Gastric Motility Disorders Post Organ Transplantation—A Comprehensive Review
Abstract
1. Introduction
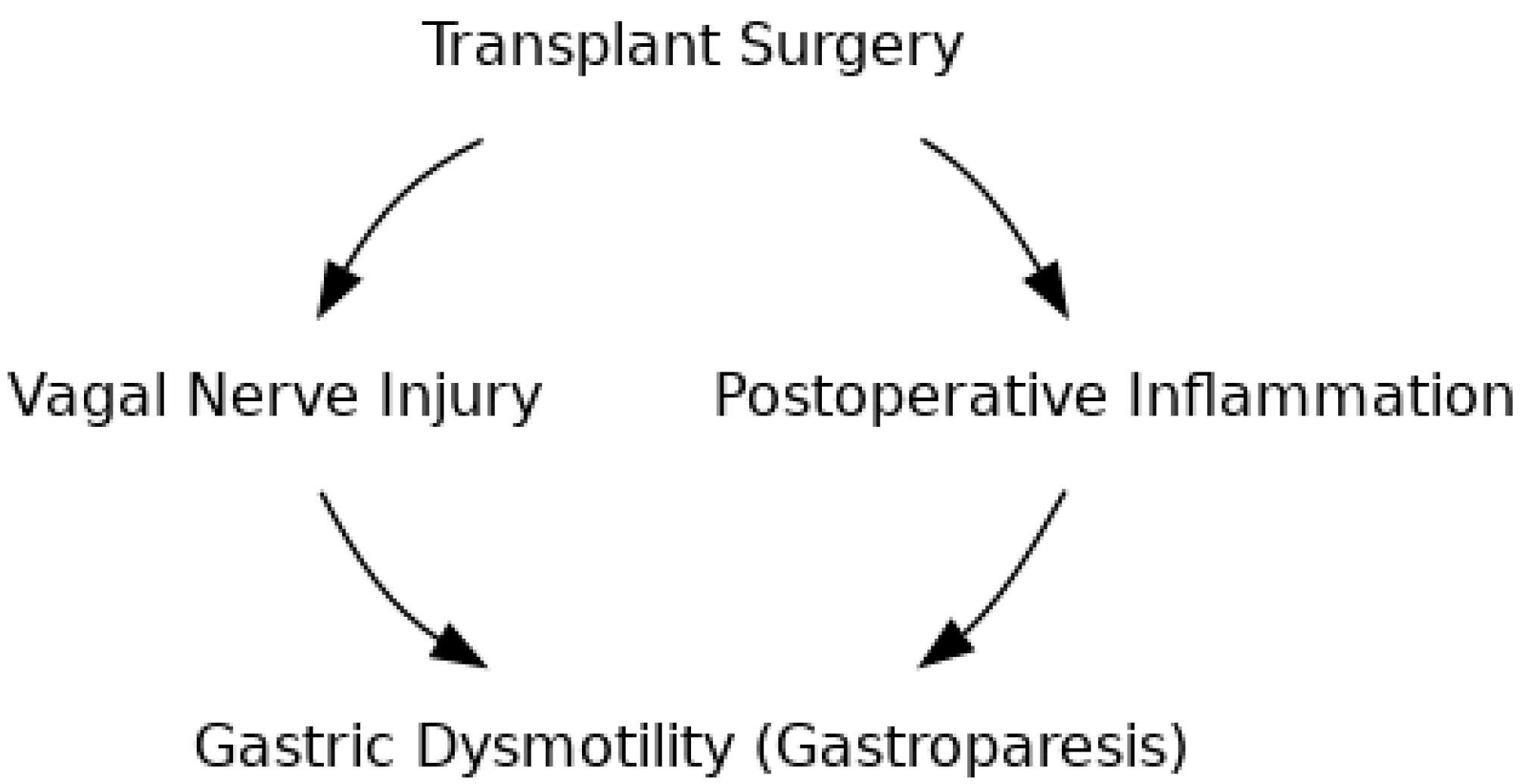
2. Pathophysiology
2.1. Vagal Nerve Injury
2.2. Immunosuppressive Medication Effects
2.3. Surgical Trauma and Inflammatory Response
2.4. Microbiome Disruption
2.5. Diabetes and Metabolic Factors
3. Clinical Manifestation and Diagnosis
3.1. Impact on Immunosuppressive Pharmacokinetics
3.1.1. Diagnostic Methodologies and Technical Considerations
3.1.2. Differential Diagnosis and Alternative Aetiologies
4. Management Strategies
4.1. Pharmacological and Nutritional Management
4.2. Endoscopic and Surgical Interventions
4.3. Emerging Therapies and Future Directions
5. Special Considerations
6. Novel Therapeutic Approaches
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Israni, A.K.; Zaun, D.; Hadley, N.; Rosendale, J.D.; Schaffhausen, C.; McKinney, W.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2018 annual data report: Deceased organ donation. Am. J. Transplant. 2020, 20, 509–541. [Google Scholar] [CrossRef]
- Sisti, J.D.; Bolet, S.I.; Amanullah, A.; Malik, Z.; Parkman, H.; Maurer, A.; Cheng, K.; Dadparvar, S. Considerations and Indications for Gastric Emptying Scintigraphy in Lung Transplant Patients. Asia Ocean. J. Nucl. Med. Biol. 2025, 13, 53. [Google Scholar]
- Raviv, Y.; D’Ovidio, F.; Pierre, A.; Chaparro, C.; Freeman, M.; Keshavjee, S.; Singer, L.G. Prevalence of gastroparesis before and after lung transplantation and its association with lung allograft outcomes. Clin. Transplant. 2012, 26, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Berkowid, N.; Schulman, L.L.; McGregor, C.; Markowid, D. Gastroparesis after lung transplantation: Potential role in postoperative respiratory complications. Chest 1995, 108, 1602–1607. [Google Scholar] [CrossRef]
- Blackett, J.W.; Benvenuto, L.; Leiva-Juarez, M.M.; D’Ovidio, F.; Arcasoy, S.; Jodorkovsky, D. Risk factors and outcomes for gastroparesis after lung transplantation. Dig. Dis. Sci. 2022, 67, 2385–2394. [Google Scholar] [CrossRef]
- Hathorn, K.E.; Chan, W.W.; Lo, W.K. Role of gastroesophageal reflux disease in lung transplantation. World J. Transplant. 2017, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Filichia, L.A.; Baz, M.A.; Cendan, J.C. Simultaneous fundoplication and gastric stimulation in a lung transplant recipient with gastroparesis and reflux. JSLS J. Soc. Laparoendosc. Surg. 2008, 12, 303. [Google Scholar]
- Au, J.; Hawkins, T.; Venables, C.; Morritt, G.; Scott, C.D.; Gascoigne, A.D.; Corris, P.A.; Hilton, C.J.; Dark, J.H. Upper gastrointestinal dysmotility in heart-lung transplant recipients. Ann. Thorac. Surg. 1993, 55, 94–97. [Google Scholar] [CrossRef]
- Duarte, A.G.; Myers, A.C. Cough reflex in lung transplant recipients. Lung 2012, 190, 23–27. [Google Scholar] [CrossRef]
- Blackett, J.W.; Benvenuto, L.; Arcasoy, S.; Jodorkovsky, D. S1356 Predictors of Gastroparesis Post-Lung Transplant. Am. J. Gastroenterol. 2020, 115, S684. [Google Scholar] [CrossRef]
- Tao, Y.; Yan, Z.; Sha, J.; Zhu, Z.; Lei, H. Severe gastroparesis causing postoperative respiratory complications in a heart-lung recipient. J. Thorac. Dis. 2010, 2, 121. [Google Scholar]
- Sodhi, S.S.; Guo, J.P.; Maurer, A.H.; O’Brien, G.; Srinivasan, R.; Parkman, H.P. Gastroparesis after combined heart and lung transplantation. J. Clin. Gastroenterol. 2002, 34, 34–39. [Google Scholar] [CrossRef]
- Joglar, J.A.; Wan, E.Y.; Chung, M.K.; Gutierrez, A.; Slaughter, M.S.; Bateson, B.P.; Loguidice, M.; Drazner, M.; Kistler, P.M.; Saour, B.; et al. Management of arrhythmias after heart transplant: Current state and considerations for future research. Circ. Arrhythmia Electrophysiol. 2021, 14, e007954. [Google Scholar] [CrossRef] [PubMed]
