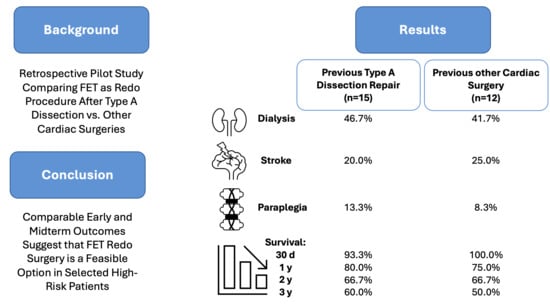
Comparable Outcomes in Redo Total Arch Replacement for Previous Aortic Dissection vs. Other Cardiac Surgeries: A Single-Center Pilot Study of the E-Vita Open Hybrid Prosthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
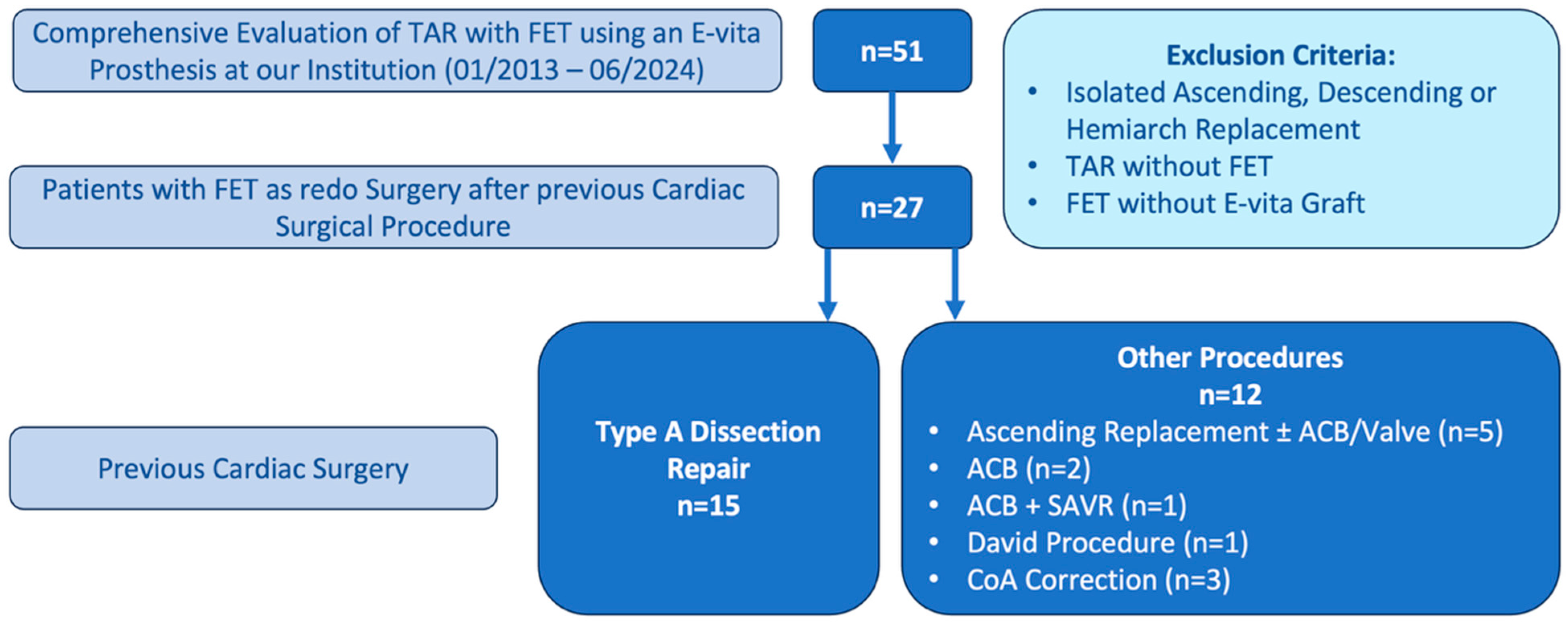
2.2. Study Design and Patient Population
2.3. Power Analysis and Sample Size Justification
2.4. Analyzed Parameters
2.5. Preoperative Management
2.6. Surgical Technique
2.7. Study Endpoints and Follow-Up
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Risk Assessment
3.2. Intraoperative Parameters and Technical Considerations
3.3. Blood Product Utilization and Hemostatic Management
3.4. Postoperative Outcomes and Complications
4. Discussion
4.1. Principal Findings and Clinical Context
4.2. Operative Outcomes and Technical Considerations
4.3. Neurological Outcomes and Protection Strategies
4.4. Survival and Long-Term Outcomes
4.5. Study Limitations and Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACB | Aorto-Coronary Bypass |
| ASCP | Antegrade Selective Cerebral Perfusion |
| BEVAR | Branched Endovascular Aortic Repair |
| Chi2 | Chi-Square-Test |
| CoA | Coarctation of the Aorta |
| COPD | Chronic Obstructive Pulmonary Disease |
| CPB | Cardiopulmonary Bypass |
| CS | Carotid-subclavian |
| CSF | Cerebrospinal Fluid |
| CT | Computed Tomography |
| FET | Frozen Elephant Trunk |
| FFP | Fresh Frozen Plasma |
| Fisher | Fisher’s Exact Test |
| GFR | Glomerular Filtration Rate |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| MRI | Magnetic Resonance Imaging |
| MW | Mann–Whitney U Test |
| n.a. | not applicable |
| NIRS | Near-Infrared Spectroscopy |
| OAD | Oral Antidiabetic Drugs |
| PCC | Prothrombin Complex Concentrate |
| RBC | Red Blood Cells |
| SAVR | Surgical Aortic Valve Replacement |
| SD | Standard Deviation |
| TAR | Total Arch Replacement |
| TEVAR | Thoracic Endovascular Aortic Repair |
| TT | T-Test |
References
- Czerny, M.; Schmidli, J.; Adler, S.; van den Berg, J.C.; Bertoglio, L.; Carrel, T.; Chiesa, R.; Clough, R.E.; Eberle, B.; Etz, C.; et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: An expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur. J. Cardiothorac. Surg. 2019, 55, 133–162. [Google Scholar] [CrossRef] [PubMed]
