Abstract
Background: Salivary gland sarcoidosis is a rare manifestation of systemic sarcoidosis that poses a challenge in terms of its diagnosis due to its similarities to disorders such as Sjögren’s syndrome, other granulomatous diseases, and infections. Objective: To systematically review reported cases of salivary gland sarcoidosis and summarize clinical presentation, diagnostic methods, treatments, and outcomes. Methods: We conducted a systematic search of PubMed, Scopus, Embase, ScienceDirect, and Medline for case reports and case series published up to April 2025. This review was registered with PROSPERO (CRD42024629263) and was conducted following PRISMA guidelines. Variables assessed included age, sex, presenting symptoms, location, duration of symptoms, treatment approaches, and outcomes. Study quality assessment was assessed using The Joanna Briggs Institute (JBI) Critical Appraisal tools. Results: A total of 28 articles involving 39 participants met the inclusion criteria, with a mean age of 42.7 years. Salivary gland sarcoidosis predominantly affected female patients (66.7%). The parotid gland was the most frequently involved site (82.1%). Common presenting features included glandular swelling that is usually painless, xerostomia, and facial palsy. Sarcoidosis was newly diagnosed in 82.1% of cases, primarily through histopathological examination revealing non-caseating granulomas. Systemic corticosteroids were the most common treatment. Outcomes were favorable in nearly all cases, with complete resolution post treatment or spontaneous remission without treatment. Conclusions: Salivary gland sarcoidosis predominantly affects middle-aged women, typically presenting as a painless parotid swelling and often serving as the initial sign of systemic disease. Diagnosis requires histopathological confirmation via biopsy, as serum ACE levels are insufficient alone. The prognosis is excellent, with most patients responding favorably to corticosteroids or even experiencing spontaneous resolution. This condition must be considered in differential diagnoses for persistent salivary gland swellings to ensure accurate diagnosis and prevent unnecessary interventions.
1. Introduction
Sarcoidosis is a complex, multisystem inflammatory disorder characterized by the formation of non-caseating granulomas within affected tissues. Despite decades of research, the precise etiology remains unknown. Current evidence suggests that sarcoidosis arises from a dysregulated immune response to unidentified antigens, influenced by a combination of genetic susceptibility and environmental exposures [1].
The epidemiology of sarcoidosis reveals geographic and demographic variability. Incidence rates are highest in Scandinavian countries (11–23 cases per 100,000 annually) and lowest in parts of Asia (0.5–1 case per 100,000) [2,3,4,5,6]. Gender and age also influence disease patterns, with peak incidence observed in men between 30 and 50 years and in women between 50 and 60 years, potentially reflecting the interplay of hormonal, genetic, and environmental factors [3]. Sarcoidosis often remains asymptomatic and is incidentally detected during routine examinations, and when symptoms do appear, they are diverse and organ specific [7,8,9,10].
The oral presentation of sarcoidosis is infrequent and typically asymptomatic, occasionally causing discomfort, pain, or impair function such as speech, swallowing, or chewing [11,12,13]. Salivary gland involvement represents a rare manifestation, reported in fewer than 6% of sarcoidosis cases [7]. The diagnosis of salivary gland sarcoidosis is particularly challenging due to its nonspecific presentation, which includes painless glandular swelling and xerostomia. Clinical presentation often mimics other autoimmune or inflammatory conditions, such as primary Sjögren, because patients may experience xerostomia, dry eyes, and glandular swelling [12,14]. Unlike Sjögren’s syndrome, which typically shows characteristic sonographic changes in 50–60% of cases, sarcoid-related glandular disease is usually self-limiting and lacks specific ultrasonographic features, complicating differential diagnosis [11,14]. Definitive confirmation requires a tissue biopsy showing non-caseating granulomas, typically obtained from the affected gland through a minimally invasive approach. Treatment is largely empirical and depends on disease severity and systemic involvement. Systemic corticosteroids remain the mainstay of therapy, although spontaneous remission can occur in mild cases. Overall, the prognosis is favorable, with most patients achieving complete recovery [9,10].
While systemic and pulmonary manifestations of sarcoidosis have been widely researched, salivary gland sarcoidosis remains underrepresented in the literature. Therefore, this systematic review aims to analyze published case reports to better understand the epidemiology, clinical presentation, diagnostic methods, treatment strategies, and outcomes of salivary gland sarcoidosis. By integrating data from existing literature, this review seeks to assist clinicians in improving diagnostic accuracy and informed patient management.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review is registered in the PROSPERO international prospective database of systematic reviews in health and social care under the registration identification number [ID: CRD42024629263]. The study adheres to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, ensuring comprehensive and transparent reporting [15].
