1. Introduction
Acute myeloid leukemia (AML) is an aggressive myeloid malignancy in which immature blasts crowd the bone marrow, disrupting normal hematopoiesis and causing life-threatening pancytopenia [
1].
Although classically a systemic disease, AML can infiltrate every ocular compartment, from adnexa and orbital tissues to choroid and optic nerve [
2], far more often than its chronic counterparts [
3,
4]. Ocular findings may precede the hematologic diagnosis or herald a covert relapse, but their presence portends poorer prognosis, being linked to higher rates of medullary relapse or central nervous system (CNS) involvement [
5,
6].
We describe a rare case of AML relapse manifesting with simultaneous orbital, adnexal, choroidal, and retinal infiltration, accompanied by hemorrhagic involvement of both the anterior and posterior segments. This constellation of findings, affecting multiple ocular structures concurrently, is exceptionally uncommon in adults and strongly suggestive of systemic recurrence. In addition to presenting this case, we review the literature on ocular involvement in leukemia, highlighting its pathogenetic mechanisms and prognostic implications.
2. Case Presentation
A 63-year-old man diagnosed with AML FLT3 ITD+, with intermediate risk according to the 2022 European LeukemiaNet (ELN) recommendations [
7], underwent consolidation chemotherapy with high-dose Cytarabine (1500 mg/m
2 every 12 h on days +1, +3, and +5) and Midostaurin (50 mg twice daily), according to the institutional consolidation protocol at Policlinico Umberto I, Rome, Italy. On day 14 of the maintenance regimen, the patient experienced a transient fever spike of 38 °C that led to treatment discontinuation. The patient underwent a CT of the chest and head, which showed no focal abnormalities suspicious for infectious foci. Blood cultures showed no evidence of active infection, but broad-spectrum antimicrobial therapy was nevertheless initiated. Bone marrow aspiration subsequently confirmed aplasia.
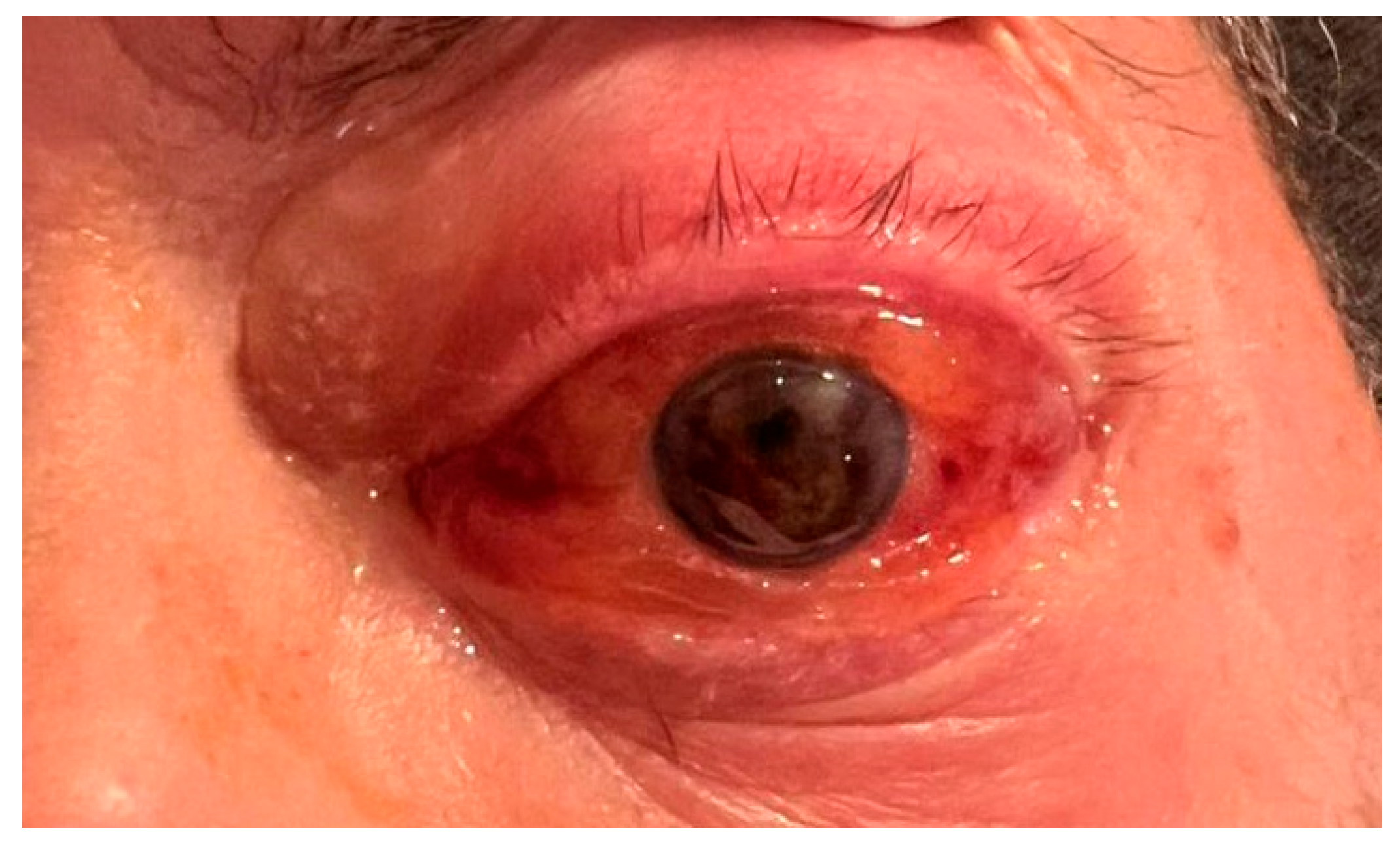
Two weeks later, the patient presented with proptosis of the left eye (LE) and blurred vision. No prior ophthalmic records were available; the patient reported a history of good vision without ocular complaints. Bedside ophthalmic examination revealed light perception visual acuity, significant proptosis, periocular edema, conjunctival chemosis and diffuse subconjunctival hemorrhage, hyphema, pupil seclusion with epipupillary membrane in the LE (
Figure 1). Indirect ophthalmoscopy could not be performed due to vitreous hemorrhage. The right eye (RE) was unremarkable for pathological signs.
