1. Introduction
The pulse wave velocity (PWV) is the rate at which pressure waves propagate through the arterial system [
1]. Clinically, PWV has been established as a reliable, non-invasive measure of arterial stiffness [
2] and an independent risk predictor of adverse cardiovascular events including hypertension [
3,
4]. Subsequent explorations into the utility of PWV have led to its study as a possible parameter in the non-invasive monitoring of key cardiovascular measurements such as arterial blood pressure (ABP) [
5]. In particular, the use of PWV in ABP measurement is a possible improvement to the current non-invasive clinical method of sphygmomanometry due to allowing for more continuous monitoring of ABP and therefore facilitating investigations into the dynamic behaviour of the arterial system.
The current gold standard for non-invasive PWV measurement is carotid-femoral PWV (cfPWV) measurement via direct tonometry [
6,
7], which involves measuring the transit time of the arterial waveform from one point on the carotid to another point on the femoral artery [
7,
8]. However, the global nature of the cfPWV limits the effectiveness of the method in conducting measurements over a long arterial segment. Firstly, the distance measurement of the cfPWV does not account for the path of blood from the aortic arch to the carotid artery; hence, assumptions are made to appropriately adjust the measured time taken, which limits the accuracy of the method [
8,
9]. Secondly, global cfPWV measurement conceals variations in arterial stiffness and mechanical properties of vessels [
7,
10] that may cause differences in the local PWV. Additionally, previous studies have concluded that the global PWV is less useful in providing significant clinical information than the local PWV, taken in a specific arterial segment, in terms of assessing arterial properties [
11,
12,
13]. Hence, the utility of the cfPWV method is limited.
Considering the value of PWV measurements in the monitoring of cardiovascular health and the limitations of the global cfPWV, further research has investigated non-invasive alternatives to local PWV determination including magnetic resonance imaging (MRI) [
14,
15], plethysmography [
4,
16], and ultrasound [
16].
Of particular interest among these methods is ultrasound, which leverages the presence of frequency shifts [
17] in reflected high-frequency sound waves to obtain measurements at specific locations on the arterial tree. Ultrasound-based local PWV methods are indirect and require intermediate measurands for calculation and determination of the PWV. These measurands include the diameter or lumen area of the artery and the velocity of blood flow in the measured region [
5,
16,
18,
19,
20,
21,
22,
23].
Given the importance of the PWV, several previous reviews have compared a myriad of non-invasive data acquisition approaches for PWV measurement including direct tonometry, plethysmography, MRI, and ultrasound [
4,
16,
24]. These reviews focused primarily on compiling prominent methodological information for the major acquisition methods, whereas more specific details on ultrasound optimisation for PWV acquisition have remained less explored. Meanwhile, considering the utility of ultrasound, other reviews explored developments in ultrasound techniques such as vector flow imaging and 3D flow imaging [
25], while others broadly detailed ultrasound techniques for studying vascular ageing [
26], with a limited discussion on considerations for developing an optimal PWV acquisition method. To date, little has been done to comprehensively discuss the optimisation of ultrasound in data acquisition for PWV estimation, particularly with respect to the flow-area (QA) and lnDiameter-velocity (lnDU) loop-based methods.
In contrast, this review seeks to evaluate different approaches for ultrasound-based acquisition while considering technical parameters and physiological realities to optimise the accuracy, reliability, and reproducibility of the parameters collected for estimation. Consequently, with a focus on the QA and lnDU loop-based methods, this paper discusses the use of ultrasound imaging in vessel data acquisition, highlights various challenges and considerations to be managed during acquisition and processing, outlines the different ultrasound-based imaging modalities for acquiring area and velocity data, and compares the simultaneous and non-simultaneous acquisition of data for PWV estimation.
2. Background of Ultrasound Measurement for PWV Estimation
2.1. Method of Processing Acquired Data
An understanding of the data-processing method used allows for a greater understanding of what data need to be acquired to improve the acquisition in the first place.
The common data processing methods used in the literature for ultrasound-based acquisition are transit time techniques and loop-based methods. Non-invasive transit time techniques include the Doppler-based cfPWV method and pulse wave imaging (PWI). Doppler-based cfPWV measurement is functionally like direct tonometry cfPWV measurement, where data are acquired via ultrasound instead of an applanation tonometer [
27]. Hence, this method is not considered, as it returns global PWV measurements rather than local measurements. Meanwhile, PWI measures and images the spatiotemporal propagation of the arterial wall distension wave to compute the local PWV [
28,
29]. However, PWI requires very high temporal resolution to accurately capture the small wall displacements, which may not be achievable with all ultrasound machines. For instance, investigations into the temporal resolution required for PWI have shown that ultrafast frame rates of more than 2 kHz are needed for optimal measurement [
29,
30]. Conversely, studies with loop-based methods were able to operate with sufficient accuracy at 60–200 Hz [
31,
32]. Additionally, in PWI, it is assumed that the detected distension corresponds with the true forward moving wave, despite the possibility that observed distension may be due to the combination of both forward and reflection waves. Thus, this paper focuses on loop-based data processing methods which can estimate the local PWV during the reflection-free period of the cardiac cycle using current clinical ultrasound systems.
2.2. Loop-Based Estimation for PWV
Loop-based methods determine the pulsatile wave from haemodynamic variables including the blood velocity and flow, the wall velocity or acceleration [
5,
16,
18,
19,
20,
21,
22,
23,
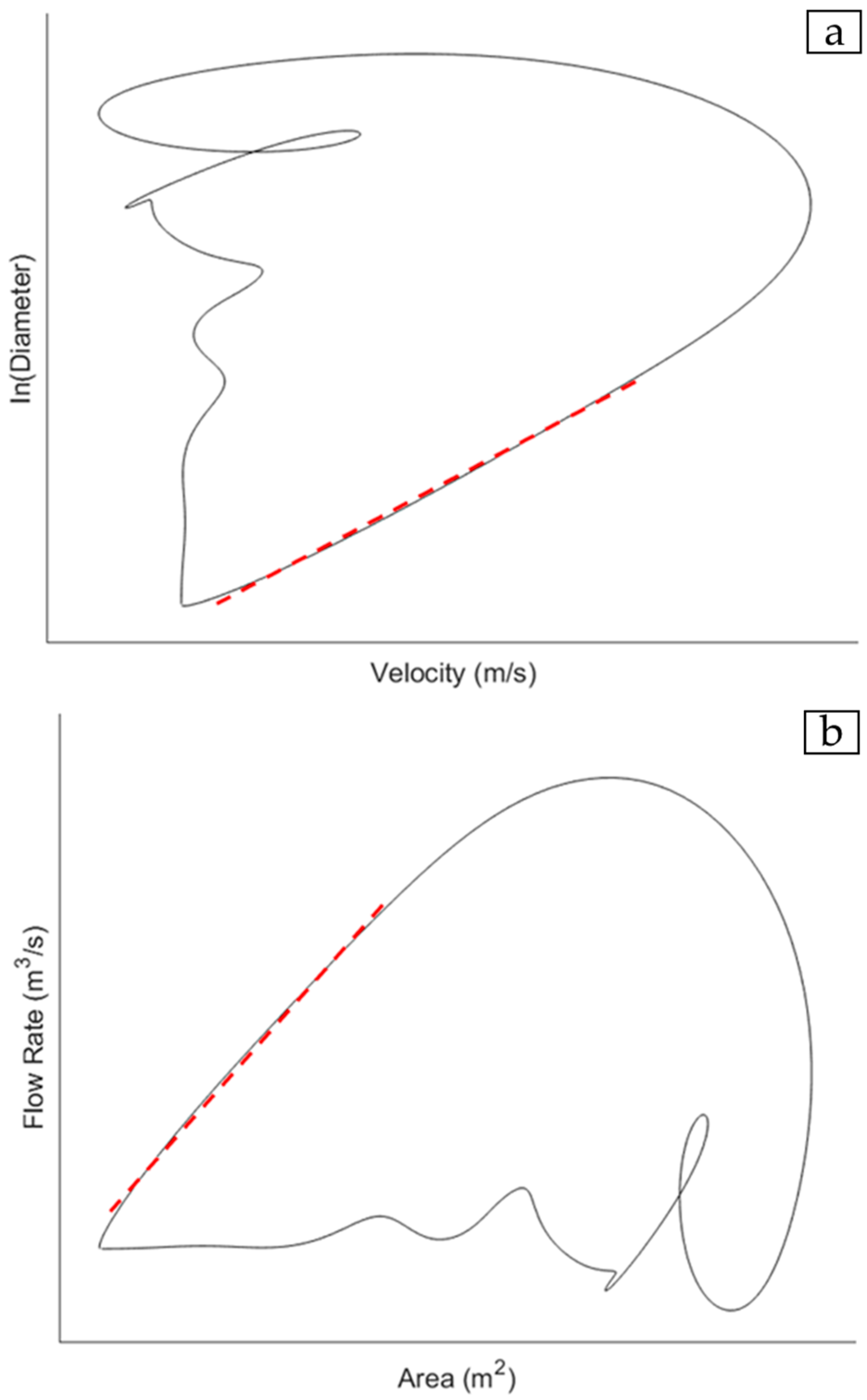
28], and the vessel lumen diameter or cross-sectional area. The waveforms for a pair of haemodynamic variables are acquired over the cardiac cycle and plotted together to give a haemodynamic loop. There are two loops of interest, namely the lnDiameter-velocity (lnDU) loop (
Figure 1a) [
20,
21,
33] and the flow rate-area (QA) loop (
Figure 1b) [
5,
19].
The full mathematical derivation of each loop can be found in [
19,
20] for the lnDU and QA loops, respectively. The estimation of PWV via the lnDU method is described by Equation (1):
where
U equals the local blood velocity within the arterial segment and
D represents the lumen diameter of the vessel. The subscripts + and − designate the respective forward and backward directions of wave travel. In contrast, PWV estimation via the QA method is described by Equation (2):
where
Q is the blood flow rate within the arterial segment and
A corresponds to the area of the arterial lumen.
A critical assumption in both equations that affects parameter optimisation is the estimation of the PWV during the reflection-free period. For the lnDU method, earlier work describes the relationship between velocity and the natural logarithm of D as being linear and proportional to the wave speed so long as the waves are unidirectional [
20,
21]. Furthermore, part of the derivation of the QA equation defines the relationship between dynamic pressure and flow in the arterial system via the use of characteristic impedance, which only holds true in the absence of reflections [
19]. Hence, from both the loops, the PWV is determined based on the gradient of the linear portion of the curve which corresponds to the early systolic period in the cardiac cycle, which is widely accepted as being reflection-free [
34,
35]. Previous studies demonstrated that both loops could detect changes in the local PWV stemming from cardiovascular diseases with sufficient accuracy [
19,
21,
31,
36]. The QA method is conceptually direct, being derived from the fundamental flow–area relationship of the water-hammer equation that links local pressure, flow, and area changes [
19]. Conversely, the lnDU method is computationally simpler, with higher reproducibility and operational consistency in practice [
21,
31]. Since both are widely used in local, loop-based PWV estimations and rely on similar measurands, both are considered in this paper within the context of acquisition optimisation.
Another loop method involves the acquisition of pressure as a parameter and includes the pressure–velocity and pressure–flow rate methods [
37]. However, unlike area and velocity, current ultrasound machines do not have a means to measure pressure directly; hence, the pressure loop methods are not considered in this paper.
2.3. Functioning Principles Behind Ultrasound Measurement
Ultrasound data acquisition involves the use of high-frequency longitudinal pressure waves or ultrasound pulses, typically in the diagnostic range of 2–15 MHz [
38], for measurement. During acquisition, the ultrasound pulses are generated and detected by a transducer. During transmission, a fast-alternating electric field is applied to the piezoelectric crystal within the transducer head, causing the crystals to vibrate at ultrasound frequencies and emit pulses into the body. During detection, the process is reversed. At the interface between media of differing acoustic impedances, the ultrasound pulse is reflected toward the piezoelectric crystal, vibrating it. Subsequently, the vibration from the returned echo is converted into electrical radiofrequency (RF) signals. By analysing the echo arrival times from successive tissue boundaries, changes in arterial lumen area and diameter can be measured for PWV estimation [
39]. For such imaging, the size of the object should be larger than the pulse wavelength to produce clear reflected echoes. In contrast, objects smaller than the pulse wavelength, such as erythrocytes, generate weak, diffuse Rayleigh scattering that results in indistinct images. However, the same scattering provides the basis for velocity and acceleration measurements taken via Doppler ultrasound [
40]. In Doppler ultrasound, a small component of the scattered signal from each moving erythrocyte is detected by the transducer. This combined backscattered signal produces a measurable echo with a frequency shift that is used to calculate velocity.