2.2. Search Strategy and Information Sources
A comprehensive electronic literature search was carried out across five major databases: PubMed, Scopus, Embase, ScienceDirect, and Medline to identify relevant studies and reduce chance of missed studies. The search utilized targeted phrases such (‘salivary gland sarcoidosis’) OR (‘parotid sarcoidosis’) OR (‘submandibular sarcoidosis’) OR (‘minor salivary gland sarcoidosis’) OR (‘major salivary glands sarcoidosis’) as keywords in the Advanced Search Builder. Filters Applied: Language: English, Article types: Case reports, case series and population: Human, with no time limitations. The final search performed was in April 2025. Studies were chosen based on predetermined inclusion and exclusion criteria set by the authors.
Records were reviewed by title and abstract by two independent reviewers (N.A. and R.A.). The selected articles were read in full to assess their eligibility. A list of excluded studies was maintained and updated periodically to prevent selection bias between the two reviewers. When the reviewers had differing views, they resolved the issue through discussion, and a third senior reviewer (M.J.) was consulted if consensus could not be reached.
2.3. Eligibility
The studies included in this review met all the predefined criteria specified by the PECOS framework (“Population,” “Exposure,” “Comparison,” “Outcomes,” and “Study design”) (Table 1).

Table 1.
PECOS inclusion and exclusion criteria.
2.4. Data Extraction
After reviewing the titles and abstracts of the search results, selected articles were read in full to assess their eligibility. Prior to synthesis, the extracted data were standardized to ensure consistency across the included case reports and case series. Data extracted included author(s) and year of publication, sample data: age and gender, location, initial symptoms, time to presentation, diagnosis of sarcoidosis (new/pre-existing), treatment, and follow-up and outcome (Table 2). Outcomes were harmonized into standardized categories (resolved, recurred, spontaneous remission, or unclear). When the reviewers had differing views, they resolved the issue by reaching a mutual agreement. In instances where a consensus was not readily reached between the two review authors, a third review author (M.J.) served as an impartial arbitrator to resolve any discrepancies. No assumptions were made about unreported diagnostic methods, treatment regimens, or long-term outcomes.

Table 2.
Study characteristics of included studies.
2.5. Study Quality Assessment
To assess the risk of bias, two review authors (R.A. and N.A.) independently conducted quality assessments for each of the included studies. The critical appraisal of case reports was assessed according to The Joanna Briggs Institute (JBI) Critical Appraisal tools issued by the Faculty of Health and Medical Sciences at the University of Adelaide, South Australia [41]. The checklist included whether demographic details, clinical presentation, diagnostic procedures, treatment, and outcomes were fully described. Discrepancies were resolved through consultation with a third review author (M.J.) (Table 3).

Table 3.
JBI Critical Appraisal for quality assessment.
3. Results
3.1. Literature Search
The databases’ search resulted in a total of 850 articles. After filtering for articles published of sarcoidosis in humans in English, and removing duplicates manually, 500 articles remained to be screened. After reviewing the title and abstracts, 363 were excluded. A list of excluded studies was maintained and updated periodically to prevent selection bias between two independent reviewers. The full texts of the remaining 137 articles were retrieved for assessment of eligibility. Of those 137 articles, 32 were not retrieved due to some being conference abstracts only (n = 11), a few were found to be irrelevant to the topic (n = 6), access limitations (n = 5), and some were older studies for which we could not locate the full text (n = 10). Leaving 105 articles to be further scrutinized according to our inclusion and exclusion criteria and to be critically appraised. Finally, a total of 28 articles were included in this systematic review following the inclusion criteria (Figure 1).
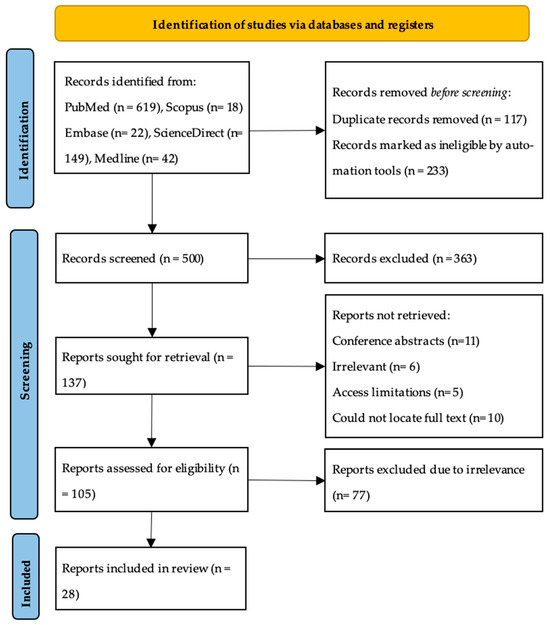
Figure 1.