- Weijs, T.J.; Ruurda, J.P.; Luyer, M.D.; Nieuwenhuijzen, G.A.; Van Hillegersberg, R.; Bleys, R.L. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J. Anat. 2015, 227, 431–439. [Google Scholar] [CrossRef]
- Kindt, S.; Tack, J. Impaired gastric accommodation and its role in dyspepsia. Gut 2006, 55, 1685–1691. [Google Scholar] [CrossRef]
- Vanden Berghe, P.; Janssen, P.; Kindt, S.; Vos, R.; Tack, J. Contribution of different triggers to the gastric accommodation reflex in humans. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G902–G906. [Google Scholar] [CrossRef][Green Version]
- Curro, D.; Ipavec, V.; Preziosi, P. Neurotransmitters of the non-adrenergic non-cholinergic relaxation of proximal stomach. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 53–62. [Google Scholar][Green Version]
- Sathnur, N.; Li, J.M.; Krishnappa, D.; Benditt, D.G. Impact of denervation by heart transplantation on post-operative atrial fibrillation susceptibility. J. Atr. Fibrillation 2020, 13, 2397. [Google Scholar] [CrossRef] [PubMed]
- Bregeon, F.; Alliez, J.R.; Héry, G.; Marqueste, T.; Ravailhe, S.; Jammes, Y. Motor and sensory re-innervation of the lung and heart after re-anastomosis of the cervical vagus nerve in rats. J. Physiol. 2007, 581, 1333–1340. [Google Scholar] [CrossRef]
- Maes, B.D.; Vanwalleghem, J.; Kuypers, D.; Ghoos, Y.; Rutgeerts, P.J.; Vanrenterghem, Y.F. Differences in gastric motor activity in renal transplant recipients treated with FK-506 versus cyclosporine. Transplantation 1999, 68, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Parkman, H.P.; Shafi, M.A.; Abell, T.L.; Gerson, L. Clinical guideline: Management of gastroparesis. Am. J. Gastroenterol. 2013, 108, 18–37. [Google Scholar] [CrossRef]
- Van Vlem, B.; Schoonjans, R.; Vanholder, R.; Vos, M.D.; Depoortere, I.; Peeters, T.L.; Lefebvre, R. In vitro evaluation of motilin agonism by macrolide immunosuppressive drugs. Nephrol. Dial. Transplant. 2002, 17, 973–977. [Google Scholar] [CrossRef][Green Version]
- Kuypers, D.R.; Claes, K.; Evenepoel, P.; Maes, B.; Vanrenterghem, Y. The rate of gastric emptying determines the timing but not the extent of oral tacrolimus absorption: Simultaneous measurement of drug exposure and gastric emptying by carbon-14-octanoic acid breath test in stable renal allograft recipients. Drug Metab. Dispos. 2004, 32, 1421–1425. [Google Scholar] [CrossRef]
- Calmet, F.H.; Yarur, A.J.; Pukazhendhi, G.; Ahmad, J.; Bhamidimarri, K.R. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 366. [Google Scholar]
- Hata, K.; Yasue, K.; Ishii, G.; Kimura, T.; Egawa, S. Everolimus-induced gastric antral vascular ectasia in advanced renal cancer. IJU Case Rep. 2020, 3, 293. [Google Scholar] [CrossRef]
- Fernández, A.R.; Sánchez-Tarjuelo, R.; Cravedi, P.; Ochando, J.; López-Hoyos, M. Ischemia reperfusion injury—A translational perspective in organ transplantation. Int. J. Mol. Sci. 2020, 21, 8549. [Google Scholar] [CrossRef]
- Hermann, G.E.; Tovar, C.A.; Rogers, R.C. LPS-induced suppression of gastric motility relieved by TNFR:Fc construct in dorsal vagal complex. AJP Gastrointest. Liver Physiol. 2002, 283, G634–G639. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCarthy, D.O.; Daun, J.M. The role of prostaglandins in interleukin-1 induced gastroparesis. Physiol. Behav. 1992, 52, 351–353. [Google Scholar] [CrossRef]
- Lehrskov, L.L.; Lyngbaek, M.P.; Soederlund, L.; Legaard, G.E.; Ehses, J.A.; Heywood, S.E.; Albrechtsen, N.J.; Holst, J.J.; Karstoft, K.; Pedersen, B.K.; et al. Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. 2018, 27, 1201–1211. [Google Scholar] [CrossRef]