Recent advances in ultrasound-based PWV estimation have arisen from improvements in both acquisition methodology and system electronics and computation. Methodological developments involve the use of simultaneous or non-simultaneous data acquisition, the number and positioning of transducers used [
41], and differing data processing methods. In parallel, upgrades in system electronics and computation aim to enhance the reliability of signal acquisition by improving the efficiency, bandwidth, sensitivity, and stability of transducers [
42], as well as improving their frame rates to achieve better temporal resolution [
16]. These upgrades encompass enhancements to existing transducer arrays [
26,
43] via modifications of the materials used for the piezoelectric component [
42] and the adoption of advanced scanning techniques such as plane wave insonification, coherent spatial compounding, multiline transmission, and motion matching techniques [
16].
2.4. Comparative Advantages of Ultrasound Imaging in Non-Invasive PWV Acquisition
Among clinically available imaging techniques, ultrasound presents distinct advantages for non-invasive PWV acquisition, including good penetration depth [
44], real-time imaging capability [
45,
46], and its established use in cardiovascular monitoring [
47].
As a clinical imaging modality, ultrasound is relatively low-cost compared to other methods such as computed tomography (CT) and MRI [
48]. Additionally, current ultrasound machines allow for repeated imaging of the arterial segment of interest without exposure to ionising radiation and the variety of transducers available enables imaging and data acquisition from both superficial arteries like the common carotid arteries [
49,
50] and deeper central arteries like the abdominal aorta [
51].
There are also several factors that make ultrasound more favourable than MRI, which is considered another non-invasive alternative in routine PWV measurement. Apart from the cost, a limiting factor of MRI’s utility compared to ultrasound is the long duration [
52,
53,
54] and the need for breath-hold in traditional MRI acquisition to minimise noise during acquisition [
15]. Breath-hold usually occurs over a period of 10–20 s and may not be possible in some patients including individuals with shortness of breath or cardiac failure [
15]. Recent developments in MRI technology such as respiratory gating and motion-correction algorithms have enabled free-breathing during acquisition [
55]. However, these techniques do not eliminate motion blurring [
56,
57] and usually sacrifice some spatial and temporal resolution for image stability [
58], which is not ideal for precise waveform acquisition in PWV estimation. Moreover, these techniques require advanced reconstruction in imaging, which increases the computational time and burden [
59,
60]. Hence, despite advancements in free-breathing MRI, ultrasound remains more practical for real-time, routine clinical acquisitions.
In terms of the quality of the acquired data, ultrasound has better temporal and spatial [
61] resolution than MRI and is effective in capturing real-time functional information [
62]. This makes ultrasound more suited to capturing the minute changes in distension of the arterial lumen and changes in blood velocity over each cardiac cycle for PWV estimation.
Moreover, in comparison to the large, generally immovable machinery used in MRI, ultrasound machines are more compact and portable [
63], with ultrasound being used regularly in hospitals and clinics for cardiology and obstetrics [
64]. Furthermore, improvements in ultrasound transducer technology have also led to the creation of handheld transducers that can be connected directly to smartphones [
65], thereby further increasing the accessibility of ultrasound.
These advantages make ultrasound favourable for clinical applications in the routine measurement of PWV and monitoring of cardiovascular health.
3. Key Challenges and Considerations in Ultrasound Imaging for PWV Measurement
In acquiring parameter data for PWV estimation, two categories of issues arise. These include challenges, which are intrinsic sources of variability in measurement that must be mitigated, and considerations, which are adjustable software or hardware factors that can be controlled to optimise data acquisition. In this section, possible mitigation strategies for the identified challenges are proposed to improve acquisition and processing to obtain greater PWV estimation accuracy.
3.1. Physiological Challenge: Wave Reflections
As previously mentioned, a key challenge in the use of the haemodynamic loops for processing the acquired data is the presence of reflected waves. Wave reflections occur due to impedance mismatches in the arterial tree such as at sites of bifurcation and tapering, where the vessel size decreases in the periphery [
66,
67]. The presence of backward reflected waves complicates PWV calculations that assume forward blood flow, thereby causing an undesired divergence in the pressure and flow waveforms [
35,
67] and affecting PWV estimations [
68]. As discussed, current loop methods attempt to mitigate this issue through estimation at the early systolic reflection-free period of the cardiac cycle [
5]. Yet, the presence and duration of the reflection-free period vary depending on the imaged vessel [
69,
70]. This limits the feasibility of the ultrasound-based data acquisition to central arteries, as peripheral vessels have significantly shorter reflection-free periods, although it is argued that the use of high sampling rates and knowledge of wave speed can assist with limiting analysis purely to forward flow [
5].
Alternatively, wave separation analysis (WSA) techniques may be employed post-acquisition to separate forward and backward flow. Manoj et al. [
71] demonstrated that adopting WSA techniques reduced the variability in PWV estimation by 65% in ultrasound-based acquisition and yielded values that aligned closely with theoretical PWV values. Thus far, WSA has been applied to transit time techniques rather than loop-based methods for PWV estimation, yet it is reasonable to propose the extension of WSA to loop-based approaches by applying separation prior to constructing loops from forward-only components. This avenue remains unexplored and requires further validation.
Overall, mitigation of wave reflections is crucial in improving the accuracy of acquired data and incorporating WSA may aid in addressing this challenge.
3.2. Physiological Challenge: Differences in Acoustic Impedance of Internal Structures
Differences in acoustic impedances within the human body represent another significant intrinsic physiological challenge that needs to be managed. Given that reflections in ultrasound are generated at tissue boundaries where the acoustic impedances differ, significant differences in acoustic impedance such as those between tissue, bone, and gas can cause unwanted artefacts [
72] like shadowing that prevent imaging of deeper tissue structures. This has implications on the feasibility of ultrasound imaging at different arteries. For instance, the thoracic aorta, which is surrounded by the rib bones and gas in the lungs, is typically not imaged by non-invasive ultrasound methods [
73,
74]. Meanwhile, while ultrasound imaging of the abdominal aorta is feasible, bowel gas may cause undesired artefacts that affect imaging [
51,
75]. Managing acoustic impedance differences within the body may involve selecting a relatively less obstructed artery such as the common carotid artery (CCA) or compensating for reflectors that block ultrasound access to the artery. For example, the effect of bowel gas on abdominal aorta imaging may be mitigated with fasting prior to imaging [
76] or with probe pressure during examination to rapidly move the gas [
77].
3.3. Technical Challenge: Operator Skill Issue
A challenge intrinsic to ultrasound measurement is operator skill, as this modality is highly operator-dependent during both image acquisition and data interpretation [
78]. This operator dependence presents a risk of accuracy reduction and can impact the intra- and inter-operator repeatability of measurements [
79]. Given the prevalent clinical use of ultrasound [
80], several studies have examined methods to tackle reliance on operator skill.
In vessel image acquisition, automatic wall tracking methods assist in obtaining more accurate, precise, and consistent measurements of vessel diameter changes over the cardiac cycle [
81,
82,
83,
84]. In Doppler ultrasound for velocity or volumetric flow measurements, it was suggested that greater operator independence may be achieved using 3D Doppler ultrasound that captures volumetric data rather than the velocity data in a specific plane to reduce the need for precise transducer alignment [
85]. However, despite these purported improvements, the method still relies on Doppler angle correction by the operator, wherein inaccuracies in angle estimation can introduce velocity measurement errors [
86]. Moreover, 3D Doppler measurement reduces but does not eliminate the fact that a poorly aligned transducer relative to the vessel can cause signal degradation and measurement errors. While these methods assist in reducing issues due to operator skill, they do not fully mitigate issues that arise from poor positioning, the angling of transducers, or sub-optimal selection of the region of interest in ultrasound technology.
A future approach to tackling operator dependence is the use of machine learning to assist in data acquisition. Machine learning systems may be able to provide real-time feedback or guidance to operators during image acquisition itself along with image quality control and automatic rather than manual selection of the vessel region of interest [
46]. For instance, deep learning methods have been proposed for automatic Doppler angle estimation from B-mode imaging to reduce operator bias [
87].
3.4. Software-Based Considerations
Prior to data acquisition, it is crucial to optimise key software parameters including the frame rate, pulse repetition frequency (PRF), gain, dynamic range, and filter settings, which have been identified to more greatly affect PWV data acquisition. The adjustment of each parameter should account for acceptable compromises, as improvement in one aspect often causes deterioration in another.
3.4.1. Frame Rate
The frame rate, defined as the number of image frames per second, directly affects the temporal resolution [
88]. In parameter acquisition, a sufficiently high temporal resolution is essential to capturing arterial changes during the short duration of the cardiac cycle, as accurate PWV estimation relies heavily on precise timing of the parameter waveforms. The frame rates utilised in previous studies ranged from 60–200 Hz [
19,
31,
32]. The frame rate can be increased by reducing the imaging depth, narrowing the width of the region of interest, and decreasing the line density [
89].
3.4.2. Pulse Repetition Frequency
Pulse repetition frequency (PRF) refers to the number of ultrasound pulses emitted over time. The PRF can be adjusted manually to minimise or eliminate aliasing, which otherwise causes inaccuracies in velocity measurement for PWV estimation. In Doppler imaging, adjustments should be made to ensure that the blood flow velocity is below the Nyquist limit, which is equal to half the PRF. During adjustments, higher PRF values reduce the effects of aliasing at the cost of shallower penetration depths [
90]. In medical imaging, the typical PRF range is 1–10 kHz [
91,
92] and the optimal PRF value varies between patients but is often within a certain range [
93]. For instance, at shallower arteries such as the CCA, previous studies have suggested the use of PRF values in the 3–4 kHz range [
91,
93]. Conversely, at deeper locations such as the abdominal aorta, a lower PRF in the range of 1–3 kHz is recommended [
91]. The PRF can be tuned by identifying aliasing, which appears as abrupt cut-offs with inversions in the spectral trace or mixed colours in colour Doppler. Once aliasing is identified, incremental adjustments to the PRF can be made until the spectrum is continuous and the colour display is uniform.
3.4.3. Gain Function and Time Gain Compensation
The gain function amplifies echoes to overcome attenuation and increase brightness. However, excessive gain increases the amount of noise and decreases the signal–noise ratio [
38]. Meanwhile, time gain compensation (TGC) selectively amplifies signals at greater depths without increasing the noise in more superficial layers [
38]. The fine-tuning of the gain and TGC is dependent on the vessel location and the tissue composition of the subject. The gain is usually adjusted from preset values to modify the overall brightness of the sonogram, whereas TGC controls the depth-specific brightness and can likewise be adjusted from preset values [
94]. In practice, the tuning of both parameters is determined by visual confirmation of the optimal contrast within the region of interest. Taken together, effective tuning of both the gain and TGC improves the imaging contrast and ensures consistent arterial wall detection in diameter and area measurement.
3.4.4. Dynamic Range
The dynamic range is the range between the maximum and minimum values of the displayed signal [
95], which affects the clarity of the displayed lumen wall. A low dynamic range for arterial lumen wall measurement is generally preferred [
96], as it provides a suitably high contrast between the vessel wall and the lumen. Given that the dynamic range adjusts how the signal amplitudes appear visually, fine-tuning of the dynamic range should be performed with visual confirmation of high contrast in B-mode imaging.
3.4.5. Filters
Filters enhance the signal quality by suppressing unwanted components and improving the signal–noise ratio. Among the filters available on clinical ultrasound systems, three have the most pronounced influence on the optimisation of parameter acquisition. The wall filter removes low-frequency motion components to isolate blood flow in Doppler imaging [
97], while the persistence filter averages consecutive frames to stabilise noisy images at the possible expense of temporal resolution [
98]. The speckle-reduction filter improves the visual clarity of lumen boundaries but, if applied in excess, can decrease the resolution of images of the arterial wall [
99]. Hence, while useful in improving acquisition accuracy, these filters must be carefully optimised to avoid the unintended erasure of relevant physiological flow components.
3.5. Hardware-Based Considerations
3.5.1. Coupling Media
An important hardware-based consideration for ultrasound imaging is the use of coupling media during imaging. For the signals to be optimally transmitted to the organ of interest, the transducer head must be in full contact with the skin surface, as the presence of air pockets between the transducer head and the skin surface will cause large acoustic impedance differences and prevent ultrasound transmission for imaging. Hence, a coupling medium is employed to decrease the difference in acoustic impedance [
100]. The gold standard for media in clinical imaging is ultrasound gel, which is spread over the measurement location. Although the gel is a fast and convenient option, it may spread inconsistently, particularly for irregular surface anatomy, and reapplication might be needed for wider area measurement. For acquisitions on irregular surfaces such as the neck, a gel pad is a possible alternative. During acquisition, the pad is laid over the area of interest or attached to the transducer head to provide a consistent and wide region for scanning [
101]. An alternative to gel media is water baths, in which the whole body or body part is submerged into water [
102,
103]. The water bath method has been shown to provide superior imaging quality when compared to gel-based imaging, particularly in peripheral vessels, and removes the need for direct skin contact [
63]. However, water immersion for imaging may be less feasible for imaging certain arteries such as the CCA, whose measurement location is at the neck.