PRISMA flow diagram.
3.2. Study Characteristics
Twenty-eight articles that presented 39 patients met the inclusion criteria (Table 2). The 28 articles were case reports and case series published Between 1969 And 2024. Of the 28 articles, 24 were case reports, presenting 1–2 cases per article, while 4 were case series. The sample size was 39 patients diagnosed with salivary gland sarcoidosis, with an age range of 5–72 years and mean of 42.7 years. Females were more affected than male, accounting for 66.7% of our sample.
Diagnosis of salivary gland involvement was established through biopsy of the affected gland and histopathological confirmation. A detailed analysis of these 32 parotid cases revealed that swelling was most frequently bilateral, occurring in 20 cases (62.5%), compared to 12 cases (37.5%) that were unilateral. The swelling was predominantly described as painless (17/32, 53.1%) rather than painful (6/32, 18.8%), with pain status not specified in the remaining cases. Other involved sites included the submandibular gland (5.1%), minor salivary glands (2.6%), and multiple glands simultaneously (10.3%). In some cases, the swelling may be accompanied by xerostomia (33.3%), facial palsy (10.3%), dry eyes, and alteration in taste. Systemic symptoms associated included generalized fatigue (15.4%), loss of appetite and weight, and fever.
In the majority of cases (82.1%), the diagnosis of sarcoidosis was established following the presentation, while the remaining patients had a previously confirmed diagnosis. The duration from symptom onset to definitive diagnosis showed wide variability among reported cases, ranging from 1 week to 12 years. The median time to diagnosis was 3 months (interquartile range: 1–9 months) among 35 cases with available data, indicating that while most cases were identified within the first year, a subset experienced considerable diagnostic delays.
Treatment of choice was corticosteroids in 69% of cases; in very few cases (12.8%), surgical intervention was the chosen modality of treatment. In other cases, no treatment was undertaken, and a combination of surgery and corticosteroids was also the treatment choice in 2 cases (5.1%). Most of the cases showed favorable outcomes, with most resolving (79.5%) with treatment, and in a few instances spontaneous remission occurred (10.3%) where no treatment was provided. And only 1 case of recurrence occurred.
3.3. Study Quality Assessment
Included studies were assessed using The Joanna Briggs Institute Critical Appraisal tools for JBI systematic reviews [41]. The tool focuses on sufficient demographics, history, presentation, diagnosis, and proper intervention. Studies scoring 7–8 were deemed as having a “high quality,” scoring 4–6 was “moderate quality,” and scores of‚ <3 were “poor quality”. Of the 28 articles, 11 scored high quality and 17 scored moderate. Moderate scores were due to a lack of data on some criteria (Table 3).
4. Discussion
Salivary gland sarcoidosis represents a rare but clinically significant manifestation of systemic sarcoidosis. Histopathologically characterized by non-caseating granulomas, it may involve major salivary glands like parotid, submandibular, and sublingual glands. The findings reveal that although salivary gland involvement in sarcoidosis is rare, it presents with distinctive clinical features and demonstrates a variable response to treatment. Despite its rarity, salivary gland involvement may represent the initial or sole manifestation of sarcoidosis, underscoring the need for heightened clinical awareness.
Most patients were diagnosed at an average (SD) age of 42.7 (16.4) years, with most of them diagnosed during the fourth decade of their lives, with a market female predominance, consistent with previous literature [1,42]. Our analysis confirms that the parotid gland is the epicenter of salivary gland sarcoidosis, involved in over 80% of cases. The high prevalence of bilateral parotid enlargement (62.5% of parotid cases) is a particularly distinctive clinical feature. This bilateral, often painless swelling should raise immediate clinical suspicion for a systemic inflammatory or granulomatous process, such as sarcoidosis, as it is less commonly a feature of benign neoplasms, which are typically unilateral [43]. The predilection for the parotid gland may be related to its high lymphatic content and its role as part of the lymphoepithelial system, making it a potential site for the deposition of unidentified antigens that trigger the granulomatous response [1,44]. When a patient presents with bilateral parotid swelling, the differential diagnosis must be systematically evaluated to distinguish sarcoidosis from other causes such as Sjögren’s syndrome, IgG4-related disease, chronic sialadenitis, and metabolic disorders like diabetes mellitus. A smaller subset of cases involved the submandibular, and minor salivary glands, though these occurrences remain notably rare in published literature. Additionally, in some instances sarcoidosis did not just affect one gland but involved a number of glands at once [13,20,28,40].