- Song, S.W.; Lee, H.; Kim, M.S.; Wong, R.H.L.; Ho, J.Y.K.; Szeto, W.Y.; Jakob, H. Next-Generation Frozen Elephant Trunk Technique in the Era of Precision Medicine. J. Chest Surg. 2024, 57, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Bachet, J.; Bavaria, J.; Carrel, T.P.; De Paulis, R.; Di Bartolomeo, R.; Etz, C.D.; Grabenwöger, M.; Grimm, M.; Haverich, A.; et al. Current status and recommendations for use of the frozen elephant trunk technique: A position paper by the Vascular Domain of EACTS. Eur. J. Cardio Thorac. Surg. 2015, 47, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for Diagnosing and Treating Acute and Chronic Syndromes of the Aortic Organ. Ann. Thorac. Surg. 2024, 118, 5–115. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, B.N.; Panfilov, D.S.; Kim, E.B. Long-term outcomes of frozen elephant trunk for aortic dissection: A single-center experience. J. Cardiothorac. Surg. 2024, 19, 559. [Google Scholar] [CrossRef] [PubMed]
- Kreibich, M.; Pitts, L.; Kempfert, J.; Yildiz, M.; Schönhoff, F.; Gaisendrees, C.; Luehr, M.; Berger, T.; Demal, T.; Jahn, J.; et al. Multicentre frozen elephant trunk technique experience as redo surgery to treat residual type A aortic dissections following ascending aortic replacement. Eur. J. Cardio Thorac. Surg. 2024, 66, ezae401. [Google Scholar] [CrossRef] [PubMed]
- Dietze, Z.; Kang, J.; Madomegov, K.; Etz, C.D.; Misfeld, M.; Borger, M.A.; Leontyev, S. Aortic arch redo surgery: Early and mid-term outcomes in 120 patients. Eur. J. Cardio Thorac. Surg. 2023, 64, ezad419. [Google Scholar] [CrossRef] [PubMed]
- Demal, T.J.; Bax, L.; Brickwedel, J.; Kölbel, T.; Vettorazzi, E.; Sitzmann, F.; Reichenspurner, H.; Detter, C. Outcome of the frozen elephant trunk procedure as a redo operation. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tsagakis, K.; Tossios, P.; Kamler, M.; Benedik, J.; Natour, D.; Eggebrecht, H.; Piotrowski, J.; Jakob, H. The DeBakey classification exactly reflects late outcome and re-intervention probability in acute aortic dissection with a slightly modified type II definition. Eur. J. Cardiothorac. Surg. 2011, 40, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Beckmann, E.; Martens, A.; Di Marco, L.; Pantaleo, A.; Reggiani, L.B.; Haverich, A.; Di Bartolomeo, R.; Pacini, D.; Shrestha, M. Total aortic arch replacement with frozen elephant trunk technique: Results from two European institutes. J. Thorac. Cardiovasc. Surg. 2020, 159, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Tsagakis, K.; Pacini, D.; Grabenwöger, M.; Borger, M.A.; Goebel, N.; Hemmer, W.; Laranjeira Santos, A.; Sioris, T.; Widenka, K.; Risteski, P.; et al. Results of frozen elephant trunk from the international E-vita Open registry. Ann. Cardiothorac. Surg. 2020, 9, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Tavolacci, S.C.; de la Pena, C.; Spielvogel, D. Outcomes of Redo Aortic Arch Repair Post Type A Dissection in the Modern Era. In Seminars in Thoracic and Cardiovascular Surgery; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Bajona, P.; Quintana, E.; Schaff, H.V.; Daly, R.C.; Dearani, J.A.; Greason, K.L.; Pochettino, A. Aortic arch surgery after previous type A dissection repair: Results up to 5 years. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 81–85; discussion 85–86. [Google Scholar] [CrossRef] [PubMed]
- Cefarelli, M.; Murana, G.; Surace, G.G.; Castrovinci, S.; Jafrancesco, G.; Kelder, J.C.; Klein, P.; Sonker, U.; Morshuis, W.J.; Heijmen, R.H. Elective Aortic Arch Repair: Factors Influencing Neurologic Outcome in 791 Patients. Ann. Thorac. Surg. 2017, 104, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Winter, M.; Mahr, S.; Stelzmueller, M.-E.; Zimpfer, D.; Ehrlich, M. Cerebral Protection Strategies in Aortic Arch Surgery—Past Developments, Current Evidence, and Future Innovation. Bioengineering 2024, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.; Nita, C.; Martinez, G. Cerebral and Spinal Cord Protection Strategies in Aortic Arch Surgery. J. Cardiovasc. Dev. Dis. 2025, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Hou, B.; Zhang, K.; Gao, S.; Cao, F.; Ji, Y.; Xie, E.; Qiu, J.; Qiu, J.; Yu, C. Protective Effect on Spinal Cord Injury of Prophylactic Cerebrospinal Fluid Drainage in Extensive Aortic Arch Repair for Type a Aortic Dissection: A Retrospective Cohort Study. J. Am. Heart Assoc. 2025, 14, e039427. [Google Scholar] [CrossRef] [PubMed]
- Murana, G.; Campanini, F.; Fiaschini, C.; Barberio, G.; Folesani, G.; Pacini, D. Spinal cord injury after frozen elephant trunk procedures-prevention and management. Ann. Cardiothorac. Surg. 2023, 12, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Juvonen, T.; Fiore, A.; Perrotti, A.; Hervé, A.; Touma, J.; Pettinari, M.; Peterss, S.; Buech, J.; Dell’Aquila, A.M.; et al. Current Outcome after Surgery for Type a Aortic Dissection. Ann. Surg. 2023, 278, e885–e892. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.J.; Poloniecki, J.D.; Gerrard, D.; Loftus, I.M.; Thompson, M.M. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br. J. Surg. 2007, 94, 395–403. [Google Scholar] [CrossRef] [PubMed]