3.5.2. Transducer Choice
Another hardware-based consideration is the transducer choice. A wide array of transducers is available for data acquisition and the decision of which type of transducer to use depends largely on the desired measurement site. More superficial measurement locations have a lower depth of focus and can be imaged with higher-frequency transducers that offer better resolution with a smaller penetration depth. Conversely, deeper measurement locations have a greater depth of focus and demand lower-frequency transducers that afford greater penetration depths with poorer resolution. For instance, PWV measurement in the abdominal aorta may require a 3.5 MHz transducer that provides sufficient tissue penetration (>15 cm) for imaging at the cost of lower image resolution [
38]. In contrast, it is more appropriate to use a higher 7.5 MHz frequency transducer [
31] that provides a lower penetration depth but higher image resolution for measurement in the more superficial CCA.
Having outlined the key physiological and technical challenges and considerations that influence the acquisition of arterial parameters for PWV estimation in practice, the following sections examine the ultrasound modes for area and velocity measurement and evaluate the simultaneous and non-simultaneous uses of these modes that operate within the discussed constraints.
4. Ultrasound Imaging Modes for Vessel Area Acquisition
Vessel area acquisition using ultrasound typically involves the transverse placement of the transducer to obtain a cross-sectional view of the arterial lumen. However, while transverse placement is useful for anatomical localisation, it is unsuitable for use in PWV estimation, particularly with loop-based methods, due to the plane mismatch in parameter acquisition. Doppler measurements for velocity and flow, as discussed in later sections, require the transducer to be positioned longitudinally for alignment with the direction of blood flow. Given that the area waveform should be acquired within the same segment of the vessel and appear in the same sagittal plane for the subsequent alignment with the velocity or flow waveform to be optimal, a longitudinal placement along the vessel axis is preferred [
5,
33,
104].
As such, since longitudinal measurements provide a one-dimensional view of the vessel, the vessel distension over the cardiac cycle is measured via changes in the diameter of the vessel lumen. The geometry of the vessel lumen is assumed to be axisymmetric and circular, with loop-based PWV estimation studies typically modelling the vessel segment as a uniform elastic cylinder [
19,
32,
105,
106]. Subsequently, loop methods requiring cross-sectional area measurements derive the area from the diameter via the equation,
.
Therefore, the following section explores area measurement techniques that involve longitudinal transducer placement and the technical developments that improve the accuracy, reliability, and repeatability of acquisition.
4.1. Imaging Modalities
In area or diameter measurement, sound waves emitted by the transducer are partially reflected and transmitted at tissue boundaries with different acoustic impedances in the beam path, which causes differences in the echo return time and facilitates detection of different tissues at different depths [
38]. There are three main imaging modes available on ultrasound machines, namely Amplitude (A-mode), Brightness (B-mode), and Motion (M-mode). A-mode ultrasound provides a one-dimensional scan line that displays the strength of the echoes as vertical peaks on a graph. While some studies have explored the use of A-mode ultrasound in simple system configurations to measure diameter [
107,
108,
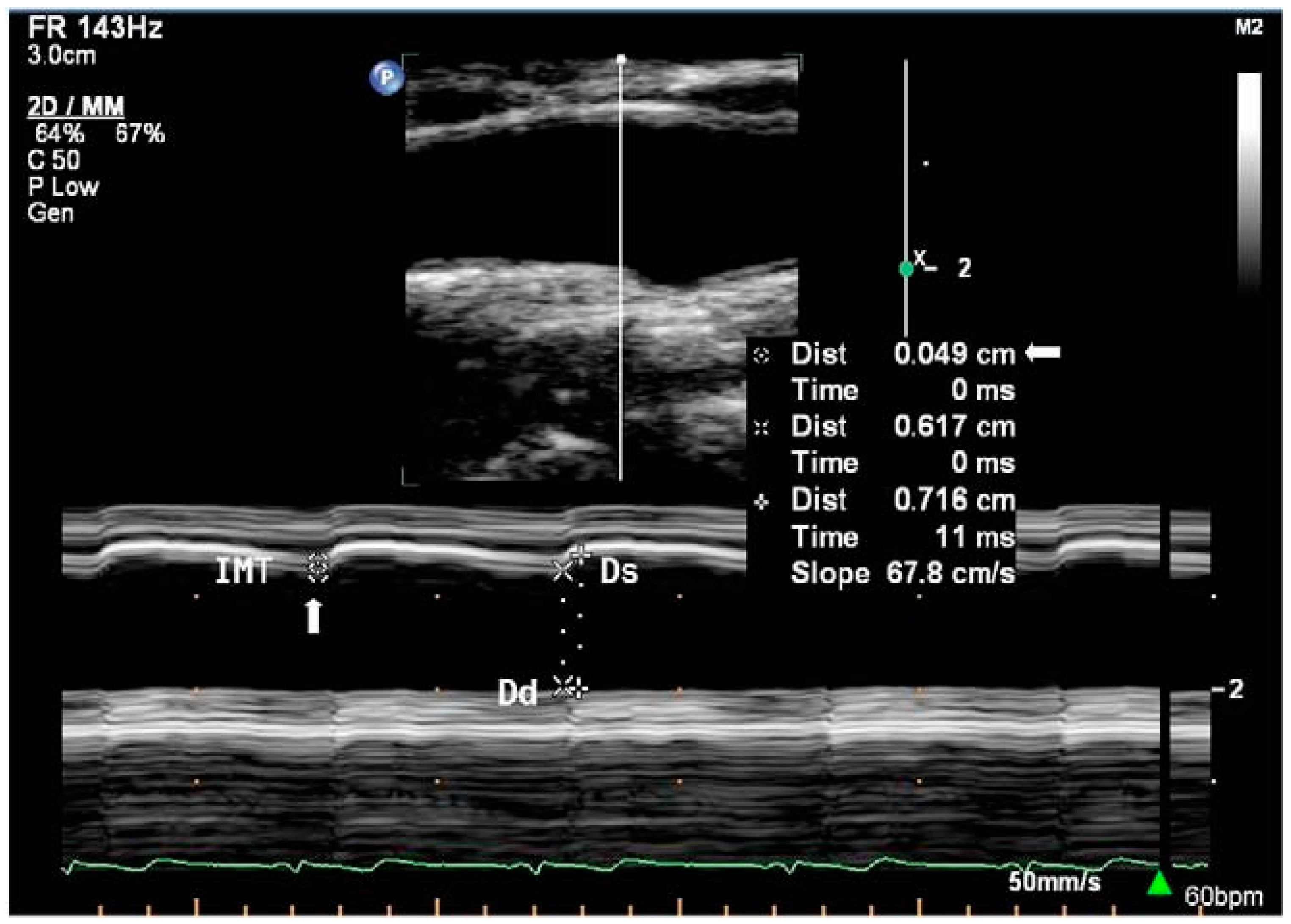
109], its use remains relatively under-represented in the literature relating to diameter and area acquisition. Hence, only B-mode and M-mode (
Figure 2) ultrasound will be discussed. The functioning principles and use of each modality in measurement are summarised in
Table 1.
4.2. Developments in B- and M-Mode Imaging
Developments in B- and M-mode imaging for area and diameter acquisition revolve around improving the resolution and contrast and involve technical improvements peri- and post-acquisition. This section briefly discusses the various explored categories of improvement to B-mode and M-mode imaging during and after acquisition which aid in improving the accuracy, reliability, and repeatability of acquisition for PWV estimation.
Peri-acquisition developments are techniques applied during acquisition to influence real-time image formation. These improvements are focused on increasing frame rates and contrast through techniques such as ultrafast plane-wave imaging [
111,
112] and spatial and frequency compounding [
113,
114], while reducing the effects of speckle artefacts via adaptive and coherence-based beamforming [
111,
115].
At the post-acquisition stage, advancements in digital signal and image processing refine raw RF data to improve image quality. For B-mode imaging, deconvolution techniques such as higher-order statistics [
116] and autoregressive parameter modelling [
117] have improved the spatial resolution and contrast ratio of images. Concurrently, various non-linear beamforming techniques such as Filtered Multiply and Sum (FMAS) [
118], Delay Multiply and Sum (DMAS) [
119], and Cross-Angular Delay Multiply and Sum (CADMAS) [
120] have been devised to enhance image contrast. In M-mode imaging, technical developments such as temporal smoothing and interpolation focus on optimising motion analysis.
Taken together, these peri- and post-acquisition developments enable greater accuracy and reliability in the tracking of arterial wall distension. Therefore, consideration of the use of one or a combination of the available techniques to optimise diameter and area acquisition is warranted.
4.3. Automatic Wall Distension Tracking Methods
In the measurement of vessel diameter, inbuilt digital callipers can be used to manually mark the desired region of interest for measurement. However, such manual tracking of wall distension in either B-mode or M-mode can be problematic due to its time-consuming and subjective manner, where small deviations in placement at the measured region of interest, due to visual identification, can cause large errors in area measurement. Hence, developments of more objective and automatic wall tracking methods are crucial for PWV estimation.
Owing to its necessity, wall tracking for ultrasound-based methods has been studied in detail, from the use of threshold detectors to study echoes from the arterial wall [
121], which is limited due to dependence on the distance to the arterial wall, which varies through the arterial cycle, to methods that use phase-locking devices which track a specific vessel wall echo [
122,
123]. Further developments also investigated combining the tracking system with B-mode imaging [
124] and pressure recordings [
125]. Alternatives to these phase-locking methods were also studied, alongside the use of conventional autocorrelation, which is independent of radiofrequency (RF) centre frequency [
126,
127] but requires lower sampling rates, and subsequently RF cross-correlation [
128,
129]. Cross-correlation techniques, however, have an estimator bias that depends nonlinearly on the actual displacement [
81]. To address this, a modification was made to the autocorrelation method to estimate the mean Doppler frequency and RF centre frequency for wall tracking [
130,
131]. In this modified method, wall tracking is conducted by integrating wall velocities as estimated by Doppler techniques. This method was found to track wall vessel motion with lower bias and variance than previous cross-correlation methods, and thus improves tissue tracking accuracy [
81].
Another type of algorithm developed to measure vessel diameter using B-mode imaging utilises the differences in pixel intensity values or brightness within a user-defined region of interest to determine the wall location and hence measure the diameter [
82,
84]. To accurately determine the wall location, the transducer is placed longitudinally to the vessel. During acquisition, the resolution of the images must be sufficiently high to observe the vessel wall layers. Additionally, electrocardiogram (ECG) gating may be employed to capture images over the cardiac cycle, with the R signal being used as a reference point. An advantage of this algorithm is its purported simplicity that facilitates possible integration into current clinical machines while being robust to differences in image quality [
82].
In automatic wall tracking, algorithms also exist for diameter measurement using a transducer placed transverse to the vessel [
83]. However, as mentioned, transverse transducer placement is not optimal for subsequent velocity measurement and movement of the transducer may cause changes in the measured region; hence, such wall tracking methods are less useful in PWV acquisition.
5. Doppler Ultrasound Imaging Modes for Velocity Acquisition
Acquisition of velocity for PWV estimation is conducted using Doppler ultrasound. Akin to area acquisition in the previous section, the volumetric blood flow in loop-based PWV estimation, specifically the QA method, is not measured directly via ultrasound imaging but derived from velocity measurements [
5]. Hence, this section explores the technical developments in the various Doppler imaging modalities as well as the higher-level approaches to measuring velocity that utilise Doppler ultrasound.
5.1. Doppler Imaging Modalities
In velocity or acceleration measurement, the Doppler ultrasound mode leverages the change the frequency of the sound wave, or Doppler shift, due to reflectors such as blood elements moving towards or away from the transducer, to perform measurement (
Figure 3) [
132]. This frequency shift is proportional to the velocity [
132]. In Doppler imaging, the magnitude and presence of the Doppler shift are affected by the Doppler angle, which is the angle between the ultrasound beam and the direction of blood flow [
132]. As mentioned, the Nyquist limit is crucial in imaging for the accurate measurement of velocity via Doppler ultrasound. Beyond the Nyquist limit, undesirable aliasing occurs which can appear as incomplete peaks in the velocity curve trace for spectral Doppler imaging or as a mixture of colours in the imaged vessel under colour Doppler mode [
133].
There are four main Doppler modes that can conduct velocity measurement, including spectral Doppler, colour Doppler, pulsed wave (PW) Doppler, and continuous wave (CW) Doppler, and these are summarised in
Table 1. In the context of PWV estimation, CW Doppler is not a preferred mode to acquire velocity as it provides measurements of velocity along the entire beam path without the depth localisation needed for quantitative velocity measurement.
5.2. Developments in Doppler Imaging Modes
For current Doppler modalities, technical developments are focused on post-processing techniques to refine raw Doppler signals to obtain improved velocity measurement. This section briefly discusses the developments in post-processing that improve the accuracy of velocity measurements.