The hallmark clinical presentation was swelling of the glandular tissue (in over 80% of the cases), which is frequently painless, but in a few cases, pain was noted although it was atypical [11,34]. This pain may possibly be attributed to localized restricted inflammation, nerve involvement (facial nerve for instance), or secondary infection [11]. This may be accompanied by xerostomia, which may be due to the replacement of salivary gland tissue with non-caseating granulomas, leading to decrease in saliva production hence dry mouth. Systemic manifestations including fatigue, low-grade fever, and weight loss were noted in a minority of cases (n = 5), while facial palsy occurred in 4 cases, which may be attributed to granulomatous infiltration or compression of the facial nerve.
Diagnosis of salivary sarcoidosis remains complex due to symptom overlap with conditions like Sjögren’s syndrome and benign salivary gland tumors. In most included reports, the diagnosis was confirmed through histopathological analysis demonstrating non-caseating granulomas within glandular tissue, which remains the gold standard [10,44]. These biopsies were obtained through surgical excision, incisional biopsies, or FNAC. The role of imaging was less frequently detailed but may support clinical suspicion in bilateral or symmetrical glandular swelling [44]. The diagnosis was often newly established (82.1%) in the context of the salivary gland presentation rather than a recurrence or known history of systemic sarcoidosis, indicating that salivary gland presentation association may be the first manifestation of systematic sarcoidosis. The non-caseating granulomas are composed predominantly of epithelioid histiocytes, often accompanied by multinucleated giant cells, and surrounded by a sparse rim of lymphocytes [45,46,47,48]. Importantly, these granulomas lack central necrosis, distinguishing them from infectious granulomatous diseases such as tuberculosis or fungal infections [49,50]. Elevated serum angiotensin-converting enzyme (ACE) levels are often used as a supportive biomarker in the diagnosis of sarcoidosis [50]. In this review, ACE levels were reported in 21 of the 39 cases, of which 15 demonstrated elevated values (71.4%), 5 were within normal limits, and one showed a decreased level. This rate of elevation is consistent with the known suboptimal sensitivity of this test, which literature suggests ranges from approximately 41% to 78%, depending on the population and disease activity [50,51]. While elevated ACE levels may reinforce clinical suspicion, particularly in cases with multisystem involvement, their sensitivity and specificity remain limited [51]. Normal ACE levels, as seen in several cases in this review, do not exclude sarcoidosis and were often present in patients with isolated salivary gland involvement or early-stage disease. Therefore, a normal serum ACE level should not deter a clinician from pursuing a diagnostic biopsy in the presence of suggestive clinical findings.
The clinical presentation of salivary gland sarcoidosis, particularly when accompanied by xerostomia and glandular swelling, creates a significant diagnostic overlap with other conditions, primarily Sjögren’s syndrome (SS) and IgG4-related disease (IgG4-RD). Accurate differentiation is critical as the management and long-term prognosis differ substantially. As summarized in Table 4, key distinguishing factors include serological markers, histopathological findings, and characteristic extraglandular manifestations. SS is typically seropositive for anti-SSA/Ro and anti-SSB/La antibodies and demonstrates focal lymphocytic sialadenitis on histology [52]. IgG4-RD is characterized by elevated serum IgG4 levels and histopathological findings of storiform fibrosis, obliterative phlebitis, and a dense IgG4+ plasma cell infiltrate [53]. In contrast, sarcoidosis is defined by non-caseating granulomas [45,46,47], lacks specific autoantibodies, and serum ACE may be elevated but lacks sensitivity [50,51]. Heerfordt’s syndrome (uveitis, parotitis, facial palsy, and fever) is a classic, though rare, extraglandular presentation of sarcoidosis [32,34], whereas SS is more commonly associated with arthralgia and Raynaud’s phenomenon [52]. A comprehensive evaluation incorporating these features is essential to avoid misdiagnosis and guide appropriate therapy.

Table 4.
Differential Diagnosis of Salivary Gland Sarcoidosis—Key Comparison with Sjögren’s Syndrome and IgG4-Related Disease.