| Variable | Previous Type A Dissection Repair (n = 15) | Previous Other Cardiac Surgery (n = 12) | p-Value |
|---|---|---|---|
| Age [years] | 59.80 ± 7.94 | 61.83 ± 14.54 | 0.669 TT |
| Gender [male] | 10 (66.7%) | 4 (33.3%) | 0.085 Chi2 |
| Height [cm] | 173.53 ± 12.35 | 169.83 ± 18.01 | 0.533 TT |
| Weight [kg] | 88.00 ± 18.83 | 84.67 ± 18.65 | 0.65 TT |
| Diabetes | 0.767 Fisher | ||
| OAD | 0 (0.0%) | 1 (8.3%) | |
| Insulin dependent | 0 (0.0%) | 0 (0.0%) | |
| Unmedicated | 2 (13.3%) | 1 (8.3%) | |
| Hypertension | 15 (100.0%) | 12 (100.0%) | n.a. |
| Hyperlipidemia | 10 (66.7%) | 9 (75.0%) | 0.696 Fisher |
| COPD | 1 (6.7%) | 0 (0.0%) | 1.0 Fisher |
| Smoking history | 5 (33.3%) | 3 (25.0%) | 0.696 Fisher |
| Stroke | 2 (13.3%) | 0 (0.0%) | 0.487 Fisher |
| Renal insufficiency | 0.342 Fisher | ||
| 0: none | 2 (13.3%) | 3 (25.0%) | |
| 1: GFR > 89 [mL/min] | 0 (0.0%) | 1 (8.3%) | |
| 2: GFR 60–89 [mL/min] | 8 (53.3%) | 7 (58.3%) | |
| 3: GFR 30–59 [mL/min] | 5 (33.3%) | 1 (8.3%) | |
| 4: GFR 15–29 [mL/min] | 0 (0.0%) | 0 (0.0%) | |
| 5: GFR < 15 [mL/min] | 0 (0.0%) | 0 (0.0%) | |
| Dialysis | 0 (0.0%) | 0 (0.0%) | |
| EuroSCORE II | 4.55 (3.86–7.28) | 5.41 (3.93–6.74) | 1.0 MW |
| Aortic pathology | <0.001 Fisher | ||
| Dissection | 1 (6.7%) | 1 (8.3%) | |
| Aneurysm | 1 (6.7%) | 9 (75.0%) | |
| Combination | 13 (86.7%) | 2 (16.7%) | |
| Type of surgery | 1.0 Fisher | ||
| Elective | 14 (93.3%) | 11 (91.7%) | |
| Urgent | 1 (6.7%) | 1 (8.3%) | |
| Emergent | 0 (0.0%) | 0 (0.0%) | |
| CS-Bypass before FET | 6 (40.0%) | 3 (25.0%) | 0.683 Fisher |
| Variable | Previous Type A Dissection Repair (n = 15) | Previous Other Cardiac Surgery (n = 12) | p-Value |
|---|---|---|---|
| CSF drainage | 12 (80%) | 9 (75%) | 1.0 Fisher |
| Duration of surgery [min] | 580.07 ± 126.84 | 481.25 ± 119.29 | 0.053 TT |
| CPB time [min] | 254.07 ± 60.64 | 234.92 ± 53.01 | 0.397 TT |
| Cross-clamp time [min] | 110.80 ± 35.31 | 115.08 ± 43.09 | 0.779 TT |
| Reperfusion [min] | 80.80 ± 35.66 | 75.50 ± 18.01 | 0.644 TT |
| Circulatory arrest [min] | 85.38 ± 25.22 (n = 8) | 64.75 ± 49.06 (n = 8) | 0.308 TT |