In Doppler measurement, angle and calibration corrections are conducted to reduce the cosine-angle error and improve the velocity measurement accuracy. The cosine-angle error stems from the reliance of the Doppler effect on the cosine of the angle between the ultrasound beam and flow direction. When the angle is not parallel to the flow direction, the measured signal is reduced. While commercial ultrasound systems have in-built angle correction features to compensate, these features provide insufficient correction in complex vessel geometries during velocity acquisition [
134], which thereby justifies the need for more advanced corrections, especially in PWV estimation where accurate velocity is vital. Hence, more complex angle correction and calibration methods such as dual-beam ultrasound, which removes the influence of the Doppler angle on the velocity measurement [
135], are needed. Other technical developments applicable across the Doppler modes include the use of the previously discussed filters to improve the signal–noise ratio and increase the accuracy of the measured velocity [
136].
Beyond angle corrections and filtering, the recent literature has increasingly leveraged machine learning and deep learning to improve the accuracy of measurement by focusing on Doppler denoising, aliasing correction, and velocity and angle estimation. For instance, deep neural networks have been used for contrast enhancement [
137], denoising, and aliasing correction [
138,
139], while different convolutional neural networks (CNNs) have been applied to facilitate high-frame-rate colour Doppler acquisition under constrained sampling to achieve improved velocity estimation [
140]. Concurrently, another approach leveraged a combination of blind deconvolution and robust principal component analysis to improve Doppler measurements by separating signals from clutter and blood flow components [
141]. Collectively, these machine learning developments enable more accurate velocity and flow measurements, which is crucial for local PWV estimation.
5.3. Doppler-Related Approaches to Velocity Acquisition
Advancements in ultrasound-based velocity acquisition have also emerged via refinement of more complex Doppler ultrasound techniques used to acquire velocity.
One such technique is vector flow imaging (VFI), which quantitatively evaluates the direction and magnitude of the true velocity vector and is an angle-independent estimation of flow velocity [
142]. VFI has been shown to consistently track omnidirectional pulsatile flow [
143] with enhanced sensitivity in evaluating structural and functional changes within major arteries such as the carotid [
144] and has enabled greater accuracy in tracking the flow profile within tortuous vessels [
25]. In the context of velocity acquisition for PWV estimation, VFI facilitates the real-time visualisation of complex flow patterns with very high frame rates [
25], thereby enabling the acquisition of velocity data at higher temporal resolutions. The angle-independent nature of VFI also reduces operator bias during velocity acquisition. While VFI application in velocity acquisition requires a high computational demand, it might still be feasible for use within a limited-size region of interest like a specific arterial segment for local PWV estimation [
145]. However, exploration into the efficacy of VFI in loop-based PWV estimation is relatively limited.
Contrast-enhanced Doppler is another technique developed to improve accuracy of Doppler measurements. This technique requires the injection of microbubbles as contrast agents that amplify the backscattered signals, which increases sensitivity [
146]. In PWV estimation, the increased sensitivity facilitates acquisition of signals for velocity measurement in deeper arteries such as the abdominal aorta and in arteries with lower velocity flow [
147].
Having summarised the acquisition modalities and their technical developments that enable greater accuracy, reliability, and repeatability of area and velocity acquisition, the subsequent section details, compares, and evaluates the simultaneous and non-simultaneous methods by which these developments can be incorporated in the context of PWV estimation.
Table 1.
Summary of ultrasound imaging modes for data acquisition.
Table 1.
Summary of ultrasound imaging modes for data acquisition.
| ULTRASOUND IMAGING MODE FOR VESSEL AREA ACQUISITION |
|---|
IMAGING
MODALITY | FUNCTIONING PRINCIPLE | USE OF MODALITY | REF. |
|---|
B-mode
(Brightness mode) | Cross-sectional (two dimensional) image of body made of multiple scanlines. Each scanline is made up of multiple points and corresponds to the relative position where transmitted echo returned from. Image brightness is relative to echo strength returning from each point.
| The vessel area is determined from the measured diameter. Diameter is measured in the longitudinal plane as distance between the anterior and posterior walls in B-mode. Use digital callipers for manual diameter measurement or wall tracking algorithms for automated measurement
| [82,148] |
M-mode
(Motion mode) | One dimensional display of motion over time. Relates ultrasound wave amplitude to imaging of moving structures. Used for fine measurements.
| | [149] |
| DOPPLER ULTRASOUND IMAGING MODES FOR VELOCITY ACQUISITION |
IMAGING
MODALITY | FUNCTIONING PRINCIPLE | USE OF MODALITY | REF. |
| Spectral Doppler | | | [150] |
| Colour Doppler | | Two complementary colours represent flow toward and away from the transducer Colour shades represent higher (lighter) or lower (darker) velocities An intermediate colour (green) indicates flow turbulence.
| [149] |
| Pulsed wave (PW) Doppler | | Requires high PRF Frequency of the transducer used should be compatible with the depth of tissue measured (i.e., higher frequencies for superficial structures) Transducer angle should be less than 60°
| [149,150] |
| Continuous wave (CW) Doppler | | Sensitive measures velocity along entire ultrasound beam Does not return specific information on depth, direction of flow or velocity.
| [150] |
6. Non-Simultaneous Versus Simultaneous Area and Velocity Acquisition
This section compares and evaluates ultrasound-based data acquisition methods for PWV calculations made via haemodynamic loop approaches. As previously discussed, the loop methods, namely QA and lnDU, both require the acquisition of velocity and diameter from the artery of interest. However, there are differences in how data are acquired, whether simultaneously or non-simultaneously. Unlike the preceding sections, which focused on optimising the acquisition of each parameter individually, this section describes and evaluates acquisition methodologies for loop-based PWV estimation.
6.1. Non-Simultaneous Acquisition Methods
Previous studies employing non-simultaneous acquisition have generally relied on repositioning a single transducer to obtain separate area and velocity recordings [
19,
31,
151]. The transducer is adjusted perpendicularly for area measurements and subsequently tilted to acquire Doppler velocity data while appropriate angle corrections are applied.
An early non-simultaneous acquisition method utilised in [
31] employs B-mode imaging for diameter acquisition and PW Doppler imaging for velocity acquisition before using the lnDU loop for PWV estimation. This method involves obtaining B-mode images with the region of interest in the focal zone, where images should clearly capture anterior and posterior walls, to reduce noise and obtain optimal diameter curve measurement. Subsequently, using the same scan projection, PW Doppler measurement is conducted with as small an angle correction as possible to prevent shifts in the scan location [
31]. In this method, angle correction is needed, as Doppler imaging requires the Doppler angle for frequency shifts and velocity measurement. Studies employing this methodology typically use high-frame-rate electrocardiogram (ECG) gating to prevent a mismatch in features of both diameter and velocity curves over each cardiac cycle by allowing for time alignment, which uses the ECG to set a trigger signal [
151,
152].
In contrast, Rabben et al. [
19] proposed a different non-simultaneous acquisition method that utilises M-mode imaging instead of B-mode imaging to record ultrasound data over three to five cardiac cycles for diameter acquisition. Akin to the previous method, after diameter acquisition, PW Doppler imaging is used to extract maximum velocities across the vessel before using the QA loop for PWV measurement. To ensure proper alignment, the intima layers for both anterior and posterior walls should be clearly visible. According to [
19], in this non-simultaneous acquisition method, the adjustment of the transducer allowed for optimal data collection, wherein the diameter distension measurements were of high accuracy and precision.
Together, these findings highlight that, while non-simultaneous setups are practical, their susceptibility to alignment errors motivates exploration of simultaneous methods.
6.2. Simultaneous Acquisition Methods
Simultaneous acquisition of diameter and velocity data is technically more complex than non-simultaneous acquisition owing to the need for acquisition of both the velocity and diameter at the same transducer angle. This has been explored in studies seeking to overcome alignment errors.
For instance, a method utilised in [
33,
104,
153] employed M-mode for diameter measurement and PW Doppler for velocity measurement. In these studies, prior to data acquisition, the transducer was positioned longitudinally in B-mode while ensuring that the vessel walls were clearly delineated. The Doppler angle used was near optimal at 58 to 60 degrees and the Doppler gating was adjusted in B-mode as well. Subsequently, during measurement, a split screen was used to obtain both diameter and velocity data at a sampling frequency of 1000 Hz. Although this method allows for automatic acquisition of the data simultaneously via the split screens, the gates and angles still need to be adjusted manually, which may affect the signals, particularly in the derivative calculations used to obtain the PWV from the lnDU loop [
33,
104]. This method was also used in separate research that tested a position near the aortic arch, wherein 10-beat cine loops were used in M-mode and PW Doppler imaging. Kowalski et al. [
153] further introduced the use of a fluid-filled phantom between a probe and vessel which acted as an intermediate medium to prevent external pressure from the transducer from distorting the aortic wall motion.
Simultaneous acquisition, as proposed in [
5], may also be conducted with constant perpendicular placement of the ultrasound transducer via the perpendicular ultrasound velocimetry (PUV) technique. In this technique, the velocity is not obtained via Doppler ultrasound but rather via cross-correlation techniques. The principle for measurement is akin to particle imaging velocimetry (PIV) [
154] and PUV uses 2D cross-correlation in the time domain on raw RF data to determine the axial velocity distribution of the flow. In PUV, the ultrasound system is operated in fast B-mode or multiple M-line mode. According to Beulen et al. [
5], the perpendicular placement allows for accurate and simultaneous assessment of diameter and velocity without the need to account for angle correction.
Rowland et al. [
34] introduced a method similar to PUV which obtains measurements of diameter and velocity from B-mode images. In this study, one-dimensional (1D) cross-correlation techniques, rather than 2D cross-correlation, were employed to track the wall motion and blood element speckle motion in the successive B-mode images [
34]. For diameter measurement, 1D cross-correlation of successive frames was conducted separately for anterior and posterior walls and the wall motion waveform for each wall was given by the cumulative summation of displacements over time. Thereafter, the diameter change waveform was obtained by the difference in wall displacement for anterior and posterior wall motion waveforms. The absolute diameter waveform was subsequently obtained with knowledge of the initial vessel diameter without distension. Meanwhile, the velocity was determined by tracking and cross-correlating the movement of the blood speckle pattern between frames, with the highest correlation indicating the mostly likely location of blood element movement [
34]. In this method, knowledge of the displacement and imaging frame rate allows for the velocity to be obtained. For accurate measurement, an ultrafast scanner was used to adequately resolve the rapid acceleration and deceleration of blood during systole [
34]. Akin to previous methods, ECG gating was used for time alignment while singular value decomposition (SVD) was applied to separate weak blood and strong tissue signals [
34].
