Ding, C. Association between COPD and CKD: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1494291. [Google Scholar] [CrossRef]
- Konstantinidou, S.K.; Argyrakopoulou, G.; Tentolouris, N.; Karalis, V.; Kokkinos, A. Interplay between baroreflex sensitivity, obesity and related cardiometabolic risk factors (Review). Exp. Ther. Med. 2022, 23, 67. [Google Scholar] [CrossRef]
- Kabbach, E.Z.; Mazzuco, A.; Borghi-Silva, A.; Cabiddu, R.; Agnoleto, A.G.; Barbosa, J.F.; Junior, L.C.S.d.C.; Mendes, R.G. Increased parasympathetic cardiac modulation in patients with acute exacerbation of COPD: How should we interpret it? Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2221–2230. [Google Scholar] [CrossRef]
- Guo, P.; Li, R.; Piao, T.H.; Wang, C.L.; Wu, X.L.; Cai, H.Y. Pathological mechanism and targeted drugs of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1565–1575. [Google Scholar] [CrossRef]
- Gao, J.; Wang, A.; Li, X.; Li, J.; Zhao, H.; Zhang, J.; Liang, J.; Chen, S.; Wu, S. The cumulative exposure to high-sensitivity C-reactive protein predicts the risk of chronic kidney diseases. Kidney Blood Press. Res. 2020, 45, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, I.I.; Qora, M.A. Prevalence of chronic renal failure in COPD patients. Egypt. J. Chest Dis. Tuberc. 2013, 62, 221–227. [Google Scholar] [CrossRef]
- Vilstrup, F.; Heerfordt, C.K.; Kamstrup, P.; Hedsund, C.; Biering-Sørensen, T.; Sørensen, R.; Kolekar, S.; Hilberg, O.; Pedersen, L.; Lund, T.K.; et al. Renin-angiotensin-system inhibitors and the risk of exacerbations in chronic obstructive pulmonary disease: A nationwide registry study. BMJ Open Respir. Res. 2023, 10, e001428. [Google Scholar] [CrossRef] [PubMed]
| Profile | Markers | Group | Cronbach’s α | McDonald’s ω |
|---|---|---|---|---|
| Pulmonary profile | lactate, SaO2 before and after the 6MWT, pCO2, pO2, diastolic blood pressure before and after 6MWT, 6MWT distance, FEV1, lung volume, systolic blood pressure before and after 6MWT, exhaled CO2, FVC, respiratory rate (before and after 6MWT), heart rate (before and after 6MWT), Borg scale (before and after 6MWT), CT densitometry metrics, fibrosis density, change in respiratory rate during 6MWT | COPD 2023 | 0.338 | 0.271 |
| COPD 2024 | 0.22 | 0.261 | ||
| COPD 2025 | 0.34 | |||
| SSc-ILD 2023 | 0.452 | 0.356 | ||
| SSc-ILD 2024 | 0.529 | |||
| SSc-ILD 2025 | 0.565 | |||
| group_COPD | 0.691 | 0.243 | ||
| group_SSc-ILD | 0.819 | 0.548 | ||
| Overall score | 0.76 | 0.501 |
| Profile | Markers | Group | Cronbach’s α | McDonald’s ω |
|---|---|---|---|---|