- Grootjans, J.; Lenaerts, K.; Derikx, J.P.; Matthijsen, R.A.; de Bruïne, A.P.; van Bijnen, A.A.; van Dam, R.M.; Dejong, C.H.; Buurman, W.A. Human intestinal ischemia-reperfusion–induced inflammation characterized: Experiences from a new translational model. Am. J. Pathol. 2010, 176, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Cowled, P.; Fitridge, R. Pathophysiology of Reperfusion Injury. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, Australia, 1992. [Google Scholar]
- Tsuchiya, Y.; Nozu, T.; Kumei, S.; Ohhira, M.; Okumura, T. IL-1 receptor antagonist blocks the lipopolysaccharide-induced inhibition of gastric motility in freely moving conscious rats. Dig. Dis. Sci. 2012, 57, 2555–2561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Docsa, T.; Sipos, A.; Cox, C.S.; Uray, K. The role of inflammatory mediators in the development of gastrointestinal motility disorders. Int. J. Mol. Sci. 2022, 23, 6917. [Google Scholar] [CrossRef]
- Tang, Q.; Dong, C.; Sun, Q. Immune response associated with ischemia and reperfusion injury during organ transplantation. Inflamm. Res. 2022, 71, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.; Hu, K.; Zuo, M.; Tian, Z.; Wei, Y.; Zhou, Q.; Li, Q. Postoperative delayed gastric emptying: May gut microbiota play a role? Front. Cell. Infect. Microbiol. 2024, 14, 1449530. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, T.; Ding, Z.; Qian, T.; Wang, P.; Wu, B.; Pan, X.; Li, X. Analysis on the change of gut microbiota and metabolome in lung transplant patients. Microbiol. Spectr. 2024, 12, e03142-23. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, J.; Zhu, S.; Xin, L.; Yu, C.; Shen, Z. The role of short chain fatty acids in irritable bowel syndrome. J. Neurogastroenterol. Motil. 2022, 28, 540. [Google Scholar] [CrossRef]
- Cherbut, C. Motor effects of short-chain fatty acids and lactate in the gastrointestinal tract. Proc. Nutr. Soc. 2003, 62, 95–99. [Google Scholar] [CrossRef]
- Lodato, R.F.; Khan, A.R.; Zembowicz, M.J.; Weisbrodt, N.W.; Pressley, T.A.; Li, Y.F.; Lodato, J.A.; Zembowicz, A.; Moody, F.G. Roles of IL-1 and TNF in the decreased ileal muscle contractility induced by lipopolysaccharide. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 276, G1356–G1362. [Google Scholar] [CrossRef]
- Torihashi, S.; Ozaki, H.; Hori, M.; Kita, M.; Ohota, S.; Karaki, H. Resident macrophages activated by lipopolysaccharide suppress muscle tension and initiate inflammatory response in the gastrointestinal muscle layer. Histochem. Cell Biol. 2000, 113, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Hamano, N.; Yamada, M.; Shirane, A.; Shingu, K. Inducible nitric oxide synthase and tumor necrosis factor-alpha in delayed gastric emptying and gastrointestinal transit induced by lipopolysaccharide in mice. Braz. J. Med. Biol. Res. 2006, 39, 1425–1434. [Google Scholar] [CrossRef]
- Grass, F.; Schäfer, M.; Cristaudi, A.; Berutto, C.; Aubert, J.D.; Gonzalez, M.; Demartines, N.; Ris, H.B.; Soccal, P.M.; Krueger, T. Incidence and risk factors of abdominal complications after lung transplantation. World J. Surg. 2015, 39, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Hirji, S.A.; Gulack, B.C.; Englum, B.R.; Speicher, P.J.; Ganapathi, A.M.; Osho, A.A.; Shimpi, R.A.; Perez, A.; Hartwig, M.G. Lung transplantation delays gastric motility in patients without prior gastrointestinal surgery—A single-center experience of 412 consecutive patients. Clin. Transplant. 2017, 31, e13065. [Google Scholar] [CrossRef]
- Acr–acnm–Snmmi–spr Practice Parameter for the Performance of Gastrointestinal tract, Hepatic, and Splenic Scintigraphy [INTERNET]. American College of Radiology. 2025. Available online: https://gravitas.acr.org/PPTS/DownloadPreviewDocument?DocId=80 (accessed on 22 October 2025).