Apart from the use of singular ultrasound beams, as with the abovementioned methods, simultaneous acquisition can also be achieved with the use of multiple ultrasound beams, as demonstrated in [
155,
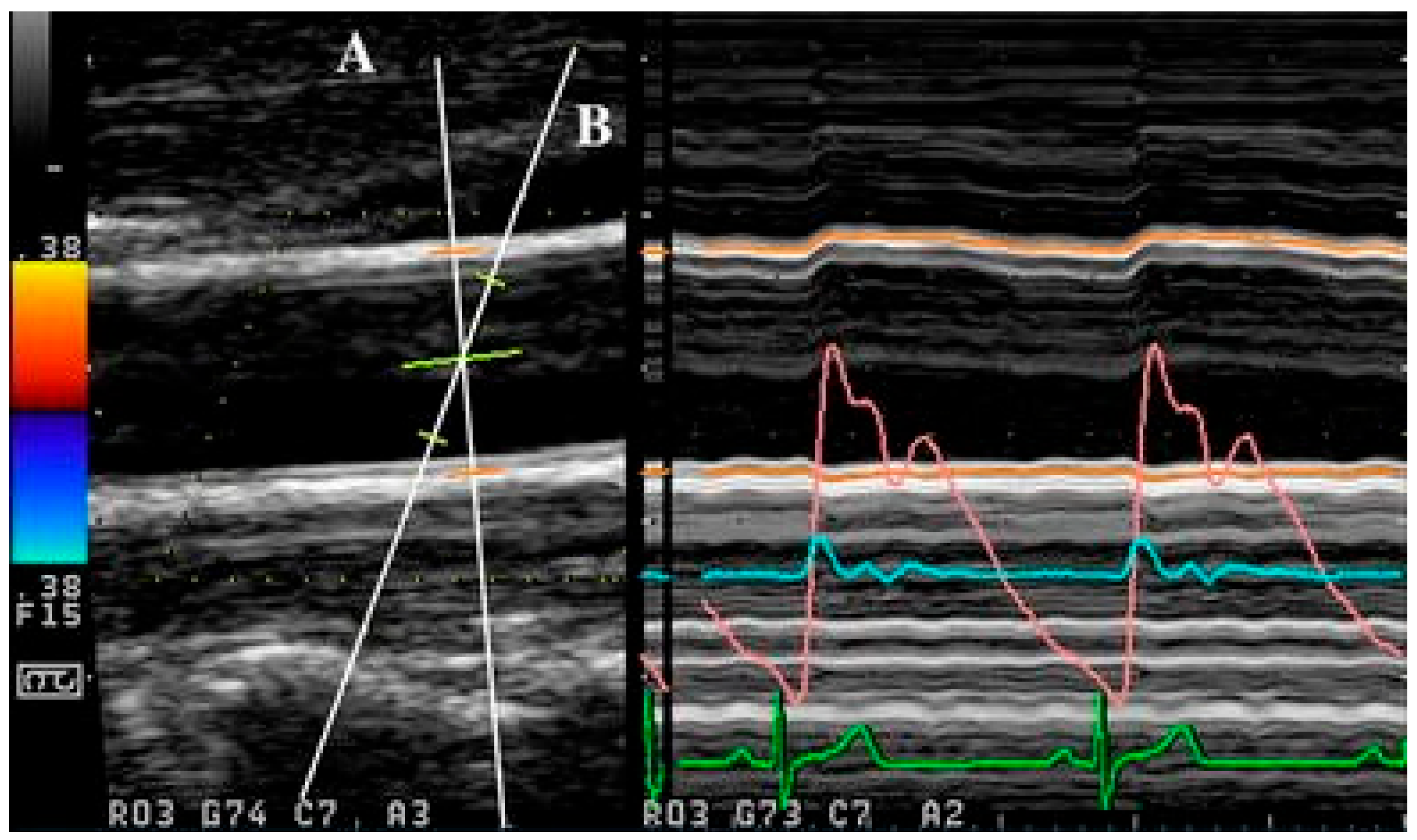
156]. In multiple beam acquisition, colour Doppler is used to obtain the blood flow velocity while diameter changes were measured using M-mode. The system consists of both the colour Doppler system and an echo-tracking subsystem that can use different ultrasound beams for velocity and diameter change, respectively. These beams can be independently manipulated, with an intersection between the beams at the range gate for both diameter and velocity measurement, as shown in
Figure 4 [
156]. From [
155], this method was found to have low variabilities in the maximum velocity and arterial diameter measurements despite the need to manipulate different beams, which thereby supports its reproducibility.
In sum, simultaneous methods can circumvent the issue of alignment errors, albeit at the cost of greater computational demand.
6.3. Comparison and Evaluation of Methods
Table 2 provides a comparison of non-simultaneous and simultaneous methods in PWV parameter acquisition. In the acquisition of parameters for PWV measurement, non-simultaneous acquisition methods present as simpler and less computationally demanding options. However, a key limitation of non-simultaneous acquisition is the fact that velocity and diameter waveforms are not obtained at the same time [
19,
31]. This lack of simultaneity is a source of error in PWV assessment due to inaccurate time alignments between the two curves despite there being methods to ensure as close an alignment as possible. Additionally, the possibility of inter-cycle variations between diameter and velocity acquisition implies that, even with accurate time alignment [
71], the measured diameter at each instance may not correspond to the velocity measured at the same timepoints. This issue may be overcome by estimating the velocity and diameter from the same data or with the use of one transducer angled oblique to the vessel and another perpendicular to the vessel for respective velocity and diameter measurement at the same region of interest [
41,
156].
In general, while simultaneous acquisition was more computationally complex, the advantage and limitations of each method varied. Of the discussed methods, the cross-correlation-based methods have a unique advantage in their ability to perform measurements with the use of B-mode imaging, which means that the transducer is kept in one position throughout acquisition. This ensures that the imaged region is constant, thereby preventing errors that may arise from Doppler angle adjustment. However, this method is computationally intense and may not be used in regions of excessively high velocities due to the limitations of the ultrasound system, which can be problematic for clinical use [
5]. In contrast, while the dual ultrasound beam method required positioning of two beams, the results had good reproducibility [
155,
156]. Additionally, the ability to independently manipulate the beams may allow for flexibility in conducting measurement.
In all, while no one method is currently optimal, simultaneous acquisition methods have a distinct advantage in preventing errors due to delays or possible physiological differences between cardiac cycles. Each discussed method has advantages that can be leveraged for improving acquisition; hence, apart from comparisons between the methods, for future work, it is crucial to note the various means by which acquisition can be further improved. For instance, the use of ECG gating allows for consistent measurements over each cardiac cycle, which improves repeatability of the measurement. Meanwhile, the use of filters such as singular value decomposition to separate tissue signals can improve the signal–noise ratio in data acquisition, particularly in Doppler ultrasound, wherein noise can result in aliasing and affect velocity measurement.
7. Conclusions
With a focus on loop-based PWV estimation methods, this review sought to evaluate different approaches for ultrasound-based acquisition while considering technical parameters and physiological realities to optimise the accuracy, reliability, and reproducibility of the parameters collected for estimation. As such, this paper has summarised the use of ultrasound imaging in vessel data acquisition, covered the different ultrasound-based imaging modalities for acquiring area and velocity data and their developments, and compared simultaneous and non-simultaneous data acquisition for PWV estimation.
Based on the reviewed literature, despite its challenges and considerations, ultrasound remains a promising means by which PWV can be obtained non-invasively. It is key, therefore, to carefully adjust the key parameters when using the various imaging modalities for measurement, particularly in the use of Doppler ultrasound for velocity measurement. In the comparison of simultaneous and non-simultaneous acquisition, it is evident that there is no optimal method for data acquisition. However, simultaneous acquisition, once optimised, would allow for the acquisition of both diameter and velocity waveforms that prevents errors from arising due to physiological differences between two cardiac cycles.
Future developments in the use of ultrasound for PWV measurement will likely focus on improvements to be made in the acquisition phase as well as improvements in processing the ultrasound data post-acquisition. Firstly, experimental investigations between non-simultaneous and simultaneous methods can be conducted to better compare the accuracy and feasibility of each method and to improve upon the best-performing method. For other improvements in haemodynamic variable acquisition via ultrasound, the discussed challenges present possible avenues of work. Given that wave reflections can greatly affect data acquisition, further research into wave analysis methods to effectively separate forward and backward flow is warranted. Meanwhile, investigations into machine learning may mitigate errors arising from operator reliance by automating part of the acquisition process or providing feedback to operators during acquisition. Apart from addressing challenges, working around the limitations of ultrasound imaging is another area of improvement, and could include considering the use of media, developments in the design and piezoelectric crystal composition of the ultrasound transducer, or developing a standardised method to limit the effect of motion or differences in acoustic impedance and ensure consistently high data quality. Another potential area for exploration is improvement in processing of ultrasound data after acquisition, which could include determining the most accurate models for PWV estimation and developments in subsequent applications of PWV like pressure estimation.
Overall, ultrasound is a promising non-invasive alternative to the current non-invasive gold standard of carotid-femoral PWV (cfPWV) measurement via direct tonometry and further research needs to be conducted to overcome current constraints and ensure accuracy, reliability, and repeatability for clinical use.
Author Contributions
Conceptualization, V.C.W.Y.N.; formal analysis, V.C.W.Y.N.; investigation, V.C.W.Y.N.; data curation, V.C.W.Y.N.; writing—original draft preparation, V.C.W.Y.N.; writing—review and editing, Y.-R.W. and H.L.L.; visualization, V.C.W.Y.N.; supervision, Y.-R.W. and H.L.L.; project administration, Y.-R.W. and H.L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank the National University of Singapore for providing institutional support and access to research resources.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviation
| ABP | Arterial Blood Pressure |
| CADMAS | Cross-Angular Delay Multiply and Sum |
| CCA | Common Carotid Artery |
| cfPWV | Carotid-Femoral Pulse Wave Velocity |
| CNN | Convolution Neural Network |
| CT | Computed Tomography |
| DMAS | Delay Multiply and Sum |
| ECG | Electrocardiography |
| FMAS | Filter Multiply and Sum |
| lnDU | lnDiameter-Velocity |
| MRI | Magnetic Resonance Imaging |
| PIV | Particle Image Velocimetry |
| PRF | Pulse Repetition Frequency |
| PUV | Perpendicular Ultrasound Velocimetry |
| PWI | Pulse Wave Imaging |
| PWV | Pulse Wave Velocity |
| QA | Flow-Area |
| RF | Radiofrequency |
| SVD | Singular Value Decomposition |
| TGC | Time Gain Compensation |
| WSA | Wave Separation Analysis |
References
- Callaghan, F.J.; Babbs, C.F.; Bourland, J.D.; Geddes, L.A. The relationship between arterial pulse-wave velocity and pulse frequency at different pressures. J. Med. Eng. Technol. 1984, 8, 15–18. [Google Scholar] [CrossRef]
- Avolio, A.P.; Kuznetsova, T.; Heyndrickx, G.R.; Kerkhof, P.L.M.; Li, J.K.-J. Arterial Flow, Pulse Pressure and Pulse Wave Velocity in Men and Women at Various Ages. In Sex-Specific Analysis of Cardiovascular Function; Springer: Cham, Switzerland, 2018; pp. 153–168. [Google Scholar] [CrossRef]
- Lu, Y.; Kiechl, S.J.; Wang, J.; Xu, Q.; Kiechl, S.; Pechlaner, R.; Aguilar, D.; Al-Hashmi, K.M.; Alvim, R.O.; Al-Zakwani, I.S.; et al. Global distributions of age- and sex-related arterial stiffness: Systematic review and meta-analysis of 167 studies with 509,743 participants. eBioMedicine 2023, 92, 104619. [Google Scholar] [CrossRef]
- Pilz, N.; Heinz, V.; Ax, T.; Fesseler, L.; Patzak, A.; Bothe, T.L. Pulse Wave Velocity: Methodology, Clinical Applications, and Interplay with Heart Rate Variability. Rev. Cardiovasc. Med. 2024, 25, 266. [Google Scholar] [CrossRef] [PubMed]
- Beulen, B.W.; Bijnens, N.; Koutsouridis, G.G.; Brands, P.J.; Rutten, M.C.; van de Vosse, F.N. Toward noninvasive blood pressure assessment in arteries by using ultrasound. Ultrasound Med. Biol. 2011, 37, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, S.; Marais, L.; Khettab, H.; Poli, F.; Li, Y.; Segers, P.; Aasmul, S.; Melis, M.; Baets, R.; Greenwald, S.; et al. Clinical Validation of Carotid-Femoral Pulse Wave Velocity Measurement Using a Multi-Beam Laser Vibrometer: The CARDIS Study. Hypertension 2024, 81, 1986–1995. [Google Scholar] [CrossRef]
- Tang, C.-J.; Lee, P.-Y.; Chuang, Y.-H.; Huang, C.-C. Measurement of local pulse wave velocity for carotid artery by using an ultrasound-based method. Ultrasonics 2020, 102, 106064. [Google Scholar] [CrossRef]
- Stoner, L.; Meyer, M.L.; Kucharska-Newton, A.; Stone, K.; Zeiff, G.; Dave, G.; Fryer, S.; Credeur, D.; Faulkner, J.; Matsushita, K.; et al. Associations Between Carotid-Femoral and Heart-Femoral Pulse Wave Velocity in Older Adults: The Atherosclerosis Risk in Communities (ARIC) Study. J. Hypertens. 2020, 38, 1786–1793. [Google Scholar] [CrossRef]
- Hirata, K.; Kawakami, M.; O’ROurke, M.F. Pulse Wave Analysis and Pulse Wave Velocity. Circ. J. 2006, 70, 1231–1239. [Google Scholar] [CrossRef]
- Darwich, M.A.; Langevin, F.; Darwich, K. Local Pulse Wave Velocity Estimation in the Carotids Using Dynamic MR Sequences. Available online: https://www.scirp.org/journal/paperinformation?paperid=55379 (accessed on 15 April 2025).