Treatment most commonly included only non-surgical (steroids) treatment (82.1%), and these patients typically demonstrated favorable outcomes, with resolution or significant reduction in glandular swelling and systemic symptoms. This confirms the well-established role of corticosteroids in sarcoidosis management [7,10]. A subset of patients underwent surgical intervention, primarily when initial suspicion was directed toward neoplastic disease. In these cases, excision was not only diagnostic but also therapeutic. In other cases, no treatment was performed, and spontaneous remission of the lesion occurred, corroborating that sarcoidosis can follow a self-limiting course [1]. Combination approaches involving both surgical and medical therapies were also employed in a few instances [16,22].
A key clinical insight from this review is the validation of a ‘watchful waiting’ or observational approach in a select subset of patients. The spontaneous remission observed in 12.8% of our cohort, all of whom had isolated salivary gland disease without severe functional impairment or threatening complications, aligns with the known self-limiting nature of sarcoidosis in many forms [1,54]. This suggests that for patients with mild, non-progressive, and asymptomatic glandular enlargement, an initial period of observation is a reasonable and evidence-supported strategy. This approach avoids the potential side effects of corticosteroid therapy, such as hyperglycemia, weight gain, and osteoporosis, which are not insignificant [10]. The decision to initiate treatment should therefore be guided by the presence of symptoms (e.g., pain, significant xerostomia), cosmetic concern, progressive enlargement, or the development of systemic or organ-threatening manifestations (e.g., uveitis, facial palsy). Future studies should aim to identify predictive factors that can reliably distinguish patients who will undergo spontaneous remission from those who will progress, thereby further refining management guidelines.
Analysis of outcomes based on treatment type reveals a uniformly favorable prognosis for salivary gland sarcoidosis, irrespective of the management strategy employed. The majority of patients (27/39, 69.2%) were treated with corticosteroids, with 79.5% (31/39) of all cases achieving complete resolution. Within the corticosteroid group, the response was excellent, leading to resolution in nearly all treated cases. This confirms the well-established role of corticosteroids as the first-line therapy for symptomatic disease [7,10]. Notably, a significant subset of patients (5/39, 12.8%) received no treatment and experienced spontaneous remission. Surgical intervention, employed in 12.8% (5/39) of cases, was primarily diagnostic, often performed under the initial suspicion of a neoplasm; however, it proved curative in these isolated gland presentations. The combination of surgery and steroids was used in two cases (5.1%) with successful outcomes. This data underscores that while corticosteroids are highly effective, the natural history of salivary gland sarcoidosis can be self-limiting, and invasive procedures are often unnecessary once the diagnosis is confirmed.
Based on the findings of this review, we propose a practical diagnostic workflow for evaluating suspected salivary gland sarcoidosis. For a patient presenting with persistent, predominantly painless salivary gland swelling (especially bilateral parotid involvement), the initial assessment should include a detailed history for systemic symptoms (e.g., fever, fatigue, visual changes, respiratory complaints) and a physical examination. Initial serological testing should aim primarily at exclusion, targeting SS (anti-SSA/SSB) [52] and, if clinically suggestive, IgG4-RD (serum IgG4 levels) [53]. Serum ACE can be ordered but should not be relied upon for rule-out, given its limited sensitivity [52]. A chest X-ray or CT scan is crucial to identify concomitant hilar lymphadenopathy or pulmonary infiltrates, which strongly support the diagnosis of sarcoidosis [1,10,44]. However, the definitive diagnostic step remains a tissue biopsy of the affected salivary gland (or concomitant suspicious lymph nodes) to demonstrate non-caseating granulomas [10,45,46,47]. This approach ensures accurate diagnosis, facilitates appropriate systemic staging, and prevents unnecessary interventions for benign or self-limiting conditions.
This review highlights the importance of considering sarcoidosis in the differential diagnosis of painless, chronic salivary gland swellings, particularly in patients with associated constitutional or ocular symptoms. Histological evaluation should be pursued early, especially when imaging or serological testing is inconclusive. Multidisciplinary collaboration with oral medicine, rheumatology, and ophthalmology is essential for accurate diagnosis and systemic evaluation. Future multicenter registries and prospective studies are needed to better define the true prevalence and optimal management of this rare presentation.
5. Limitations
Several limitations must be acknowledged. First, the review is based entirely on case reports and case series, which inherently carry a high risk of selection and publication bias. Most reports lacked standardized follow-up and did not consistently report clinical metrics or treatment protocols, reducing comparability across cases. The heterogeneity in clinical presentations, diagnostic workups, and treatment modalities further precluded a meta-analysis.
Additionally, a large number of potentially relevant articles could not be retrieved, possibly excluding additional informative cases. Finally, the JBI-based risk of bias assessment revealed that most reports lacked comprehensive data on adverse effects and long-term outcomes, further limiting the strength of evidence.