- Akindipe, O.A.; Faul, J.L.; Vierra, M.A.; Triadafilopoulos, G.; Theodore, J. The surgical management of severe gastroparesis in heart/lung transplant recipients. Chest 2000, 117, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Stovold, R.; Forrest, I.A.; Corris, P.A.; Murphy, D.M.; Smith, J.A.; Decalmer, S.; Johnson, G.E.; Dark, J.H.; Pearson, J.P.; Ward, C. Pepsin, a biomarker of gastric aspiration in lung allografts: A putative association with rejection. Am. J. Respir. Crit. Care Med. 2007, 175, 1298–1303. [Google Scholar] [CrossRef]
- Chun, P.; Chan, M.; Nunley, D.; Balasubramanian, G. Changes in esophageal contractile and barrier function in lung transplant recipients. Esophagus 2025, 22, 657–665. [Google Scholar] [CrossRef]
- Dellon, E.S.; Morgan, D.R.; Mohanty, S.P.; Davis, K.; Aris, R.M. High incidence of gastric bezoars in cystic fibrosis patients after lung transplantation. Transplantation 2006, 81, 1141–1146. [Google Scholar] [CrossRef]
- Jarrett, S.A.; Lo, K.B.; Body, C.; Kim, J.J.; Zheng, Z.; Kundu, S.; Huang, E.; Basu, A.; Flynn, M.; Died-Lindo, K.A.; et al. Nausea, vomiting, and dyspepsia following solid organ abdominal transplant. Cureus 2022, 14, e24274. [Google Scholar] [CrossRef] [PubMed]
- Cashion, A.K.; Holmes, S.L.; Hathaway, D.K.; Gaber, A.O. Gastroparesis following kidney/pancreas transplant. Clin. Transplant. 2004, 18, 306–311. [Google Scholar] [CrossRef]
- Van Hooff, J.P.; Christiaans, M.H.; van Duijnhoven, E.M. Evaluating mechanisms of post-transplant diabetes mellitus. Nephrol. Dial. Transplant. 2004, 19 (Suppl. S6), vi8–vi12. [Google Scholar] [CrossRef]
- Cho, Y.M.; Park, K.S.; Jung, H.S.; Jeon, H.J.; Ahn, C.; Ha, J.; Kim, S.J.; Rhee, B.D.; Kim, S.Y.; Lee, H.K. High incidence of tacrolimus-associated posttransplantation diabetes in the Korean renal allograft recipients according to American Diabetes Association criteria. Diabetes Care 2003, 26, 1123–1128. [Google Scholar] [CrossRef]
- Fridell, J.A.; Chen, J.M.; Kerby, E.A.; Marshall, W.A.; Lud, A.J.; Powelson, J.A.; Mangus, R.S. Impact of Gastroparesis on Outcomes After Pancreas Transplantation. Transplant. Direct. 2025, 11, e1788. [Google Scholar] [CrossRef]
- Drury, A.M.; Albunni, H.; Al-Haddad, M.; Powelson, J.A.; Lud, A.; Fridell, J.A. Role of gastric peroral endoscopic myotomy (GPOEM) in chronic gastroparesis management after pancreas transplantation. Clin. Transplant. 2024, 38, e15176. [Google Scholar] [CrossRef]
- Loucks, J.; Yost, S.; Kaplan, B. An introduction to basic pharmacokinetics. Transplantation 2015, 99, 903–907. [Google Scholar] [CrossRef]
- Gautam, A. Gastrointestinal complications following transplantation. Surg. Clin. 2006, 86, 1195–1206. [Google Scholar] [CrossRef]
- Burlen, J.; Chennubhotla, S.; Ahmed, S.; Landes, S.; Ramirez, A.; Stocker, A.M.; Abell, T.L. Investigating defects of esophageal motility in lung transplant recipients. Gastroenterol. Res. 2022, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Pandolfino, J.E. High-resolution manometry in clinical practice. Gastroenterol. Hepatol. 2015, 11, 374. [Google Scholar]
- Bilnik, K.; Klimacka-Nawrot, E.; Kurek, J.; Blonska-Fajfrowska, B.; Stadnicki, A. PTU-152 High Resolution Manometry Pattern of Esophagogastric Junction and Esophageal Motility in Patients before and after Fundoplication. Gut 2013, 62, A110. [Google Scholar] [CrossRef]
- Saad, R.J.; Hasler, W.L. A technical review and clinical assessment of the wireless motility capsule. Gastroenterol. Hepatol. 2011, 7, 795. [Google Scholar]
- Tran, K.; Brun, R.; Kuo, B. Evaluation of regional and whole gut motility using the wireless motility capsule: Relevance in clinical practice. Ther. Adv. Gastroenterol. 2012, 5, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Scott, S.M.; Hobson, A.R. Gastrointestinal motility revisited: The wireless motility capsule. United Eur. Gastroenterol. J. 2013, 1, 413–421. [Google Scholar] [CrossRef]
- Georgiou, M.F.; Sfakianaki, E.; Diaz-Kanelidis, M.N.; Moshiree, B. Gastric emptying scintigraphy protocol optimization using machine learning for the detection of delayed gastric emptying. Diagnostics 2024, 14, 1240. [Google Scholar] [CrossRef]
- Gioco, R.; Corona, D.; Ekser, B.; Puzzo, L.; Inserra, G.; Pinto, F.; Schipa, C.; Privitera, F.; Veroux, P.; Veroux, M. Gastrointestinal complications after kidney transplantation. World J. Gastroenterol. 2020, 26, 5797. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.H. Gastrointestinal infectious disease complications following transplantation and their differentiation from immuno-suppressant-induced gastrointestinal toxicities. Clin. Transplant. 2001, 15, 11–22. [Google Scholar] [CrossRef]
- Kaplan, C.S.; Petersen, E.A.; Icenogle, T.B.; Copeland, J.G.; Villar, H.V.; Sampliner, R.; Minnich, L.; Ray, C.G. Gastrointestinal cytomegalovirus infection in heart and heart-lung transplant recipients. Arch. Intern. Med. 1989, 149, 2095–2100. [Google Scholar] [CrossRef]
- Sakr, M.; Hassanein, T.; Gavaler, J.; Abu-Elmagd, K.; Fung, J.; Gordon, R.; Starzl, T.; Van Thiel, D. Cytomegalovirus infection of the upper gastrointestinal tract following liver transplantation—Incidence, location, and severity in cyclosporine-and FK506-treated patients. Transplantation 1992, 53, 786. [Google Scholar] [CrossRef]
- Craven, H.; Iftikhar, H.; Bhatnagar, P. Profound opiate toxicity in gastroparesis following therapeutic dose. Case Rep. 2016, 2016, bcr2016215308. [Google Scholar]