| Cardiac profile | LVEF, systolic pulmonary artery pressure (sPAP), HRV indices (IN, HF, LF, VLF, TP), NT-proBNP, MR-proANP, hsTnT | COPD 2023 | 0.41 | 0.06 |
| COPD 2024 | 0.18 | 0.08 | ||
| COPD 2025 | 0.13 | 0.06 | ||
| SSc-ILD 2023 | 0.11 | 0.001 | ||
| SSc-ILD 2024 | 0.21 | 0.46 | ||
| SSc-ILD 2025 | 0.21 | 0.43 | ||
| group_COPD | 0.06 | 0.02 | ||
| group_SSc-ILD | 0.11 | 0.35 | ||
| Overall score | 0.08 | 0.003 |
| Profile | Group | Cronbach’s α | McDonald’s ω | Subprofiles | Markers | Group | Cronbach’s α | McDonald’s ω |
|---|---|---|---|---|---|---|---|---|
| Cardiac profile | SSC-ILD | 0.78 | 0.82 | C1 | hsTnT; sPAP | SSC-ILD | 0.74 | 0.81 |
| COPD | 0.72 | 0.78 | ||||||
| All | 0.72 | 0.79 | ||||||
| C2 | LVEF | SSC-ILD | 0.76 | 0.87 | ||||
| COPD | 0.72 | 0.78 | COPD | 0.75 | 0.86 | |||
| All | 0.76 | 0.86 | ||||||
| C3 | MR-proANP. ntproBNP | SSC-ILD | 0.86 | 0.93 | ||||
| COPD | 0.98 | 0.99 | ||||||
| All | 0.66 | 0.74 | All | 0.96 | 0.97 | |||
| C4 | HRV indices (LF. HF. TP) | SSC-ILD | 0.39 | 0.18 | ||||
| COPD | 0.25 | 0.04 | ||||||
| All | 0.32 | 0.07 | ||||||
| Pulmonary profile | SSC-ILD | 0.92 | 0.93 | R1 | FVC; FEV1 | SSC-ILD | 0.97 | 0.98 |
| COPD | 0.86 | 0.91 | ||||||
| All | 0.9 | 0.93 | ||||||
| R2 | pO2 | SSC-ILD | 0.94 | 0.96 | ||||
| COPD | 0.91 | 0.95 | ||||||
| All | 0.93 | 0.95 | ||||||
| R3 | pCO2 | SSC-ILD | 0.9 | 0.94 | ||||
| COPD | 0.92 | 0.93 | COPD | 0.94 | 0.96 | |||
| All | 0.93 | 0.96 | ||||||
| R4 | pH | SSC-ILD | 0.87 | 0.92 | ||||
| COPD | 0.89 | 0.93 | ||||||
| All | 0.88 | 0.93 | ||||||
| R5 | Lactate | SSC-ILD | 0.95 | 0.97 | ||||
| COPD | 0.94 | 0.96 | ||||||
| All | 0.95 | 0.97 | ||||||
| R6 | Exhaled CO2 | SSC-ILD | 0.97 | 0.98 | ||||
| COPD | 0.98 | 0.99 | ||||||
| All | 0.98 | 0.99 | ||||||
| R7 | SaO2 before 6MWT; SaO2 after 6MWT | SSC-ILD | 0.98 | 0.98 | ||||
| COPD | 0.95 | 0.96 | ||||||
| All | 0.91 | 0.92 | All | 0.97 | 0.97 | |||
| R8 | 6MWT distance | SSC-ILD | 0.97 | 0.98 | ||||
| COPD | 0.98 | 0.99 | ||||||
| All | 0.98 | 0.98 | ||||||
| R9 | Borg scale. respiratory rate after 6MWT | SSC-ILD | 0.92 | 0.94 | ||||
| COPD | 0.88 | 0.91 | ||||||
| All | 0.91 | 0.94 | ||||||
| R10 | Lung volume | SSC-ILD | 0.96 | 0.97 | ||||
| COPD | 0.98 | 0.99 | ||||||
| All | 0.99 | 0.99 | ||||||
| R11 | CT densitometry; fibrosis density | SSC-ILD | 0.89 | 0.92 | ||||
| COPD | 0.83 | 0.88 | ||||||
| All | 0.89 | 0.92 | ||||||
| Renal profile | SSC-ILD | 0.80 | 0.83 | N1 | eGFR; Serum creatinine; Urinary creatinine; ACR | SSC-ILD | 0.82 | 0.85 |
| COPD | 0.82 | 0.86 | ||||||
| COPD | 0.53 | 0.78 | All | 0.82 | 0.85 | |||
| N2 | Right kidney CT density; Left kidney CT densityи | SSC-ILD | 0.87 | 0.91 | ||||