| Cerebral perfusion [min] | 89.60 ± 20.30 | 78.27 ± 38.70 | 0.391 TT |
| Lowest Temperature [°C] | 27.5 (26.0–28.5) | 26.25 (25.83–28.0) | 0.183 MW |
| Perfusion Strategy | 0.218 Fisher | ||
| Unilateral | 10 (66.7%) | 8 (66.7%) | |
| Bilateral | 3 (20.0%) | 3 (25.0%) | |
| Trilateral | 2 (13.3%) | 4 (33.3%) | |
| Prosthesis type | 0.87 Fisher | ||
| Open | 9 (60.0%) | 6 (50.0%) | |
| Open Plus | 2 (13.3%) | 3 (25.0%) | |
| Open Neo | 4 (26.7%) | 3 (25.0%) | |
| Ishimaru zone of distal anastomosis | 0.376 Fisher | ||
| 0 | 2 (13.3%) | 3 (25.0%) | |
| 2 | 7 (46.7%) | 3 (25.0%) | |
| 3 | 3 (20.0%) | 5 (41.7%) | |
| Arch vessel reimplantation | 0.13 Fisher | ||
| Truncus, carotid, subclavia | 4 (26.7%) | 7 (58.3%) | |
| Truncus, carotid | 11 (73.3%) | 5 (41.7%) | |
| Concomitant procedure | 0.332 Fisher | ||
| ACB | 0 (0.0%) | 1 (8.3%) | |
| Valve | 2 (13.3%) | 0 (0.0%) | |
| Max. Lactate [mmol/L] | 8.24 ± 2.55 | 7.04 ± 3.70 | 0.352 TT |
| Variable | Previous Type A Dissection Repair (n = 15) | Previous Other Cardiac Surgery (n = 12) | p-Value |
|---|---|---|---|
| RBC [mL] | 1650 (825–3600) 14 (93.3%) | 1350 (900–3975) 12 (100.0%) | 0.899 MW 1.0 Fisher |
| Platelets [mL] | 1275 ± 773.29 14 (93.3%) | 1425 ± 822.34 10 (83.3%) | 0.653 TT 0.569 Fisher |
| FFP [mL] | 2760 ± 1347.59 10 (66.7%) | 2416.67 ± 1942.59 6 (50.0%) | 0.681 TT 0.452 Fisher |
| PCC [I.U.] | 6000 (4000–8000) 15 (100.0%) | 6000 (4000–9000) 11 (91.7%) | 0.919 MW 0.444 Fisher |
| Fibrinogen [g] | 6.0 (4.0–16.0) 15 (100.0%) | 5.5 (4.0–7.3) 10 (83.3%) | 0.428 MW 0.188 Fisher |
| NovoSeven [mg] | 8 (6.5–18) 5 (33.3%) | 6.1 (4.2–n.a.) 2 (16.7%) | 0.381 MW 0.408 Fisher |
| Haemate [I.U.] | 3500 (3000–5000) 8 (53.3%) | 2000 (1250–3500) 4 (33.3%) | 0.073 MW 0.441 Fisher |
| Fibrogammin [I.U.] | 1875 (1250–n.a.) 2 (13.3%) | 1875 (1250–n.a.) 2 (16.7%) | 1.0 MW 1.0 Fisher |
| Variable | Previous Type A Dissection Repair (n = 15) | Previous Other Cardiac Surgery (n = 12) | p-Value |
|---|---|---|---|
| LOS ICU [d] | 10 (8–24) | 6.5 (2.25–17.75) | 0.114 MW |
| Time to discharge [d] | 24 (18–40.5) | 22.5 (16.75–33.0) | 0.631 MW |
| Re-Sternotomy | 4 (26.7%) | 2 (16.7%) | 0.662 Fisher |
| Stroke | 3 (20.0%) | 3 (25.0%) | 1.0 Fisher |
| Renal insufficiency | 0.334 Fisher | ||
| 0: none | 0 (0.0%) | 0 (0.0%) | |
| 1: GFR > 89 [mL/min] | 0 (0.0%) | 0 (0.0%) | |