- Hasler, W.L.; Wilson, L.A.; Nguyen, L.A.; Snape, W.J.; Abell, T.L.; Koch, K.L.; McCallum, R.W.; Pasricha, P.J.; Sarosiek, I.; Farrugia, G.; et al. Opioid use and potency are associated with clinical features, quality of life, and use of resources in patients with gastroparesis. Clin. Gastroenterol. Hepatol. 2019, 17, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Sandoval Terra Campos Guelli, M.; Correa, T.; Silva, I.; Zampier, D.; Costa, L.; Dias, L.; Guidoreni, A. MO558: Chronic kidney disease and gastroparesis: What is the association? Nephrol. Dial. Transplant. 2022, 37 (Suppl. S3), gfac074.003. [Google Scholar] [CrossRef]
- Turshudzhyan, A.; Inyangetor, D. Uremic and post-transplant gastropathy in patients with chronic kidney disease and end-stage renal disease. Cureus 2020, 12, e10578. [Google Scholar]
- Naymagon, S.; Naymagon, L.; Wong, S.Y.; Ko, H.M.; Renteria, A.; Levine, J.; Colombel, J.F.; Ferrara, J. Acute graft-versus-host disease of the gut: Considerations for the gastroenterologist. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 711–726. [Google Scholar] [CrossRef]
- Barbash, B.; Kramer, S.; Tzimas, D.; Saitta, P. Graft-versus-host disease of the upper gastrointestinal tract after an autologous stem cell transplant. ACG Case Rep. J. 2014, 2, 55–57. [Google Scholar] [CrossRef]
- Camilleri, M.; Kuo, B.; Nguyen, L.; Vaughn, V.M.; Petrey, J.; Greer, K.; Yadlapati, R.; Abell, T.L. ACG clinical guideline: Gastroparesis. Am. J. Gastroenterol. 2022, 117, 1197–1220. [Google Scholar] [CrossRef]
- Cangemi, D.J.; Lacy, B.E. Gastroparesis: Myths, Misconceptions, and Management. Clin. Exp. Gastroenterol. 2023, 16, 65–78. [Google Scholar] [CrossRef]
- Hasler, W.L. Gastroparesis: Symptoms, evaluation, and treatment. Gastroenterol. Clin. N. Am. 2007, 36, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Lidor, A.O.; Ensor, C.R.; Sheer, A.J.; Orens, J.B.; Clarke, J.O.; McDyer, J.F. Domperidone for delayed gastric emptying in lung transplant recipients with and without gastroesophageal reflux. Prog. Transplant. 2014, 24, 27–32. [Google Scholar] [CrossRef]
- Weber, F.H., Jr.; Richards, R.D.; McCallum, R.W. Erythromycin: A motilin agonist and gastrointestinal prokinetic agent. Am. J. Gastroenterol. 1993, 88, 485–490. [Google Scholar] [PubMed]
- Bhagat, V.; Pandit, R.A.; Ambapurkar, S.; Sengar, M.; Kulkarni, A.P. Drug interactions between antimicrobial and immunosuppressive agents in solid organ transplant recipients. Indian J. Crit. Care Med. 2021, 25, 67–76. [Google Scholar] [CrossRef]
- Cruz Jr, R.J.; Poloyac, K.; Roberts, M.; Stein, W.; Humar, A. Safe Use of Erythromycin For Refractory Gastroparesis After Small Bowel Transplantation. Exp. Clin. Transplant. 2021, 20, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Van den Houte, K.; Clevers, E.; Andrews, C.N.; Papathanasopoulos, A.; Holvoet, L.; Van Oudenhove, L.; Caenepeel, P.; Arts, J.; Vanuytsel, T.; et al. Prucalopride in gastroparesis: A randomized placebo-controlled crossover study. Am. J. Gastroenterol. 2019, 114, 1265–1274. [Google Scholar] [CrossRef]
- Camilleri, M.; McCallum, R.W.; Tack, J.; Spence, S.C.; Gottesdiener, K.; Fiedorek, F.T. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: A randomized, placebo-controlled study. Gastroenterology 2017, 153, 1240–1250. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. The neurokinin-1 receptor antagonist aprepitant: An intelligent bullet against cancer? Cancers 2020, 12, 2682. [Google Scholar] [CrossRef] [PubMed]
- Bateman, D.N.; Rawlins, M.D.; Simpsonjm, J.M. Extrapyramidal reactions to prochlorperazine and haloperidol in the United Kingdom. QJM Int. J. Med. 1986, 59, 549–556. [Google Scholar]
- Sadiya, A. Nutritional therapy for the management of diabetic gastroparesis: Clinical review. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Salehi, O.; Gao, W.L.; Kenfield, C.; Hebbard, G. Roux-en-Y jejunostomy in gastroparesis: Insight into patient perspectives and outcomes. World J. Gastrointest. Surg. 2025, 17, 102543. [Google Scholar] [CrossRef]
- Comerlato, P.H.; Stefani, J.; Viana, L.V. Mortality and overall and specific infection complication rates in patients who receive parenteral nutrition: Systematic review and meta-analysis with trial sequential analysis. Am. J. Clin. Nutr. 2021, 114, 1535–1545. [Google Scholar] [CrossRef]
- Mohajir, W.A.; Khurana, S.; Singh, K.; Chong, R.W.; Bhutani, M.S. Botulinum toxin a use in the gastrointestinal tract: A reappraisal after three decades. Gastroenterol. Hepatol. 2023, 19, 198. [Google Scholar]
- Ukleja, A.; Tandon, K.; Shah, K.; Alvarez, A. Endoscopic botox injections in therapy of refractory gastroparesis. World J. Gastrointest. Endosc. 2015, 7, 790. [Google Scholar] [CrossRef]
- Ichkhanian, Y.; Hwang, J.H.; Ofosu, A.; Li, A.A.; Szvarca, D.; Draganov, P.V.; Yang, D.; Alsheik, E.; Zuchelli, T.; Piraka, C.; et al. Role of gastric per-oral endoscopic myotomy (G-POEM) in post-lung transplant patients: A multicenter experience. Endosc. Int. Open 2022, 10, E832–E839. [Google Scholar] [CrossRef]
- Mason, R.J.; Lipham, J.; Eckerling, G.; Schward, A.; DeMeester, T.R. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch. Surg. 2005, 140, 841–848. [Google Scholar] [CrossRef][Green Version]
- Shada, A.L.; Dunst, C.M.; Pescarus, R.; Speer, E.A.; Cassera, M.; Reavis, K.M.; Swanstrom, L.L. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg. Endosc. 2016, 30, 1326–1332. [Google Scholar] [CrossRef]