| All | 0.81 | 0.81 | COPD | 0.85 | 0.96 | |||
| All | 0.86 | 0.9 |
| Year | Profile | ILD-SSD Median [Q1–Q3] n = 59 | COPD Median [Q1–Q3] n = 62 | Cliff’s Delta (ILD vs. COPD) | Effect Size (Rank-Biserial r) | MWU p | Median Diff (ILD-SSD-COPD) | Bio Diff Bucket | q (FDR-BH) |
|---|---|---|---|---|---|---|---|---|---|
| 2023 | Cardiac profile | 0.077 [−0.167–0.342] | −0.093 [−0.265–0.155] | 0.27 | 0.27 | 0.01 * | 0.169 | 0.048 * | |
| 2024 | 0.035 [−0.129–0.157] | −0.059 [−0.309–0.216] | 0.184 | 0.184 | 0.082 | 0.094 | 0.246 | ||
| 2025 | −0.007 [−0.176–0.18] | −0.087 [−0.232–0.199] | 0.071 | 0.071 | 0.505 | 0.080 | 0.722 | ||
| 2023 | Renal profile | 0.217 [0.013–0.369] | −0.331 [−0.458–−0.088] | 0.685 | 0.685 | 0.0008 | 0.548 | medium | 0.0007 |
| 2024 | 0.048 [−0.347–0.358] | 0.051 [−0.337–0.302] | 0.038 | 0.038 | 0.722 | −0.003 | 0.722 | ||
| 2025 | 0.036 [−0.405–0.331] | 0.096 [−0.307–0.4] | −0.101 | −0.101 | 0.339 | −0.060 | 0.722 | ||
| 2023 | Pulmonary profile | −0.055 [−0.219–0.239] | −0.041 [−0.244–0.171] | 0.067 | 0.067 | 0.529 | −0.014 | 0.722 | |
| 2024 | −0.06 [−0.323–0.214] | −0.009 [−0.199–0.2] | −0.045 | −0.045 | 0.673 | −0.051 | 0.722 | ||
| 2025 | −0.103 [−0.335–0.194] | −0.003 [−0.23–0.218] | −0.049 | −0.049 | 0.643 | −0.099 | 0.722 |
| Subprofile | Year | SSc-ILD Median [Q1–Q3] n = 59 | COPD Median [Q1–Q3] n = 62 | Cliff’s Delta (SSc-ILD vs. COPD) | Effect Size (Rank-Biserial r) | MWU p | Median Diff (SSc-SSc-ILD-COPD) | Bio Diff Bucket | q (FDR-BH) |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 2023 | −0.173 [−0.475–0.32] | −0.148 [−0.41–0.493] | −0.055 | −0.055 | 0.602 | −0.024 | 0.672 | |
| 2024 | −0.227 [−0.526–0.247] | −0.123 [−0.529–0.358] | −0.071 | −0.071 | 0.505 | −0.104 | 0.605 | ||
| 2025 | −0.326 [−0.616–0.189] | −0.1 [−0.407–0.455] | −0.214 | −0.214 | 0.042 | −0.226 | small | 0.090 | |
| C2 | 2023 | 0.356 [−0.336–0.702] | −0.163 [−0.855–0.529] | 0.276 | 0.276 | 0.009 | 0.519 | medium | 0.024 |
| 2024 | 0.254 [−0.405–0.818] | −0.123 [−0.829–0.63] | 0.200 | 0.200 | 0.058 | 0.376 | small | 0.109 | |
| 2025 | 0.519 [−0.866–0.98] | 0.057 [−0.808–0.519] | 0.147 | 0.147 | 0.163 | 0.461 | small | 0.252 | |
| C3 | 2023 | −0.217 [−0.471–0.497] | −0.406 [−0.513–−0.131] | 0.237 | 0.237 | 0.025 | 0.188 | 0.056 | |
| 2024 | −0.122 [−0.357–0.146] | −0.286 [−0.457–−0.028] | 0.253 | 0.253 | 0.016 | 0.164 | 0.042 | ||
| 2025 | −0.058 [−0.281–0.167] | −0.278 [−0.479–−0.04] | 0.283 | 0.283 | 0.007 | 0.220 | small | 0.023 | |
| C4 | 2023 | −0.037 [−0.287–0.403] | 0.02 [−0.35–0.511] | −0.051 | −0.051 | 0.632 | −0.057 | 0.686 | |
| 2024 | 0.137 [−0.335–0.412] | 0.1 [−0.366–0.568] | −0.019 | −0.019 | 0.860 | 0.037 | 0.860 | ||