| 2: GFR 60–89 [mL/min] | 0 (0.0%) | 3 (25.0%) | |
| 3: GFR 30–59 [mL/min] | 3 (20.0%) | 3 (25.0%) | |
| 4: GFR 15–29 [mL/min] | 3 (20.0%) | 1 (8.3%) | |
| 5: GFR < 15 [mL/min] | 1 (6.7%) | 0 (0.0%) | |
| Dialysis | 7 (46.7%) | 5 (41.7%) | |
| Delirium | 5 (33.3%) | 3 (25.0%) | 0.696 Fisher |
| Multiorgan failure | 1 (6.7%) | 1 (8.3%) | 1.0 Fisher |
| Paraplegia | 2 (13.3%) | 1 (8.3%) | 1.0 Fisher |
| Survival | |||
| 30 d | 14 (93.3%) | 12 (100.0%) | 1.0 Fisher |
| 1 y | 12 (80.0%) | 9 (75.0%) | 0.502 Fisher |
| 2 y | 10 (66.7%) | 8 (66.7%) | 0.495 Fisher |
| 3 y | 9 (60.0%) | 6 (50.0%) | 1.0 Fisher |
| In-Hospital Mortality | 1 (6.7%) | 0 (0.0%) | 1.0 Fisher |
| Subsequent endovascular procedure (TEVAR/BEVAR) | 8 (53.3%) | 6 (50.0%) | 0.678 Fisher |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan, M.; Vöhringer, L.; Baumgaertner, M.; Salewski, C.; Marinos, S.L.; Rustenbach, C.J.; Schlensak, C.; Doll, I. Comparable Outcomes in Redo Total Arch Replacement for Previous Aortic Dissection vs. Other Cardiac Surgeries: A Single-Center Pilot Study of the E-Vita Open Hybrid Prosthesis. J. Clin. Med. 2025, 14, 7588. https://doi.org/10.3390/jcm14217588
Radwan M, Vöhringer L, Baumgaertner M, Salewski C, Marinos SL, Rustenbach CJ, Schlensak C, Doll I. Comparable Outcomes in Redo Total Arch Replacement for Previous Aortic Dissection vs. Other Cardiac Surgeries: A Single-Center Pilot Study of the E-Vita Open Hybrid Prosthesis. Journal of Clinical Medicine. 2025; 14(21):7588. https://doi.org/10.3390/jcm14217588
Chicago/Turabian StyleRadwan, Medhat, Luise Vöhringer, Michael Baumgaertner, Christoph Salewski, Spiros Lukas Marinos, Christian Jörg Rustenbach, Christian Schlensak, and Isabelle Doll. 2025. "Comparable Outcomes in Redo Total Arch Replacement for Previous Aortic Dissection vs. Other Cardiac Surgeries: A Single-Center Pilot Study of the E-Vita Open Hybrid Prosthesis" Journal of Clinical Medicine 14, no. 21: 7588. https://doi.org/10.3390/jcm14217588
APA StyleRadwan, M., Vöhringer, L., Baumgaertner, M., Salewski, C., Marinos, S. L., Rustenbach, C. J., Schlensak, C., & Doll, I. (2025). Comparable Outcomes in Redo Total Arch Replacement for Previous Aortic Dissection vs. Other Cardiac Surgeries: A Single-Center Pilot Study of the E-Vita Open Hybrid Prosthesis. Journal of Clinical Medicine, 14(21), 7588. https://doi.org/10.3390/jcm14217588