- Toro, J.P.; Lytle, N.W.; Patel, A.D.; Davis Jr, S.S.; Christie, J.A.; Waring, J.P.; Sweeney, J.F.; Lin, E. Efficacy of laparoscopic pyloroplasty for the treatment of gastroparesis. J. Am. Coll. Surg. 2014, 218, 652–660. [Google Scholar] [CrossRef]
- Weinkauf, J.G.; Yiannopoulos, A.; Faul, J.L. Transcutaneous electrical nerve stimulation for severe gastroparesis after lung transplantation. J. Heart Lung Transplant. 2005, 24, 1444-e1. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, X.; Ye, Q.; Gao, Y.; Deng, R. Kidney transplantation and gut microbiota. Clin. Kidney J. 2024, 17, sfae214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Swarte, J.C.; Gacesa, R.; Knobbe, T.J.; Kremer, D.; Jansen, B.H.; de Borst, M.H.; TransplantLines Investigators; Harmsen, H.J.; Erasmus, M.E.; et al. The gut microbiome in end-stage lung disease and lung transplantation. MSystems 2024, 9, e0131223. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Wu, J.; Chalson, H.; Merigan, L.; Mitchell, A. Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobiliary Surg. Nutr. 2013, 2, 142. [Google Scholar] [PubMed]
- Vijay, C.P.; Amrutha, A.; Vinutha, D.C.; Rohith, V.; Sriraam, N. Wearable electrogastrogram perspective for healthcare applications. In Recent Trends in Computational Sciences; CRC Press: Boca Raton, FL, USA, 2023; pp. 42–48. [Google Scholar]
- Klouda, T.; Ryan, E.M.; Leonard, J.B.; Freiberger, D.; Midyat, L.; Dahlberg, S.; Rosen, R.; Visner, G. Gastrointestinal complications in pediatric lung transplant recipients: Incidence, risk factors, and effects on patient outcomes. Pediatr. Transplant. 2024, 28, e14665. [Google Scholar] [CrossRef]
- Rodriguez, L.; Heinz, N.; Colliard, K.; Amicangelo, M.; Nurko, S. Diagnostic and clinical utility of the wireless motility capsule in children: A study in patients with functional gastrointestinal disorders. Neurogastroenterol. Motilit 2021, 33, e14032. [Google Scholar] [CrossRef]
- Green, A.D.; Belkind-Gerson, J.; Surjanhata, B.C.; Mousa, H.; Kuo, B.; Di Lorenzo, C. Wireless motility capsule test in children with upper gastrointestinal symptoms. J. Pediatr. 2013, 162, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Basseri, B.; Conklin, J.L.; Pimentel, M.; Tabrizi, R.; Phillips, E.H.; Simsir, S.A.; Chaux, G.E.; Falk, J.A.; Ghandehari, S.; Soukiasian, H.J. Esophageal motor dysfunction and gastroesophageal reflux are prevalent in lung transplant candidates. Ann. Thorac. Surg. 2010, 90, 1630–1636. [Google Scholar] [CrossRef]
- Dy, F.J.; Freiberger, D.; Liu, E.; Boyer, D.; Visner, G.; Rosen, R. Impact of gastroesophageal reflux and delayed gastric emptying on pediatric lung transplant outcomes. J. Heart Lung Transplant. 2017, 36, 854–861. [Google Scholar]
- Angleitner, P.; Arnoldner, M.A.; Zuckermann, A.O.; Aliabadi-Zuckermann, A.Z. Severe gastroparesis after orthotopic heart transplantation. Eur. J. Cardio-Thorac. Surg. 2021, 59, 717–719. [Google Scholar] [CrossRef]
- Ye, Y.; Yin, Y.; Huh, S.Y.; Almansa, C.; Bennett, D.; Camilleri, M. Epidemiology, etiology, and treatment of gastroparesis: Real-world evidence from a large US national claims database. Gastroenterology 2022, 162, 109–121. [Google Scholar] [CrossRef]
- Yiannopoulos, A.; Shafazand, S.; Ziedalski, T.; Berry, G.J.; Robbins, R.C.; Theodore, J.; Faul, J.L. Gastric pacing for severe gastroparesis in a heart–lung transplant recipient. J. Heart Lung Transplant. 2004, 23, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Song, G. S1557 Understanding the Impact of Liver Transplantation on GI Motility: Prevalence and Risk of GERD and Gastroparesis in Transplant Recipients. Am. J. Gastroenterol. 2023, 118, S1175. [Google Scholar] [CrossRef]
- Vitton, V.; Benoit D’Journo, X.; Reynaud-Gaubert, M.; Barthet, M.; Gonzalez, J.M. Gastric peroral endoscopic myotomy (GPOEM) for severe gastroparesis after lung transplantation: A promising minimally invasive option. Clin. Transplant. 2021, 35, e14434. [Google Scholar] [CrossRef] [PubMed]
- Peppas, S.; Ahmad, A.I.; Altork, N.; Cho, W.K. Efficacy and safety of gastric per-oral endoscopic myotomy (GPOEM) in lung transplant patients with refractory gastroparesis: A systematic review and meta-analysis. Surg. Endosc. 2023, 37, 6695–6703. [Google Scholar] [CrossRef]
- Derousseau, T.; Chan, W.W.; Cangemi, D.; Kaza, V.; Lo, W.K.; Gavini, S. Delayed gastric emptying in prelung transplant patients is associated with posttransplant acute cellular rejection independent of reflux. J. Clin. Gastroenterol. 2022, 56, e121–e125. [Google Scholar] [CrossRef]
- Martinek, J.; Hustak, R.; Mares, J.; Vackova, Z.; Spicak, J.; Kieslichova, E.; Buncova, M.; Pohl, D.; Amin, S.; Tack, J. Endoscopic pyloromyotomy for the treatment of severe and refractory gastroparesis: A pilot, randomised, sham-controlled trial. Gut 2022, 71, 2170–2178. [Google Scholar] [CrossRef]
- Stojilkovic, T.; Staudinger, K.; Dennis, J.; Dennis, J.F. A systematic review of the long-term clinical success of gastric peroral endoscopic myotomy for refractory gastroparesis. Cureus 2023, 15, e39709. [Google Scholar] [CrossRef]
- Abell, T.L.; Johnson, W.D.; Kedar, A.; Runnels, J.M.; Thompson, J.; Weeks, E.S.; Minocha, A.; Griswold, M.E. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest. Endosc. 2011, 74, 496–503. [Google Scholar] [CrossRef]