6. Conclusions
Salivary gland sarcoidosis predominantly affects middle-aged women, typically presenting as a painless parotid swelling and often serving as the initial sign of systemic disease. Diagnosis requires histopathological confirmation via biopsy, as serum ACE levels are insufficient alone. The prognosis is excellent. While systemic corticosteroids are the mainstay of treatment for significant or symptomatic disease, our findings strongly indicate that a conservative, “watchful waiting” approach is a viable and often underutilized strategy in select patients with mild, isolated gland involvement, given the notable rate of spontaneous remission. This condition must be considered in differential diagnoses for persistent salivary gland swellings to ensure accurate diagnosis, prevent unnecessary interventions, and tailor management to the patient’s specific clinical scenario.
Author Contributions
Conceptualization, M.J. and N.A.; methodology, M.J. and N.A.; validation, M.J., N.A., and R.B.A.; formal analysis, N.A.; investigation, N.A. and R.B.A.; data curation, N.A.; writing—original draft preparation, M.J. and N.A.; writing—review and editing, M.J.; supervision, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors are grateful to Ajman University for supporting this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rossides, M.; Darlington, P.; Kullberg, S.; Arkema, E.V. Sarcoidosis: Epidemiology and Clinical Insights. J. Intern. Med. 2023, 293, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Sikjær, M.G.; Hilberg, O.; Ibsen, R.; Løkke, A. Sarcoidosis: A Nationwide Registry-Based Study of Incidence, Prevalence and Diagnostic Work-Up. Respir. Med. 2021, 187, 106548. [Google Scholar] [CrossRef]
- Arkema, E.V.; Grunewald, J.; Kullberg, S.; Eklund, A.; Askling, J. Sarcoidosis Incidence and Prevalence: A Nationwide Register-Based Assessment in Sweden. Eur. Respir. J. 2016, 48, 1690–1699. [Google Scholar] [CrossRef]
- Salonen, J.; Kaarteenaho, R. National Retrospective Registry Survey on the Epidemiology of Sarcoidosis in Finland 2002–2022. BMJ Open Respir. Res. 2024, 11, e002461. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Kim, H.M.; Kim, Y.J.; Song, J.W. Prevalence and Incidence of Sarcoidosis in Korea: A Nationwide Population-Based Study. Respir. Res. 2018, 19, 158. [Google Scholar] [CrossRef]
- Jeon, M.H.; Kang, T.; Yoo, S.H.; Swan, H.S.; Kim, H.J.; Ahn, H.S. The Incidence, Comorbidity and Mortality of Sarcoidosis in Korea, 2008–2015: A Nationwide Population-Based Study. Sarcoidosis Vasc. Diffus. Lung Dis. 2020, 37, 24–26. [Google Scholar] [CrossRef]
- Baughman, R.P.; Field, S.; Costabel, U.; Crystal, R.G.; Culver, D.A.; Drent, M.; Judson, M.A.; Wolff, G. Sarcoidosis in America: Analysis Based on Health Care Use. Ann. Am. Thorac. Soc. 2016, 13, 1244–1252. [Google Scholar] [CrossRef]
- Judson, M.A. The Clinical Features of Sarcoidosis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2015, 49, 63–78. [Google Scholar] [CrossRef]
- Sève, P.; Pacheco, Y.; Durupt, F.; Jamilloux, Y.; Gerfaud-Valentin, M.; Isaac, S.; Boussel, L.; Calender, A.; Androdias, G.; Valeyre, D.; et al. Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis. Cells 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.N.; Fujita, A.; Sung, E.K.; Siegel, C.; Nadgir, R.N.; Saito, N.; Sakai, O. Sarcoidosis in the Head and Neck: An Illustrative Review of Clinical Presentations and Imaging Findings. AJR Am. J. Roentgenol. 2017, 208, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Folwaczny, M.; Sommer, A.; Sander, C.A.; Kellner, H. Parotid Sarcoidosis Mimicking Sjögren’s Syndrome: Report of a Case. J. Oral Maxillofac. Surg. 2002, 60, 117–120. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Rinaggio, J. Diagnosis of Systemic Sarcoidosis Prompted by Orofacial Manifestations: A Review of the Literature. J. Am. Dent. Assoc. 