| 2025 | −0.047 [−0.34–0.401] | 0.066 [−0.333–0.571] | −0.061 | −0.061 | 0.567 | −0.113 | 0.657 | ||
| N1 | 2023 | −0.136 [−0.442–0.187] | −0.073 [−0.327–0.414] | −0.183 | −0.183 | 0.084 | −0.063 | 0.147 | |
| 2024 | −0.154 [−0.531–0.338] | −0.018 [−0.249–0.448] | −0.166 | −0.166 | 0.116 | −0.136 | 0.190 | ||
| 2025 | −0.174 [−0.571–0.382] | −0.037 [−0.251–0.472] | −0.198 | −0.198 | 0.061 | −0.137 | 0.111 | ||
| N2 | 2023 | 0.513 [0.513–0.513] | −0.589 [−0.589–−0.589] | 0.898 | 0.898 | 0.000 | 1.102 | large | 0.000 |
| 2024 | 0.141 [−0.317–0.859] | −0.17 [−0.578–0.244] | 0.220 | 0.220 | 0.056 | 0.311 | small | 0.109 | |
| 2025 | 0.036 [−0.425–0.761] | −0.07 [−0.48–0.351] | 0.060 | 0.060 | 0.606 | 0.106 | 0.672 | ||
| R1 | 2023 | −0.24 [−0.76–0.395] | 0.45 [−0.549–0.805] | −0.280 | −0.280 | 0.009 | −0.690 | medium | 0.024 |
| 2024 | −0.308 [−0.742–0.3] | 0.529 [−0.255–1.07] | −0.407 | −0.407 | 0.000 | −0.836 | medium | 0.001 | |
| 2025 | −0.38 [−0.75–0.33] | 0.494 [−0.342–1.102] | −0.390 | −0.390 | 0.000 | −0.874 | medium | 0.001 | |
| R10 | 2023 | 0.836 [0.527–1.125] | −0.748 [−1.174–−0.364] | 0.921 | 0.921 | 0.000 | 1.584 | large | 0.000 |
| 2024 | 0.806 [0.542–1.122] | −0.895 [−1.135–−0.326] | 0.922 | 0.922 | 0.000 | 1.701 | large | 0.000 | |
| 2025 | 0.806 [0.593–1.14] | −0.849 [−1.11–−0.285] | 0.912 | 0.912 | 0.000 | 1.655 | large | 0.000 | |
| R11 | 2023 | 0.198 [−0.155–0.823] | −0.425 [−0.764–0.094] | 0.548 | 0.548 | 0.000 | 0.624 | medium | 0.000 |
| 2024 | 0.283 [−0.172–0.792] | −0.449 [−0.674–−0.019] | 0.571 | 0.571 | 0.000 | 0.733 | medium | 0.000 | |
| 2025 | 0.295 [−0.144–0.997] | −0.408 [−0.711–−0.026] | 0.563 | 0.563 | 0.000 | 0.703 | medium | 0.000 | |
| R2 | 2023 | −0.199 [−0.606–0.177] | −0.078 [−0.575–0.42] | −0.070 | −0.070 | 0.510 | −0.121 | 0.605 | |
| 2024 | −0.379 [−0.634–0.256] | −0.051 [−0.699–0.515] | −0.081 | −0.081 | 0.450 | −0.328 | small | 0.560 | |
| 2025 | −0.355 [−0.761–0.247] | −0.018 [−0.691–0.579] | −0.082 | −0.082 | 0.444 | −0.338 | small | 0.560 | |
| R3 | 2023 | −0.277 [−0.642–0.54] | −0.015 [−0.865–0.923] | −0.042 | −0.042 | 0.696 | −0.263 | small | 0.740 |
| 2024 | −0.28 [−0.687–0.361] | −0.002 [−0.878–0.805] | −0.096 | −0.096 | 0.369 | −0.277 | small | 0.483 | |
| 2025 | −0.283 [−0.588–0.401] | −0.039 [−0.686–0.648] | −0.097 | −0.097 | 0.365 | −0.244 | small | 0.483 | |
| R4 | 2023 | −0.001 [−0.5–0.902] | 0.13 [−0.934–0.671] | 0.099 | 0.099 | 0.351 | −0.131 | 0.483 | |
| 2024 | 0.132 [−0.419–0.649] | 0.222 [−0.986–0.699] | 0.031 | 0.031 | 0.776 | −0.090 | 0.808 | ||
| 2025 | 0 [−0.383–0.654] | 0.169 [−0.856–0.722] | 0.028 | 0.028 | 0.792 | −0.169 | 0.808 | ||