- Radenkovic, G.; Radenkovic, D.; Velickov, A. Development of interstitial cells of Cajal in the human digestive tract as the result of reciprocal induction of mesenchymal and neural crest cells. J. Cell. Mol. Med. 2018, 22, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Dasari, S.; Bernard, C.E.; Chikkamenahalli, L.L.; Yates, K.P.; Pasricha, P.J.; Sarosiek, I.; McCallum, R.; Koch, K.L.; Abell, T.L.; et al. Proteomics in gastroparesis: Unique and overlapping protein signatures in diabetic and idiopathic gastroparesis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G716–G726. [Google Scholar] [CrossRef] [PubMed]
| Transplant Type | Prevalence/Onset of Gastroparesis | Key Contributing Factors/Comments |
|---|---|---|
| Lung Transplant | Delayed gastric emptying in 40–50% of adult lung recipients (23–91% range in studies); 52.9% in pediatric lung transplants. Symptoms often begin immediately post-surgery (within days to weeks). | Bilateral vagal nerve injury is common due to mediastinal dissection, leading to high gastroparesis rates. Contributes to aspiration risk and chronic lung allograft dysfunction (e.g., 2.7-fold higher risk of CLAD). Often severe and refractory, sometimes requiring surgical interventions (e.g., pyloroplasty, jejunostomy). |
| Heart Transplant | Gastroparesis in approximately 8–17% of adult heart recipients. May present acutely in the early postoperative period (including rare cases of acute gastric dilation/rupture), though often less frequent and less severe than in lung transplant. | Partial vagal nerve disruption occurs (dependent on surgical technique—e.g., bicaval approach may preserve some vagal fibres). Typically milder gastric emptying delays since only cardiac branches of the vagus are cut. Management is usually conservative initially, and severe gastroparesis is less common than in lung or combined transplants. |
| Heart–Lung Transplant | Very high prevalence, reported in over 80% of combined heart–lung recipients. Gastroparesis symptoms often appear early (within weeks) after transplant, reflecting the extensive surgical impact. | Complete bilateral vagal transection is almost inevitable, causing profound gastric denervation. This leads to severe, persistent gastroparesis (gastric retention up to 93% at 2 h has been noted). Many cases are refractory to medical therapy, frequently necessitating interventions like GES (gastric electrical stimulation) or pyloroplasty to maintain nutrition. |
| Liver Transplant | Clinically significant gastroparesis is less common in liver transplant patients (exact prevalence not well-defined, but considerably lower than thoracic transplants). When it occurs, onset is often delayed—typically months (e.g., 9–13 months) post-transplant. | Direct vagal innervation of the stomach is usually preserved in liver transplantation (no thoracic vagotomy). Metabolic factors are often responsible for delayed gastroparesis in these patients, e.g., development of post-transplant diabetes or side effects of immunosuppressants (tacrolimus toxicity). Additionally, celiac plexus manipulation during surgery and ischemia–reperfusion injury can contribute to transient motility impairment. Overall risk is moderate, and cases may improve with metabolic stabilisation. |
| Kidney Transplant (alone) | Definitive gastroparesis diagnosis in roughly 5–10% of kidney recipients (one large series found 6% with confirmed gastroparesis, although up to 19% had evidence of gastric food retention on endoscopy). Onset can be subacute to chronic: some patients have pre-existing diabetic gastroparesis (carrying into the post-transplant period), while others develop symptoms months after transplant due to new-onset diabetes. | Many kidney transplant recipients have a history of diabetes mellitus or develop new-onset diabetes after transplantation (NODAT) (incidence 10–30%). Diabetic autonomic neuropathy (damage to vagal nerve fibres from years of diabetes) often underlies gastroparesis. Immunosuppressive drugs like calcineurin inhibitors (tacrolimus, cyclosporine) and steroids can precipitate or worsen diabetes, thereby contributing to gastric dysmotility. Improved glycemic control post-transplant can sometimes ameliorate symptoms, but if neuropathy is advanced, gastroparesis may persist. |
| Kidney–Pancreas Transplant | In combined kidney–pancreas recipients (performed for Type 1 diabetics), gastroparesis may persist in ~30% of patients despite restored euglycemia. Some studies report roughly one-third of these patients continue to experience delayed gastric emptying post-transplant. Typically, this is observed in the first year post-transplant if it is going to persist. | The pancreas transplant often improves diabetic gastroparesis by normalising blood sugar, but in a subset of patients, irreversible neural damage from long-term diabetes means gastric emptying remains delayed. Additionally, surgical factors and immunosuppressive medication side effects can contribute. In those with persistent symptoms, adjunct therapies (prokinetics, G-POEM, etc.) may be required for symptom relief. This highlights that gastroparesis in these patients is multifactorial, involving both prior neuropathy and ongoing post-transplant factors. |
| Mechanism | Key Features | Transplant Relevance | Pathophysiological Impact |
|---|---|---|---|
| Vagal Nerve Injury | Predictable during thoracic surgery; bilateral injury common in heart–lung and lung transplants | High in lung (up to 91%) and heart–lung (83%) transplants; variable in heart; indirect in liver | Disrupts vagovagal reflexes, accommodation reflex, and antroduodenal coordination; severe, persistent gastroparesis; limited reinnervation potential |