- Vappou, J.; Luo, J.; Okajima, K.; Di Tullio, M.; Konofagou, E. Aortic pulse wave velocity measured by pulse wave imaging (PWI): A comparison with applanation tonometry. Artery Res. 2011, 5, 65–71. [Google Scholar] [CrossRef]
- Williams, R.; Needles, A.; Cherin, E.; Zhou, Y.Q.; Henkelman, R.M.; Adamson, S.L.; Foster, F.S. Noninvasive Ultrasonic Measurement of Regional and Local Pulse-Wave Velocity in Mice. Ultrasound Med. Biol. 2007, 33, 1368–1375. [Google Scholar] [CrossRef]
- Pereira, T.; Correia, C.; Cardoso, J. Novel Methods for Pulse Wave Velocity Measurement. J. Med. Biol. Eng. 2015, 35, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Wentland, A.L.; Grist, T.M.; Wieben, O. Review of MRI-based measurements of pulse wave velocity: A biomarker of arterial stiffness. Cardiovasc. Diagn. Ther. 2014, 4, 193–206. [Google Scholar] [CrossRef]
- Stankovic, Z.; Allen, B.D.; Garcia, J.; Jarvis, K.B.; Markl, M. 4D flow imaging with MRI. Cardiovasc. Diagn. Ther. 2014, 4, 173–192. [Google Scholar] [CrossRef]
- Nabeel, P.M.; Kiran, V.R.; Joseph, J.; Abhidev, V.V.; Sivaprakasam, M. Sivaprakasam. Local Pulse Wave Velocity: Theory, Methods, Advancements, and Clinical Applications. IEEE Rev. Biomed. Eng. 2020, 13, 74–112. [Google Scholar] [CrossRef]
- Katsi, V.; Felekos, I.; Kallikazaros, I. Christian Andreas Doppler: A legendary man inspired by the dazzling light of the stars. Hippokratia 2013, 17, 113–114. [Google Scholar]
- Bramwell, J.C. The velocity of pulse wave in man. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1922, 93, 298–306. [Google Scholar] [CrossRef]
- Rabben, S.I.; Stergiopulos, N.; Hellevik, L.R.; Smiseth, O.A.; Slørdahl, S.; Urheim, S.; Angelsen, B. An ultrasound-based method for determining pulse wave velocity in superficial arteries. J. Biomech. 2004, 37, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Khir, A. Determination of wave speed and wave separation in the arteries using diameter and velocity. J. Biomech. 2010, 43, 455–462. [Google Scholar] [CrossRef]
- Giudici, A.; Palombo, C.; Morizzo, C.; Kozakova, M.; Cruickshank, J.K.; Wilkinson, I.B.; Khir, A.W. Transfer-function-free technique for the noninvasive determination of the human arterial pressure waveform. Physiol. Rep. 2021, 9, e15040. [Google Scholar] [CrossRef]
- Meusel, M.; Wegerich, P.; Bode, B.; Stawschenko, E.; Kusche-Vihrog, K.; Hellbrück, H.; Gehring, H. Measurement of Blood Pressure by Ultrasound-The Applicability of Devices, Algorithms and a View in Local Hemodynamics. Diagn. Basel Switz. 2021, 11, 2255. [Google Scholar] [CrossRef]
- Seabra, A.C.G.; da Silva, A.F.; Stieglitz, T.; Amado-Rey, A.B. In Silico Blood Pressure Models Comparison. IEEE Sens. J. 2022, 22, 23486–23493. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Sequí-Domínguez, I.; Saz-Lara, A.; Garcia-Klepzig, J.L.; Lucerón-Lucas-Torres, M.; Martínez-García, I.; Álvarez-Bueno, C.; Martínez-Vizcaíno, V. Concordance among pulse wave velocity assessment methods: A network meta-analysis. Chin. Med. J. 2024, 137, 2137–2139. [Google Scholar] [CrossRef] [PubMed]
- Oktar, S.Ö.; Cerit, M.N.; Şendur, H.N.; Kılıç, A.C.K. Recent advances in vascular ultrasound imaging technology and their clinical implications. Diagn. Interv. Radiol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Bianchini, E.; Guala, A.; Golemati, S.; Alastruey, J.; Climie, R.E.; Dalakleidi, K.; Francesconi, M.; Fuchs, D.; Hartman, Y.; Malik, A.E.F.; et al. The Ultrasound Window into Vascular Ageing: A Technology Review by the VascAgeNet COST Action. J. Ultrasound Med. 2023, 42, 2183–2213. [Google Scholar] [CrossRef] [PubMed]
- Calabia, J.; Torguet, P.; Garcia, M.; Garcia, I.; Martin, N.; Guasch, B.; Faur, D.; Vallés, M. Doppler ultrasound in the measurement of pulse wave velocity: Agreement with the Complior method. Cardiovasc. Ultrasound 2011, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Vappou, J.; Luo, J.; Okajima, K.; Di Tullio, M.; Konofagou, E.E. Non-invasive measurement of local pulse pressure by pulse wave-based ultrasound manometry (PWUM). Physiol. Meas. 2011, 32, 1653–1662. [Google Scholar] [CrossRef]
- van den Bos–van de Steeg, M.G.M.; Fekkes, S.; Saris, A.E.; de Korte, C.L.; Hansen, H.H. In Vivo Comparison of Pulse Wave Velocity Estimation Based on Ultrafast Plane Wave Imaging and High-Frame-Rate Focused Transmissions. Ultrasound Med. Biol. 2022, 48, 2335–2343. [Google Scholar] [CrossRef]
- Couade, M. The advent of ultrafast ultrasound in vascular imaging: A review. J. Vasc. Diagn. Interv. 2016, 4, 9–22. [Google Scholar] [CrossRef]
- Di Lascio, N.; Kusmic, C.; Stea, F.; Faita, F. Ultrasound-based Pulse Wave Velocity Evaluation in Mice. J. Vis. Exp. 2017, 120, 54362. [Google Scholar] [CrossRef]
- Teixeira, R.; Vieira, M.J.; Gonçalves, A.; Cardim, N.; Gonçalves, L. Ultrasonographic vascular mechanics to assess arterial stiffness: A review. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 233–246. [Google Scholar] [CrossRef]
- Pomella, N.; Wilhelm, E.N.; Kolyva, C.; González-Alonso, J.; Rakobowchuk, M.; Khir, A.W. Noninvasive assessment of the common carotid artery hemodynamics with increasing exercise work rate using wave intensity analysis. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H233–H241. [Google Scholar] [CrossRef] [PubMed]
- Rowland, E.M.; Riemer, K.; Lichtenstein, K.; Tang, M.-X.; Weinberg, P.D. Non-invasive Assessment by B-Mode Ultrasound of Arterial Pulse Wave Intensity and Its Reduction During Ventricular Dysfunction. Ultrasound Med. Biol. 2023, 49, 473–488. [Google Scholar] [CrossRef]
- Manoj, R.; Raj, K.V.; Nabeel, P.M.; Sivaprakasam, M.; Joseph, J. Measurement of pressure dependent variations in local pulse wave velocity within a cardiac cycle from forward travelling pulse waves. Sci. Rep. 2025, 15, 3066. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, Y.; He, B.; Qi, G. A Comparison of Ultrasound-Based QA and ln(D)U Methods for Measurement Local Pulse Wave Velocity. In Proceedings of the 2nd International Conference on Biomedical Engineering and Bioinformatics, Tianjin, China, 19–21 September 2018; Association for Computing Machinery: New York, NY, USA, 2018; pp. 144–147. [Google Scholar] [CrossRef]
- Mynard, J.P.; Kondiboyina, A.; Kowalski, R.; Cheung, M.M.H.; Smolich, J.J. Measurement, Analysis and Interpretation of Pressure/Flow Waves in Blood Vessels. Front. Physiol. 2020, 11, 1085. [Google Scholar] [CrossRef]
- Poggi, C.; Palavecino, M. Ultrasound principles and instrumentation. Surg. Open Sci. 2024, 18, 123–128. [Google Scholar] [CrossRef]
- Beulen, B.; Bijnens, N.; Rutten, M.; Brands, P.; van de Vosse, F. Perpendicular ultrasound velocity measurement by 2D cross correlation of RF data. Part A: Validation in a straight tube. Exp. Fluids 2010, 49, 1177–1186. [Google Scholar] [CrossRef]
- Meola, M.; Ibeas, J.; Lasalle, G.; Petrucci, I. Basics for performing a high-quality color Doppler sonography of the vascular access. J. Vasc. Access 2021, 22, 18–31. [Google Scholar] [CrossRef]
- Tortoli, P.; Morganti, T.; Bambi, G.; Palombo, C.; Ramnarine, K.V. Noninvasive simultaneous assessment of wall shear rate and wall distension in carotid arteries. Ultrasound Med. Biol. 2006, 32, 1661–1670. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Zhang, T.; Shung, K.K.; Zhu, B. Recent Advancements in Ultrasound Transducer: From Material Strategies to Biomedical Applications. BME Front. 2022, 9764501. [Google Scholar] [CrossRef]
- Lee, W.; Roh, Y. Ultrasonic transducers for medical diagnostic imaging. Biomed. Eng. Lett. 2017, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.; Kollorz, E. Ultrasound. In Medical Imaging Systems: An Introductory Guide; Maier, A., Steidl, S., Christlein, V., Hornegger, J., Eds.; Springer: Cham, Switzerland, 2018; Available online: http://www.ncbi.nlm.nih.gov/books/NBK546144/ (accessed on 15 April 2025).
- Løvstakken, L. Signal Processing in Diagnostic Ultrasound: Algorithms for Real-Time Estimation and Visualization of Blood Flow Velocity. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2007. [Google Scholar]
- Brattain, L.J.; Telfer, B.A.; Dhyani, M.; Grajo, J.R.; Samir, A.E. Machine Learning for Medical Ultrasound: Status, Methods, and Future Opportunities. Abdom. Radiol. 2018, 43, 786–799. [Google Scholar] [CrossRef]
- Nazer, B.; Gerstenfeld, E.P.; Hata, A.; A Crum, L.; Matula, T.J. Cardiovascular applications of therapeutic ultrasound. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2013, 39, 287–294. [Google Scholar] [CrossRef]
- Bierig, S.M.; Jones, A. Accuracy and Cost Comparison of Ultrasound Versus Alternative Imaging Modalities, Including CT, MR, PET, and Angiography. J. Diagn. Med. Sonogr. 2009, 25, 138–144. [Google Scholar] [CrossRef]
- Kim, G.-H.; Youn, H.-J. Is Carotid Artery Ultrasound Still Useful Method for Evaluation of Atherosclerosis? Korean Circ. J. 2017, 47, 1–8. [Google Scholar] [CrossRef]
- Tate, Q.; Kim, S.; Treiman, G.; Parker, D.L.; Hadley, J.R. Increased Vessel Depiction of the Carotid Bifurcation with a Specialized 16-Channel Phased Array Coil at 3T. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 2012, 69, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Fadel, B.M.; Mohty, D.; Kazzi, B.E.; Alamro, B.; Arshi, F.; Mustafa, M.; Echahidi, N.; Aboyans, V. Ultrasound Imaging of the Abdominal Aorta: A Comprehensive Review. J. Am. Soc. Echocardiogr. 2021, 34, 1119–1136. [Google Scholar] [CrossRef]
- Jimeno, M.M.; Vaughan, J.T.; Geethanath, S. Superconducting magnet designs and MRI accessibility: A review. NMR Biomed. 2023, 36, e4921. [Google Scholar] [CrossRef]
- Jalloul, M.; Miranda-Schaeubinger, M.; Noor, A.M.; Stein, J.M.; Amiruddin, R.; Derbew, H.M.; Mango, V.L.; Akinola, A.; Hart, K.; Weygand, J.; et al. MRI scarcity in low- and middle-income countries. NMR Biomed. 2023, 36, e5022. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.G. Comparing costs and efficacy of MRI. Am. J. Roentgenol. 1986, 146, 1307–1310. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Li, K.; Jin, N.; Zhang, Y.; Zhang, T.; Miller, F.H.; Larson, A.C. Respiratory Self-Gating for Free-Breathing Magnetization Transfer MRI of the Abdomen. Magn. Reson. Med. 2015, 73, 2249–2254. [Google Scholar] [CrossRef]
- Kocaoglu, M.; Pednekar, A.S.; Wang, H.; Alsaied, T.; Taylor, M.D.; Rattan, M.S. Breath-hold and free-breathing quantitative assessment of biventricular volume and function using compressed SENSE: A clinical validation in children and young adults. J. Cardiovasc. Magn. Reson. 2020, 22, 54. [Google Scholar] [CrossRef] [PubMed]
- Moghari, M.H.; Barthur, A.; Amaral, M.E.; Geva, T.; Powell, A.J. Free-breathing whole-heart 3D cine magnetic resonance imaging with prospective respiratory motion compensation. Magn. Reson. Med. 2017, 80, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Contijoch, F.; Rasche, V.; Seiberlich, N.; Peters, D.C. The future of CMR: All-in-one vs. real-time CMR (Part 2). J. Cardiovasc. Magn. Reson. 2024, 26, 100998. [Google Scholar] [CrossRef] [PubMed]
- Henningsson, M.; Botnar, R.M. Advanced Respiratory Motion Compensation for Coronary MR Angiography. Sensors 2013, 13, 6882–6899. [Google Scholar] [CrossRef]
- Stroud, R.E.; Piccini, D.; Schoepf, U.J.; Heerfordt, J.; Yerly, J.; Di Sopra, L.; Rollins, J.D.; Fischer, A.M.; Suranyi, P.; Varga-Szemes, A. Correcting versus resolving respiratory motion in free-breathing whole-heart MRA: A comparison in patients with thoracic aortic disease. Eur. Radiol. Exp. 2019, 3, 29. [Google Scholar] [CrossRef]
- Alkhalifah, B. Comparison of Magnetic Resonance Imaging and High-Resolution Ultrasound for the Diagnosis of Pathologies of the Peripheral Nerve. J. Pharm. Bioallied Sci. 2023, 15 (Suppl. S2), S1277–S1279. [Google Scholar] [CrossRef]
- Deffieux, T.; Demené, C.; Tanter, M. Functional Ultrasound Imaging: A New Imaging Modality for Neuroscience. Neuroscience 2021, 474, 110–121. [Google Scholar] [CrossRef]
- Nagaoka, R.; Masuno, G.; Kobayashi, K.; Yoshizawa, S.; Umemura, S.-I.; Saijo, Y. Measurement of regional pulse-wave velocity using spatial compound imaging of the common carotid artery in vivo. Ultrasonics 2015, 55, 92–103. [Google Scholar] [CrossRef]
- Ultrasound. National Institute of Biomedical Imaging and Bioengineering. Available online: https://www.nibib.nih.gov/science-education/science-topics/ultrasound (accessed on 15 April 2025).