| R5 | 2023 | −0.358 [−0.751–0.275] | −0.112 [−0.522–0.662] | −0.173 | −0.173 | 0.104 | −0.245 | small | 0.177 |
| 2024 | −0.398 [−0.775–0.221] | −0.275 [−0.615–0.66] | −0.153 | −0.153 | 0.151 | −0.123 | 0.241 | ||
| 2025 | −0.432 [−0.75–0.235] | −0.295 [−0.594–0.653] | −0.119 | −0.119 | 0.262 | −0.137 | 0.371 | ||
| R6 | 2023 | 0.125 [−0.287–1.023] | −0.1 [−1.073–0.723] | 0.209 | 0.209 | 0.049 | 0.225 | small | 0.100 |
| 2024 | 0.102 [−0.293–1.07] | 0.03 [−1.046–0.639] | 0.143 | 0.143 | 0.176 | 0.072 | 0.265 | ||
| 2025 | 0.082 [−0.297–1.106] | 0.006 [−1.055–0.613] | 0.128 | 0.128 | 0.228 | 0.076 | 0.333 | ||
| R7 | 2023 | −0.757 [−0.943–0.013] | 0.14 [−0.151–0.851] | −0.460 | −0.460 | 0.000 | −0.897 | medium | 0.000 |
| 2024 | −0.772 [−0.983–−0.196] | 0.211 [−0.4–0.801] | −0.519 | −0.519 | 0.000 | −0.983 | medium | 0.000 | |
| 2025 | −0.675 [−0.85–−0.047] | 0.259 [−0.383–0.989] | −0.486 | −0.486 | 0.000 | −0.934 | medium | 0.000 | |
| R8 | 2023 | −0.21 [−0.738–0.386] | 0.294 [−0.394–0.891] | −0.248 | −0.248 | 0.019 | −0.505 | medium | 0.046 |
| 2024 | −0.392 [−0.976–0.427] | 0.252 [−0.567–1.012] | −0.277 | −0.277 | 0.009 | −0.643 | medium | 0.024 | |
| 2025 | −0.26 [−0.91–0.434] | 0.258 [−0.501–0.989] | −0.298 | −0.298 | 0.005 | −0.526 | medium | 0.016 | |
| R9 | 2023 | −0.338 [−0.844–0.52] | 0.216 [−0.338–0.566] | −0.236 | −0.236 | 0.025 | −0.554 | medium | 0.056 |
| 2024 | −0.472 [−1.147–0.24] | 0.153 [−0.123–0.657] | −0.450 | −0.450 | 0.000 | −0.624 | medium | 0.000 | |
| 2025 | −0.469 [−1.09–0.278] | 0.2 [−0.217–0.704] | −0.423 | −0.423 | 0.000 | −0.669 | medium | 0.000 |
| Profile | Cardiac Profile | Renal Profile | Pulmonary Profile |
|---|---|---|---|
| Cardiac profile | 1 | −0.10038 | 0.10462 |
| Renal profile | −0.10038 | 1 | −0.11329 |
| Pulmonary profile | 0.10462 | −0.11329 | 1 |
| Subprofiles | C1 | C2 | C3 | C4 | N1 | N2 | R1 | R10 | R11 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 1.00 | −0.19 | 0.12 | −0.18 | −0.14 | −0.09 | 0.30 * | −0.13 | 0.00 | 0.16 | 0.00 | −0.03 | −0.01 | 0.10 | 0.30 * | 0.18 | 0.25 |
| C2 | −0.19 | 1.00 | 0.04 | 0.04 | −0.11 | 0.04 | −0.04 | 0.16 | 0.06 | −0.06 | 0.03 | 0.08 | 0.01 | 0.03 | −0.14 | −0.02 | −0.08 |
| C3 | 0.12 | 0.04 | 1.00 | −0.33 * | −0.06 | 0.12 | 0.03 | 0.32 * | 0.18 | −0.14 | 0.28 | −0.15 | 0.15 | −0.10 | 0.03 | 0.17 | −0.07 |
| C4 | −0.18 | 0.04 | −0.33 * | 1.00 | 0.04 | 0.13 | −0.07 | −0.16 | −0.14 | −0.13 | −0.06 | −0.03 | 0.03 | 0.14 | −0.09 | −0.12 | −0.02 |
| N1 | −0.14 | −0.11 | −0.06 | 0.04 | 1.00 | −0.02 | −0.13 | −0.11 | 0.10 | −0.14 | 0.10 | −0.10 | 0.10 | −0.31 * | 0.11 | 0.00 | 0.13 |