| Immunosuppressive Medications | Tacrolimus accelerates motility; cyclosporine and MMF impair it; corticosteroids/mTOR inhibitors enhance motility variably | Kidney, liver, and heart transplants | Drug-dependent effects on gastric emptying; mucosal injury, enteric neuron modulation, and cytokine interactions complicate motility outcomes |
| Surgical Trauma and Inflammatory Response | Ischaemia–reperfusion injury, celiac plexus trauma, cytokine cascades (TNF-α, IL-1β, IL-6) | Highest in multivisceral; moderate in liver; low in isolated kidney/heart transplants | Acute cytokine surge impairs vagovagal reflexes and smooth muscle; chronic inflammation and neural injury prolong gastroparesis |
| Microbiome Dysbiosis | Reduced diversity; loss of Bacteroides, Faecalibacterium, Prevotella; overgrowth of Enterococcus, Streptococcus | Lung > heart/liver transplants; exacerbated by antibiotics and immunosuppression | Alters SCFA production, neurotransmitter signalling, gut permeability; fuels systemic inflammation and motility inhibition |
| Diabetes and Metabolic Factors | Pre-existing or post-transplant diabetes; autonomic neuropathy | Common in kidney/pancreas transplants; PTDM risk with tacrolimus/steroids | Impaired gastric accommodation and delayed emptying from autonomic dysfunction; pancreas transplant may reverse effects |
| Domain | Consolidated Findings | Key Evidence/Metrics |
|---|---|---|
| Clinical Manifestations | Core symptoms include nausea, vomiting, early satiety, bloating, epigastric pain. Malnutrition, weight loss, and micronutrient deficiencies are prevalent, particularly in multivisceral transplant recipients. | Common across transplant types; jejunostomy often required in severe nutritional compromise. |
| Organ-Specific Presentations | -Lung & Heart–lung: Most severe and early-onset symptoms; linked to vagal injury and aspiration. -Liver: Delayed onset (9–13 months); linked to metabolic derangement, immunosuppressive toxicity. -Kidney-Pancreas: Symptoms persist despite glucose control. -Stem Cell (GVHD): ~60% have GI involvement, presenting as ileus, nausea, diarrhoea. -Renal (CKD): Uremic gastroparesis due to autonomic neuropathy, anaemia, hormonal shifts. | -Lung: Gastric emptying delay in 52.9–67%; bilateral lung: 73%; HRM abnormalities in 83%. -Heart–lung: Gastric retention up to 93% at 2 h (normal <50%). -Liver: CMV gastritis in 20–28%, onset 2–3 months; relapse up to 44%. -Kidney-Pancreas: Multifactorial aetiology. -GVHD: Biopsy-confirmed GI involvement. |
| Complications Mimicking Gastroparesis | GERD and microaspiration (especially in lung transplants) can contribute to chronic rejection. Bezoars can develop rapidly in CF recipients. PTLD may present similarly with gastric involvement. Opioid use can significantly contribute to motility delay. | -Elevated pepsin in BAL linked to acute rejection (grade A2+). -Bezoars form ~34 days post-op; PTLD affects 23–30% (stomach in 11.9%). -Opioids implicated in 41% (82% due to potent opioids). |
| Pharmacological Impact | Gastroparesis affects immunosuppressant Tmax but not bioavailability. | Tacrolimus Tmax vs. gastric emptying: r2 = 0.30, p < 0.0001; 4 h level correlation: r2 = 0.96, p < 0.0001. |
| Diagnostic Modalities | -Gold Standard: Gastric scintigraphy (85–95% sensitivity; 80–90% specificity). -Adjuncts: HRM (83% abnormal in lung recipients), WMC (pH, pressure, transit), electrogastrography (dysrhythmia detection), endoscopy (rules out obstruction/bezoars), AI models. -Special Protocols: 90 min dynamic oatmeal + 24 h chest imaging to detect aspiration. | -Scintigraphy: <60% retention at 2 h, <10% at 4 h = normal. -AI: AUC = 90.7%, accuracy = 80% for delayed emptying; pyloric therapy response: sensitivity 96%, specificity 75%. |
| Technological Integration | Electrogastrography allows symptom-timing correlation. Artificial intelligence improves diagnostic and therapeutic predictions. | AI models outperform human-based scoring in scintigraphy analysis and response prediction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharadwaj, H.R.; Koo, T.H.; Dahiya, D.S.; Dalal, P.; Fuad, M.; Arab, S.; Chhatwal, K.; Bhatti, T.; Malik, M.; Singh, S.; et al. Gastric Motility Disorders Post Organ Transplantation—A Comprehensive Review. J. Clin. Med. 2025, 14, 7581. https://doi.org/10.3390/jcm14217581
Bharadwaj HR, Koo TH, Dahiya DS, Dalal P, Fuad M, Arab S, Chhatwal K, Bhatti T, Malik M, Singh S, et al. Gastric Motility Disorders Post Organ Transplantation—A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(21):7581. https://doi.org/10.3390/jcm14217581
Chicago/Turabian StyleBharadwaj, Hareesha Rishab, Thai Hau Koo, Dushyant Singh Dahiya, Priyal Dalal, Muhtasim Fuad, Sammy Arab, Karanjot Chhatwal, Taha Bhatti, Maham Malik, Simardeep Singh, and et al. 2025. "Gastric Motility Disorders Post Organ Transplantation—A Comprehensive Review" Journal of Clinical Medicine 14, no. 21: 7581. https://doi.org/10.3390/jcm14217581
APA StyleBharadwaj, H. R., Koo, T. H., Dahiya, D. S., Dalal, P., Fuad, M., Arab, S., Chhatwal, K., Bhatti, T., Malik, M., Singh, S., Hasan, F., Tofani, C., & Infantolino, A. (2025). Gastric Motility Disorders Post Organ Transplantation—A Comprehensive Review. Journal of Clinical Medicine, 14(21), 7581. https://doi.org/10.3390/jcm14217581