- Díaz-Gómez, J.L.; Mayo, P.H.; Koenig, S.J. Point-of-Care Ultrasonography. N. Engl. J. Med. 2021, 385, 1593–1602. [Google Scholar] [CrossRef]
- Hoctor, R.; Dentinger, A.; Thomenius, K. Array signal processing approaches to ultrasound-based arterial pulse wave velocity estimation. In Proceedings of the (ICASSP ’05) IEEE International Conference on Acoustics, Speech, and Signal Processing, Philadelphia, PA, USA, 23 March 2005; Volume 5, pp. 1018–1027. [Google Scholar] [CrossRef]
- Mynard, J.P.; Kondiboyina, A. Chapter 11—Wave reflection in the arterial tree. In Textbook of Arterial Stiffness and Pulsatile Hemodynamics in Health and Disease; Chirinos, J.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 169–194. [Google Scholar] [CrossRef]
- Segers, P.; Swillens, A.; Taelman, L.; Vierendeels, J. Wave reflection leads to over- and underestimation of local wave speed by the PU- and QA-loop methods: Theoretical basis and solution to the problem. Physiol. Meas. 2014, 35, 847–861. [Google Scholar] [CrossRef]
- Alastruey, J.; Hunt, A.A.E.; Weinberg, P.D. Weinberg. Novel wave intensity analysis of arterial pulse wave propagation accounting for peripheral reflections. Int. J. Numer. Methods Biomed. Eng. 2013, 30, 249–279. [Google Scholar] [CrossRef]
- Hermeling, E.; Reesink, K.D.; Reneman, R.S.; Hoeks, A.P. Confluence of incident and reflected waves interferes with systolic foot detection of the carotid artery distension waveform. J. Hypertens. 2008, 26, 2374–2380. [Google Scholar] [CrossRef]
- Manoj, R.; V, R.K.; M, N.P.; Sivaprakasam, M.; Joseph, J. Measurement of Inter and Intra-cycle Variations in Local Pulse Wave Velocity from Forward Travelling Pulse Waves. In Proceedings of the 2024 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Eindhoven, The Netherlands, 26–28 June 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Hwang, J.H. 1—Principles of Ultrasound. In Endosonography, 4th ed.; Hawes, R.H., Fockens, P., Varadarajulu, S., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 2–12. [Google Scholar] [CrossRef]
- Barbry, T.; Bouhemad, B.; Leleu, K.; de Castro, V.; Rémérand, F.; Rouby, J.-J. Transthoracic ultrasound approach of thoracic aorta in critically ill patients with lung consolidation. J. Crit. Care 2006, 21, 203–208. [Google Scholar] [CrossRef]
- Sawada, H.; Franklin, M.K.; Moorleghen, J.J.; Howatt, D.A.; Kukida, M.; Lu, H.S.; Daugherty, A. Ultrasound Monitoring of Descending Aortic Aneurysms and Dissections in Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2557–2559. [Google Scholar] [CrossRef]
- Rosenberry, C.; Ball, V. False Positive Aortic Dissection on Abdominal Ultrasound. West. J. Emerg. Med. 2010, 11, 110–111. [Google Scholar]
- Goldschmidt, E.; Al-Embideen, S.; Abbas, J.; Russell, T.; Paolini, D.; Al-Balbissi, L.; Lurie, F. The Effect of Patient Oral Intake Status on Abdominal Aortic Ultrasound Visualization. Ann. Vasc. Surg. 2021, 74, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Goudie, A. Detection of intraperitoneal free gas by ultrasound. Australas. J. Ultrasound Med. 2013, 16, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Drukker, L.; Papageorghiou, A.; Hu, Y.; Noble, J.A. Task model-specific operator skill assessment in routine fetal ultrasound scanning. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1437–1444. [Google Scholar] [CrossRef]
- Mead, G.E.; Lewis, S.C.; Wardlaw, J.M. Variability in Doppler ultrasound influences referral of patients for carotid surgery. Eur. J. Ultrasound 2000, 12, 137–143. [Google Scholar] [CrossRef]
- Rozycki, G.S. Surgeon-Performed Ultrasound: Its Use in Clinical Practice. Ann. Surg. 1998, 228, 16. [Google Scholar] [CrossRef] [PubMed]
- Rabben, S.I.; Bjærum, S.; Sørhus, V.; Torp, H. Ultrasound-based vessel wall tracking: An auto-correlation technique with RF center frequency estimation. Ultrasound Med. Biol. 2002, 28, 507–517. [Google Scholar] [CrossRef]
- Hunt, B.E.; Flavin, D.C.; Bauschatz, E.; Whitney, H.M. Accuracy and robustness of a simple algorithm to measure vessel diameter from B-mode ultrasound images. J. Appl. Physiol. 2016, 120, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Pasynkov, D.V.; Kolchev, A.A.; Egoshin, I.A.; Klyushkin, I.V.; Pasynkova, O.O. A Method for Automatic Calculation of the Diameter of a Pulsating Blood Vessel on Ultrasound Images in Video Stream Mode. Biomed. Eng. 2023, 57, 23–27. [Google Scholar] [CrossRef]
- Graf, S.; Gariepy, J.; Massonneau, M.; Armentano, R.L.; Mansour, S.; Barra, J.G.; Simon, A.; Levenson, J. Experimental and clinical validation of arterial diameter waveform and intimal media thickness obtained from B-mode ultrasound image processing. Ultrasound Med. Biol. 1999, 25, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Lee, B. Signal Processing Techniques for Operator Independent Doppler Ultrasound: Potential for Use in Transcranial Doppler Ultrasound. Available online: https://rshare.library.torontomu.ca/articles/thesis/Signal_processing_techniques_for_operator_independent_Doppler_ultrasound_potential_for_use_in_transcranial_Doppler_ultrasound/14664744/1?file=28151097 (accessed on 16 April 2025).
- Yamamoto, M.; Carrillo, J.; Insunza, A.; Mari, G.; Ville, Y. Error introduced into velocity measurements by inappropriate Doppler angle assignment. Ultrasound Obstet. Gynecol. 2006, 28, 853–854. [Google Scholar] [CrossRef]
- Automated Ultrasound Doppler Angle Estimation Using Deep Learning. IEEE Conference Publication. IEEE Xplore. Available online: https://ieeexplore-ieee-org.libproxy1.nus.edu.sg/document/8857587 (accessed on 13 October 2025).
- Huang, C.; Ren, T.; Luo, J. Effects of key parameters on the accuracy and precision of local pulse wave velocity measurement by ultrasound imaging. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2877–2880. [Google Scholar] [CrossRef]
- Temporal Super-Resolution of 2D/3D echocardiography Using Cubic B-Spline Interpolation—ScienceDirect. Available online: https://www-sciencedirect-com.libproxy1.nus.edu.sg/science/article/pii/S1746809420300240 (accessed on 13 October 2025).
- O’brien, W.D.; A Frizzell, L.; Schaeffer, D.J.; Zachary, J.F. Superthreshold behavior of ultrasound-induced lung hemorrhage in adult mice and rats: Role of pulse repetition frequency and exposure duration. Ultrasound Med. Biol. 2001, 27, 267–277. [Google Scholar] [CrossRef]
- Schmidt, W.A. SP0021 How to Optimize Doppler Settings for Fast Flow. Ann. Rheum. Dis. 2014, 73, 6. [Google Scholar] [CrossRef]
- Chan, V.; Perlas, A. Basics of Ultrasound Imaging. In Atlas of Ultrasound-Guided Procedures in Interventional Pain Management; Chan, V.; Perlas, A. Springer: New York, NY, USA, 2011; pp. 13–19. [Google Scholar] [CrossRef]
- Terslev, L.; Diamantopoulos, A.P.; Døhn, U.M.; Schmidt, W.A.; Torp-Pedersen, S. Settings and artefacts relevant for Doppler ultrasound in large vessel vasculitis. Arthritis Res. Ther. 2017, 19, 167. [Google Scholar] [CrossRef]
- Karmakar, M.K.; Kwok, W.H. CHAPTER 43—Ultrasound-Guided Regional Anesthesia. In A Practice of Anesthesia for Infants and Children, 4th ed.; Coté, C.J., Lerman, J., Todres, I.D., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 911–938. [Google Scholar] [CrossRef]
- Lee, Y.; Kang, J.; Yoo, Y. Automatic dynamic range adjustment for ultrasound B-mode imaging. Ultrasonics 2015, 56, 435–443. [Google Scholar] [CrossRef]
- Zander, D.; Hüske, S.; Hoffmann, B.; Cui, X.-W.; Dong, Y.; Lim, A.; Jenssen, C.; Löwe, A.; Koch, J.B.H.; Dietrich, C.F. Ultrasound Image Optimization (‘Knobology’): B-Mode. Ultrasound Int. Open 2020, 06, E14–E24. [Google Scholar] [CrossRef]
- Tao, Q.; Wang, Y.; Fish, P.; Wang, W.; Cardoso, J. The wall signal removal in Doppler ultrasound systems based on recursive PCA. Ultrasound Med. Biol. 2004, 30, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Sun, Y.-N.; Lin, C.-J. A Motion Compounding Technique for Speckle Reduction in Ultrasound Images. J. Digit. Imaging 2009, 23, 246–257. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Xu, X.; Chun, L.; Tang, J.; Deng, Y. A robust detail preserving anisotropic diffusion for speckle reduction in ultrasound images. BMC Genom. 2011, 12, S14. [Google Scholar] [CrossRef]
- Afzal, S.; Zahid, M.; Rehan, Z.A.; Shakir, H.M.F.; Javed, H.; Aljohani, M.M.H.; Mustafa, S.K.; Ahmad, M.; Hassan, M.M. Preparation and Evaluation of Polymer-Based Ultrasound Gel and Its Application in Ultrasonography. Gels 2022, 8, 42. [Google Scholar] [CrossRef]
- Tsui, B.C.H.; Tsui, J. A flexible gel pad as an effective medium for scanning irregular surface anatomy. Can. J. Anaesth. J. Can. Anesth. 2011, 59, 226–227. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Yoo, J.H.; Thapa, M.; Callahan, M.J. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr. Radiol. 2013, 43, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Shrimal, P.; Bhoi, S.; Sinha, T.P.; Murmu, L.R.; Nayer, J.; Ekka, M.; Mishra, P.; Kumar, A.; Trikha, V.; Aggarwal, P. Sensitivity and specificity of waterbath ultrasound technique in comparison to the conventional methods in diagnosing extremity fractures. Am. J. Emerg. Med. 2022, 53, 118–121. [Google Scholar] [CrossRef]
- Pomella, N.; Wilhelm, E.N.; Kolyva, C.; González-Alonso, J.; Rakobowchuk, M.; Khir, A.W. Common Carotid Artery Diameter, Blood Flow Velocity and Wave Intensity Responses at Rest and during Exercise in Young Healthy Humans: A Reproducibility Study. Ultrasound Med. Biol. 2017, 43, 943–957. [Google Scholar] [CrossRef]
- Cinthio, M.; Jansson, T.; Ahlgren, Å.R.; Lindström, K.; Persson, H.W. A Method for Arterial Diameter Change Measurements Using Ultrasonic B-Mode Data. Ultrasound Med. Biol. 2010, 36, 1504–1512. [Google Scholar] [CrossRef]
- Meinders, J.M.; Hoeks, A.P. Simultaneous assessment of diameter and pressure waveforms in the carotid artery. Ultrasound Med. Biol. 2004, 30, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Ando, T.; Tsuruoka, N.; Inaba, K.; Haga, Y. A Simulation Model for a Blood Vessel Diameter Sensor. Sens. Mater. 2024, 36, 427–442. [Google Scholar] [CrossRef]
- Sahani, A.K.; Joseph, J.; Sivaprakasam, M. Automatic measurement of lumen diameter of carotid artery in A-Mode ultrasound. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 3873–3876. [Google Scholar] [CrossRef]
- Raj, K.V.; Nabeel, P.M.; Chandran, D.; Sivaprakasam, M.; Joseph, J. High-frame-rate A-mode ultrasound for calibration-free cuffless carotid pressure: Feasibility study using lower body negative pressure intervention. Blood Press. 2022, 31, 19–30. [Google Scholar] [CrossRef]