| N2 | −0.09 | 0.04 | 0.12 | 0.13 | −0.02 | 1.00 | −0.34 * | 0.37 * | 0.12 | −0.02 | 0.05 | −0.03 | 0.03 | 0.10 | −0.24 | −0.08 | −0.07 |
| R1 | 0.30 * | −0.04 | 0.03 | −0.07 | −0.13 | −0.34 * | 1.00 | −0.32 * | −0.14 | −0.08 | 0.11 | 0.12 | 0.19 | −0.03 | 0.68 * | 0.41 * | 0.51 * |
| R10 | −0.13 | 0.16 | 0.32 * | −0.16 | −0.11 | 0.37 * | −0.32 * | 1.00 | 0.56 * | −0.10 | −0.02 | 0.08 | −0.02 | 0.05 | −0.41 * | −0.26 | −0.33 * |
| R11 | 0.00 | 0.06 | 0.18 | −0.14 | 0.10 | 0.12 | −0.14 | 0.56 * | 1.00 | −0.05 | −0.03 | 0.17 | −0.03 | 0.09 | −0.11 | −0.06 | 0.03 |
| R2 | 0.16 | −0.06 | −0.14 | −0.13 | −0.14 | −0.02 | −0.08 | −0.10 | −0.05 | 1.00 | −0.31 * | 0.21 | −0.04 | 0.26 | 0.03 | −0.05 | 0.02 |
| R3 | 0.00 | 0.03 | 0.28 | −0.06 | 0.10 | 0.05 | 0.11 | −0.02 | −0.03 | −0.31 * | 1.00 | −0.56 * | 0.28 | −0.08 | 0.18 | 0.11 | 0.12 |
| R4 | −0.03 | 0.08 | −0.15 | −0.03 | −0.10 | −0.03 | 0.12 | 0.08 | 0.17 | 0.21 | −0.56 * | 1.00 | −0.21 | 0.06 | 0.04 | 0.14 | 0.12 |
| R5 | −0.01 | 0.01 | 0.15 | 0.03 | 0.10 | 0.03 | 0.19 | −0.02 | −0.03 | −0.04 | 0.28 | −0.21 | 1.00 | −0.02 | 0.22 | 0.25 | 0.20 |
| R6 | 0.10 | 0.03 | −0.10 | 0.14 | −0.31 * | 0.10 | −0.03 | 0.05 | 0.09 | 0.26 | −0.08 | 0.06 | −0.02 | 1.00 | −0.09 | 0.04 | −0.01 |
| R7 | 0.30 * | −0.14 | 0.03 | −0.09 | 0.11 | −0.24 | 0.68 * | −0.41 * | −0.11 | 0.03 | 0.18 | 0.04 | 0.22 | −0.09 | 1.00 | 0.54 * | 0.73 * |
| R8 | 0.18 | −0.02 | 0.17 | −0.12 | 0.00 | −0.08 | 0.41 * | −0.26 * | −0.06 | −0.05 | 0.11 | 0.14 | 0.25 | 0.04 | 0.54 * | 1.00 | 0.51 * |
| R9 | 0.25 | −0.08 | −0.07 | −0.02 | 0.13 | −0.07 | 0.51 * | −0.33 * | 0.03 | 0.02 | 0.12 | 0.12 | 0.20 | −0.01 | 0.73 * | 0.51 * | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrayeva, L.; Bacheva, I.; Alina, A.; Klassen, O. Pulmo–Cardio–Renal Continuum in Chronic Lung Diseases: A 3-Year Prospective Cohort Study. J. Clin. Med. 2025, 14, 7631. https://doi.org/10.3390/jcm14217631
Ibrayeva L, Bacheva I, Alina A, Klassen O. Pulmo–Cardio–Renal Continuum in Chronic Lung Diseases: A 3-Year Prospective Cohort Study. Journal of Clinical Medicine. 2025; 14(21):7631. https://doi.org/10.3390/jcm14217631
Chicago/Turabian StyleIbrayeva, Lyazat, Irina Bacheva, Assel Alina, and Olga Klassen. 2025. "Pulmo–Cardio–Renal Continuum in Chronic Lung Diseases: A 3-Year Prospective Cohort Study" Journal of Clinical Medicine 14, no. 21: 7631. https://doi.org/10.3390/jcm14217631
APA StyleIbrayeva, L., Bacheva, I., Alina, A., & Klassen, O. (2025). Pulmo–Cardio–Renal Continuum in Chronic Lung Diseases: A 3-Year Prospective Cohort Study. Journal of Clinical Medicine, 14(21), 7631. https://doi.org/10.3390/jcm14217631