2006, 137, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Shahril, N.S.; Hussein, H.; Said, M.S. Case Review of Sarcoidosis Resembling Sjögren’s Syndrome. J. Clin. Med. Res. 2010, 2, 284–288. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hoggins, G.S.; Allan, D. Sarcoidosis of the Maxillary Region. Oral Surg. Oral Med. Oral Pathol. 1969, 28, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Som, P.M.; Shugar, J.M.; Biller, H.F. Parotid Gland Sarcoidosis and the CT Sialogram. J. Comput. Assist. Tomogr. 1981, 5, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Melsom, R.D.; Speight, P.M.; Ryan, J.; Perry, J.D. Sarcoidosis in a Patient Presenting with Clinical and Histological Features of Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 1988, 47, 166–168. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Yanadori, A.; Tabata, H.; Yamakage, A.; Yamazaki, S. Sarcoidosis with Giant Parotomegaly. Cutis 2001, 68, 199–200. [Google Scholar]
- Surattanont, F.; Mandel, L.; Wolinsky, B. Bilateral Parotid Swelling Caused by Sarcoidosis. J. Am. Dent. Assoc. 2002, 133, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Gaur, S.N. Sarcoidosis Presenting as Acute Bilateral Parotid Swelling. Asian Pac. J. Allergy Immunol. 2004, 22, 171–174. [Google Scholar]
- Vairaktaris, E.; Vassiliou, S.; Yapijakis, C.; Papakosta, V.; Kavantzas, N.; Martis, C.; Patsouris, E. Salivary Gland Manifestations of Sarcoidosis: Report of Three Cases. J. Oral Maxillofac. Surg. 2005, 63, 1016–1021. [Google Scholar] [CrossRef]
- Yates, J.M.; Dickenson, A.J. Sarcoidosis Presenting as an Isolated Facial Swelling—An Unexpected Diagnosis? Dent. Update 2006, 33, 112–114. [Google Scholar] [CrossRef]
- McCormick, J.T.; Newton, E.D.; Geyer, S.; Caushaj, P.F. Sarcoidosis Presenting as a Solitary Parotid Mass. Ear Nose Throat J. 2006, 85, 664–665. [Google Scholar] [CrossRef]
- Rudralingam, M.; Nolan, A.; Macleod, I.; Greenwood, M.; Heath, N. A Case of Sarcoidosis Presenting with Diffuse, Bilateral Swelling of the Salivary Glands. Dent. Update 2007, 34, 439–442. [Google Scholar] [CrossRef]
- Poate, T.W.; Sharma, R.; Moutasim, K.A.; Escudier, M.P.; Warnakulasuriya, S. Orofacial Presentations of Sarcoidosis—A Case Series and Review of the Literature. Br. Dent. J. 2008, 205, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Teymoortash, A.; Werner, J.A. Parotid Gland Involvement in Sarcoidosis: Sonographic Features. J. Clin. Ultrasound 2009, 37, 507–510. [Google Scholar] [CrossRef]
- Geraldes Filho, J.C.; Barbosa, H.S.; Souza, D.O.; Prates, A.A. Chronic Sialadenitis Caused by Sarcoidosis: A Case Report. Braz. J. Otorhinolaryngol. 2010, 76, 196. [Google Scholar] [CrossRef]
- Banks, G.C.; Kirse, D.J.; Anthony, E.; Bergman, S.; Shetty, A.K. Bilateral Parotitis as the Initial Presentation of Childhood Sarcoidosis. Am. J. Otolaryngol. 2013, 34, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Whatley, B.; Kashani, S.; Howlett, D. A 22-Year-Old Woman with Bilateral Panuveitis and Parotid Swelling. BMJ 2015, 351, h4178. [Google Scholar] [CrossRef]
- Sharma, T.; Joshi, D.; Khurana, A.; Gupta, V.; Kapoor, N. Bilaterally Enlarged Parotids and Sicca Symptoms as a Presentation of Sarcoidosis: Pivotal Role of Aspiration Cytology in Diagnosis. J. Cytol. 2015, 32, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Chappity, P.; Kumar, R.; Sahoo, A.K. Heerfordt’s Syndrome Presenting with Recurrent Facial Nerve Palsy: Case Report and 10-Year Literature Review. Sultan Qaboos Univ. Med. J. 2015, 15, e124–e128. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.H.; Lee, J.K. Isolated Parotid Gland Sarcoidosis Mimicking Parotid Tumor. J. Korean Med. Sci. 2016, 31, 644–645. [Google Scholar] [CrossRef]