- Baltgaile, G. Arterial wall dynamics. Perspect. Med. 2012, 1, 146–151. [Google Scholar] [CrossRef]
- Hashemseresht, M.; Afrakhteh, S.; Behnam, H. High-resolution and high-contrast ultrafast ultrasound imaging using coherent plane wave adaptive compounding. Biomed. Signal Process. Control 2022, 73, 103446. [Google Scholar] [CrossRef]
- Foiret, J.; Cai, X.; Bendjador, H.; Park, E.-Y.; Kamaya, A.; Ferrara, K.W. Improving plane wave ultrasound imaging through real-time beamformation across multiple arrays. Sci. Rep. 2022, 12, 13386. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Guenther, D.; Trahey, G. Adaptive imaging and spatial compounding in the presence of aberration. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1131–1144. [Google Scholar] [CrossRef]
- Yoon, C.; Kim, G.-D.; Yoo, Y.; Song, T.-K.; Chang, J.H. Frequency equalized compounding for effective speckle reduction in medical ultrasound imaging. Biomed. Signal Process. Control 2013, 8, 876–887. [Google Scholar] [CrossRef]
- Paridar, R.; Asl, B.M. Frame rate improvement in ultrafast coherent plane wave compounding. Ultrasonics 2023, 135, 107136. [Google Scholar] [CrossRef] [PubMed]
- Abeyratne, U.; Petropulu, A.; Reid, J. Higher order spectra based deconvolution of ultrasound images. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 1064–1075. [Google Scholar] [CrossRef]
- Kouame, D.; Gregoire, J.; Pourcelot, L.; Lethiecq, M.; Ossant, F. Ultrasound imaging: Signal acquisition, new advanced processing for biomedical and industrial applications. In Proceedings of the (ICASSP ’05) IEEE International Conference on Acoustics, Speech, and Signal Processing, Philadelphia, PA, USA, 23 March 2005; Volume 5, pp. v/993–v/996. [Google Scholar] [CrossRef]
- Mohamed Moubark, A.; Nie, L.; Mohd Zaman, M.H.; Islam, M.T.; Zulkifley, M.A.; Baharuddin, M.H.; Alomari, Z.; Freear, S. Enhancement of Ultrasound B-Mode Image Quality Using Nonlinear Filtered-Multiply-and-Sum Compounding for Improved Carotid Artery Segmentation. Diagnostics 2023, 13, 1161. [Google Scholar] [CrossRef]
- Jeon, S.; Park, E.-Y.; Choi, W.; Managuli, R.; Lee, K.J.; Kim, C. Real-time delay-multiply-and-sum beamforming with coherence factor for in vivo clinical photoacoustic imaging of humans. Photoacoustics 2019, 15, 100136. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.B.; Toulemonde, M.; Lerendegui, M.; Riemer, K.; Malounda, D.; Weinberg, P.D.; Shapiro, M.G.; Tang, M.-X. Enhanced Ultrasound Image Formation with Computationally Efficient Cross-Angular Delay Multiply and Sum Beamforming. Ultrasound Med. Biol. 2025, 51, 1523–1528. [Google Scholar] [CrossRef]
- Arndt, J.O.; Klauske, J.; Mersch, F. The diameter of the intact carotid artery in man and its change with pulse pressure. Pflug. Arch. Gesamte Physiol. Menschen Tiere 1968, 301, 230–240. [Google Scholar] [CrossRef]
- Hokanson, D.E.; Mozersky, D.J.; Sumner, D.S.; Strandness, D.E., Jr. A phase-locked echo tracking system for recording arterial diameter changes in vivo. J. Appl. Physiol. 1972, 32, 728–733. [Google Scholar] [CrossRef]
- Groves, D.; Powalowski, T.; White, D. A digital technique for tracking moving interfaces. Ultrasound Med. Biol. 1982, 8, 185–190. [Google Scholar] [CrossRef]
- Imura, T.; Yamamoto, K.; Kanamori, K.; Mikami, T.; Yasuda, H. Non-invasive ultrasonic measurement of the elastic properties of the human abdominal aorta. Cardiovasc. Res. 1986, 20, 208–214. [Google Scholar] [CrossRef]
- Tardy, Y.; Meister, J.J.; Perret, F.; Brunner, H.R.; Arditi, M. Non-invasive estimate of the mechanical properties of peripheral arteries from ultrasonic and photoplethysmographic measurements. Clin. Phys. Physiol. Meas. 1991, 12, 39–54. [Google Scholar] [CrossRef]
- Hoeks, A.; Ruissen, C.; Hick, P.; Reneman, R. Transcutaneous detection of relative changes in artery diameter. Ultrasound Med. Biol. 1985, 11, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, A.; Brands, P.; Smeets, F.; Reneman, R. Assessment of the distensibility of superficial arteries. Ultrasound Med. Biol. 1990, 16, 121–128. [Google Scholar] [CrossRef]
- Moddemeijer, R. On the determination of the position of extrema of sampled correlators. IEEE Trans. Signal Process. 1991, 39, 216–219. [Google Scholar] [CrossRef]
- Dejong, P.; Arts, T.; Hoeks, A.; Reneman, R. Determination of tissue motion velocity by correlation interpolation of pulsed ultrasonic echo signals. Ultrason. Imaging 1990, 12, 84–98. [Google Scholar] [CrossRef]
- Loupas, T.; Powers, J.; Gill, R. An axial velocity estimator for ultrasound blood flow imaging, based on a full evaluation of the Doppler equation by means of a two-dimensional autocorrelation approach. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 672–688. [Google Scholar] [CrossRef]
- Lai, X.; Torp, H.; Kristoffersen, K. An extended autocorrelation method for estimation of blood velocity. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997, 44, 1332–1342. [Google Scholar] [CrossRef]
- Schober, P.; Loer, S.A.; Schwarte, L.A. Transesophageal Doppler devices: A technical review. J. Clin. Monit. Comput. 2009, 23, 391–401. [Google Scholar] [CrossRef]
- Botz, B. Aliasing Phenomenon (Ultrasound). Radiology Reference Article. Radiopaedia.org. Radiopaedia. Available online: https://radiopaedia.org/articles/aliasing-phenomenon-ultrasound?lang=gb (accessed on 7 February 2024).
- Polak, J.F.; Kremkau, F.W. The 60° Doppler Angle Correction Paradigm. J. Ultrasound Med. 2021, 40, 2227–2233. [Google Scholar] [CrossRef]
- Wang, F.; Jin, P.; Feng, Y.; Fu, J.; Wang, P.; Liu, X.; Zhang, Y.; Ma, Y.; Yang, Y.; Yang, A.; et al. Flexible Doppler ultrasound device for the monitoring of blood flow velocity. Sci. Adv. 2021, 7, eabi9283. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Taneja, H.; Chand, L. Performance Enhancement and Analysis of Filters in Ultrasound Image Denoising. Procedia Comput. Sci. 2018, 132, 643–652. [Google Scholar] [CrossRef]
- MBlons, M.; Deffieux, T.; Osmanski, B.-F.; Tanter, M.; Berthon, B. PerceptFlow: Real-Time Ultrafast Doppler Image Enhancement Using Deep Convolutional Neural Network and Perceptual Loss. Ultrasound Med. Biol. 2022, 49, 225–236. [Google Scholar] [CrossRef]
- Vollbrecht, T.M.; Hart, C.; Zhang, S.; Katemann, C.; Sprinkart, A.M.; Isaak, A.; Attenberger, U.; Pieper, C.C.; Kuetting, D.; Geipel, A.; et al. Deep learning denoising reconstruction for improved image quality in fetal cardiac cine MRI. Front. Cardiovasc. Med. 2024, 11, 1323443. [Google Scholar] [CrossRef]
- Nahas, H.; Yiu, B.Y.; Chee, A.J.; Au, J.S.; Yu, A.C. Deep-learning-assisted and GPU-accelerated vector Doppler imaging with aliasing-resistant velocity estimation. Ultrasonics 2023, 134, 107050. [Google Scholar] [CrossRef]
- Puig, J.; Friboulet, D.; Jung Ling, H.; Varray, F.; Mougharbel, M.; Poree, J.; Provost, J.; Garcia, D.; Millioz, F. Boosting Cardiac Color Doppler Frame Rates with Deep Learning. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.-H.; Basarab, A.; Zemmoura, I.; Remenieras, J.-P.; Kouame, D. Joint Blind Deconvolution and Robust Principal Component Analysis for Blood Flow Estimation in Medical Ultrasound Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 969–978. [Google Scholar] [CrossRef]
- Goddi, A.; Fanizza, M.; Bortolotto, C.; Raciti, M.V.; Fiorina, I.; He, X.; Du, Y.; Calliada, F. Vector flow imaging techniques: An innovative ultrasonographic technique for the study of blood flow. J. Clin. Ultrasound 2017, 45, 582–588. [Google Scholar] [CrossRef]
- Haniel, J.; Yiu, B.Y.S.; Chee, A.J.Y.; Huebner, R.; Yu, A.C.H. Efficacy of ultrasound vector flow imaging in tracking omnidirectional pulsatile flow. Med. Phys. 2023, 50, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Gan, L.; Liao, X.; Peng, Y.; Yang, F.; Liu, W.; Wang, M.; Song, J.; Zhang, J. Quantitative Analysis on Vessel Stiffness and Vector Flow Imaging in the Assessment of Carotid Artery Structural and Functional Changes in Patients with Type 2 Diabetes. Ultrasound Med. Biol. 2025, 51, 85–93. [Google Scholar] [CrossRef]
- Rossi, S.; Tortoli, P.; Ramalli, A. Lowering the Computational Load in High Frame Rate 2-D Vector Flow Imaging. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Abou Ali, A.N.; Fittipaldi, A.; Rocha-Neves, J.; Ruaro, B.; Benedetto, F.; Al Ghadban, Z.; Simon, G.; Lepidi, S.; D’Oria, M. Clinical applications of contrast-enhanced ultrasound in vascular surgery: State-of-the-art narrative and pictorial review. JVS Vasc. Insights 2025, 3, 100254. [Google Scholar] [CrossRef]
- Rafailidis, V.; Partovi, S.; Dikkes, A.; Nakamoto, D.A.; Azar, N.; Staub, D. Evolving clinical applications of contrast-enhanced ultrasound (CEUS) in the abdominal aorta. Cardiovasc. Diagn. Ther. 2018, 8 (Suppl. S1), S118–S130. [Google Scholar] [CrossRef]
- Martin, K. 1 Introduction to B-mode imaging. In Diagnostic Ultrasound; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Ashley, E.A.; Niebauer, J. Understanding the Echocardiogram. In Cardiology Explained; Remedica: London, UK, 2004; Available online: https://www.ncbi.nlm.nih.gov/books/NBK2215/ (accessed on 16 April 2025).
- Moorthy, R. Doppler Ultrasound. Med. J. Armed Forces India 2002, 58, 1–2. [Google Scholar] [CrossRef]
- Huang, C.-C.; Cheng, H.-F.; Zhu, B.-P.; Chen, P.-Y.; Beh, S.T.; Kuo, Y.-M.; Huang, C.-C. Studying Arterial Stiffness Using High-Frequency Ultrasound in Mice with Alzheimer Disease. Ultrasound Med. Biol. 2017, 43, 2054–2064. [Google Scholar] [CrossRef]
- Chérin, E.; Williams, R.; Needles, A.; Liu, G.; White, C.; Brown, A.S.; Zhou, Y.-Q.; Foster, F.S. Ultrahigh frame rate retrospective ultrasound microimaging and blood flow visualization in mice in vivo. Ultrasound Med. Biol. 2006, 32, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.; Mynard, J.P.; Smolich, J.J.; Cheung, M.M.H. Comparison of invasive and non-invasive aortic wave intensity and wave power analyses in sheep. Physiol. Meas. 2019, 40, 015005. [Google Scholar] [CrossRef] [PubMed]
- Adrian, R.J. Twenty years of particle image velocimetry. Exp. Fluids 2005, 39, 159–169. [Google Scholar] [CrossRef]
- Niki, K.; Sugawara, M.; Chang, D.; Harada, A.; Okada, T.; Sakai, R.; Uchida, K.; Tanaka, R.; Mumford, C.E. A new noninvasive measurement system for wave intensity: Evaluation of carotid arterial wave intensity and reproducibility. Heart Vessel. 2002, 17, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Niki, K.; Ohte, N.; Okada, T.; Harada, A. Clinical usefulness of wave intensity analysis. Med. Biol. Eng. Comput. 2009, 47, 197–206. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).