- Broggi, G.; Reggio, E.; Giuliano, L.; Palmucci, S.; Caltabiano, R.; Lanzafame, S. Parotid Gland Involvement in Heerfordt Syndrome: A Case Report. Pathologica 2017, 109, 418–420. [Google Scholar]
- Assiri, K.; Al-Ahmari, M. Primary Parotid Gland Sarcoidosis—Case Report. Egypt. J. Hosp. Med. 2018, 72, 4593–4595. [Google Scholar] [CrossRef]
- Brown, J.R.; Thottam, P.J.; Poulik, J.; Haupert, M. Pediatric Sarcoidosis Presenting as Bilateral Parotid Swelling. Otolaryngol. Case Rep. 2017, 6, 19–21. [Google Scholar] [CrossRef]
- Diamantopoulos, P.T.; Charakopoulos, E.; Viniou, N.A.; Diamantopoulou, L.; Gaggadi, M. An Unusual Case Report of Unilateral Parotid Gland Sarcoidosis with Spontaneous Remission. Medicine 2019, 98, e18172. [Google Scholar] [CrossRef]
- Derbel, A.; Frikha, F.; Ghribi, M.; Salah, R.B.; Bahloul, Z. Bilateral Parotid Swelling: Don’t Forget Systemic Sarcoidosis! Ann. Clin. Cases 2021, 2, 1027. [Google Scholar]
- Abraham, S.M.; Faizal, B.; Mithun, C.B.; Smitha, N.V. Sarcoidosis Masquerading as Parotid Gland Disorder: A Unique Clinical Scenario. Kerala J. ENT Head Neck Surg. 2024, 2, 23–26. [Google Scholar]
- Vidović Juras, D.; Ivković, I.; Hećimović, A.; Gjadrov Kuveždić, K.; Andabak Rogulj, A.; Lončar Brzak, B.; Brailo, V.; Škrinjar, I.; Špiljak, B. Unusual Parotid Gland and Sublingual Mucosa Swelling in a 48-Year-Old Woman. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2024, 138, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Vourexakis, Z.; Dulguerov, P.; Bouayed, S.; Burkhardt, K.; Landis, B.N. Sarcoidosis of the Submandibular Gland: A Systematic Review. Am. J. Otolaryngol. 2010, 31, 424–428. [Google Scholar] [CrossRef]
- Ogle, O.E. Salivary Gland Diseases. Dent. Clin. N. Am. 2020, 64, 87–104. [Google Scholar] [CrossRef]
- Prasse, A. The Diagnosis, Differential Diagnosis, and Treatment of Sarcoidosis. Dtsch. Arztebl. Int. 2016, 113, 565–574. [Google Scholar] [CrossRef]
- Welter, S.M.; DeLuca-Johnson, J.; Thompson, K. Histologic Review of Sarcoidosis in a Neck Lymph Node. Head Neck Pathol. 2018, 12, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Costabel, U.; Ohshimo, S.; Guzman, J. Diagnosis of Sarcoidosis. Curr. Opin. Pulm. Med. 2008, 14, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Donatiello, I.; Caputo, A.; Tana, M.; Naccarelli, T.; Mantini, C.; Ricci, F.; Ticinesi, A.; Meschi, T.; Cipollone, F.; et al. Clinical Features, Histopathology and Differential Diagnosis of Sarcoidosis. Cells 2022, 11, 59. [Google Scholar] [CrossRef]
- Dow, C.T.; Lin, N.W.; Chan, E.D. Sarcoidosis, Mycobacterium Paratuberculosis and Noncaseating Granulomas: Who Moved My Cheese. Microorganisms 2023, 11, 829. [Google Scholar] [CrossRef]
- van der Walt, J.D.; Leake, J. Granulomatous Sialadenitis of the Major Salivary Glands. A Clinicopathological Study of 57 Cases. Histopathology 1987, 11, 131–144. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Du, X.; Dong, J.Z. Re-Evaluating Serum Angiotensin-Converting Enzyme in Sarcoidosis. Front. Immunol. 2023, 14, 950095. [Google Scholar] [CrossRef]
- Druyan, A.; Shuv, N.; Lidar, M. Put Down the ACE: Low Clinical Utility for Angiotensin-Converting Enzyme Levels in Sarcoidosis: A Single-Center Retrospective Cohort Study. J. Clin. Med. 2024, 13, 7657. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Umehara, H.; Okazaki, K.; Kawa, S.; Takahashi, H.; Goto, H.; Matsui, S.; Tsubouchi, H.; Tsuji, Y.; Kiyosawa, K.; Kawano, M.; et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod. Rheumatol. 2021, 31, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Pérez-Alvarez, R.; Pallarés, L.; Ramos-Casals, M. Sarcoidosis: An update on current pharmacotherapy options and future directions. Expert Opin. Pharmacother. 2016, 17, 2431–2448. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).