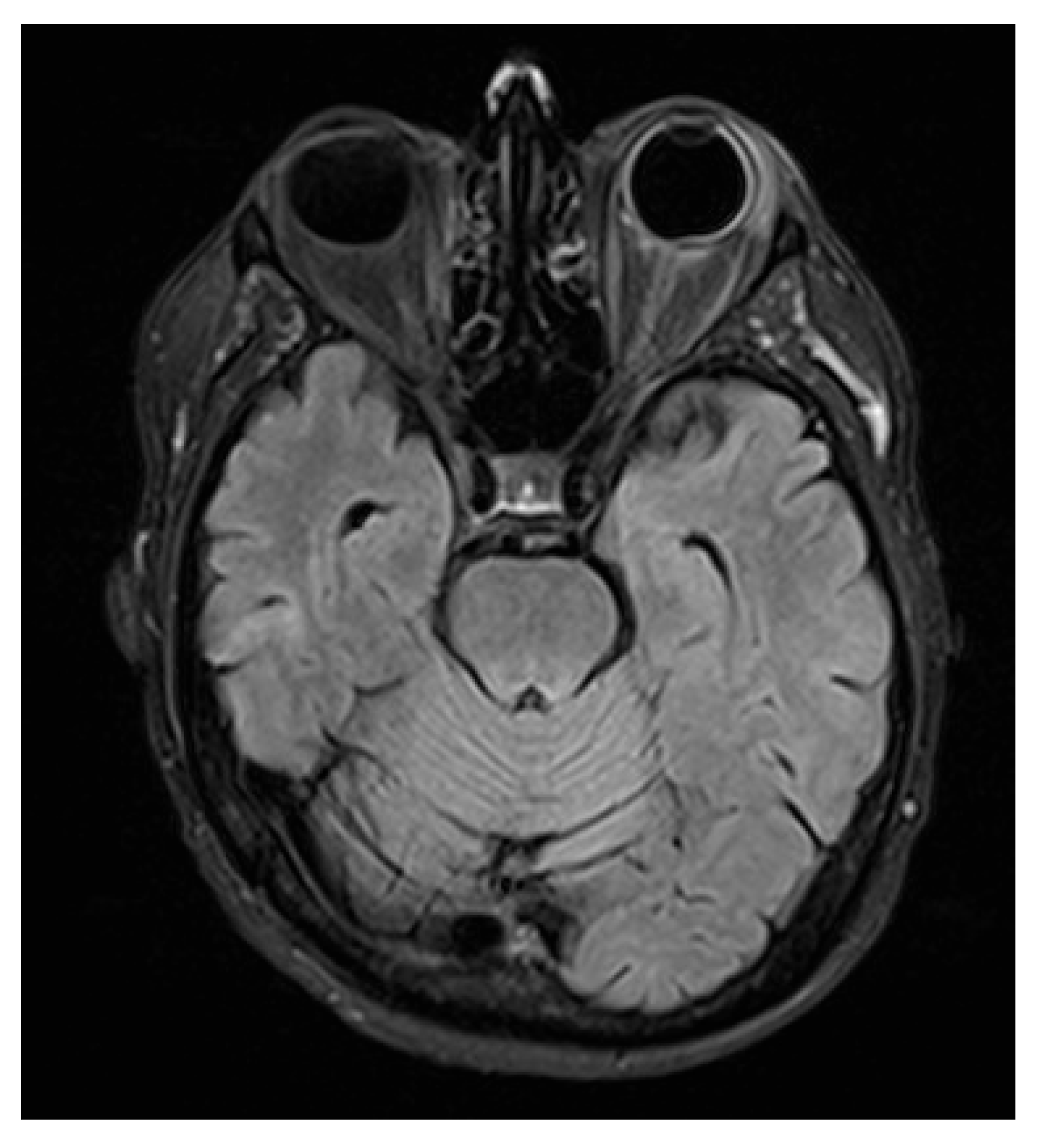
MRI of the brain and orbits was obtained. Neuroimaging demonstrated minute, focal hypointense lesions in both supratentorial and infratentorial regions, compatible with microbleeds, without signal abnormalities suggestive of CNS leukemic localization.
Orbital T2-FLAIR MRI sequences showed mild edema and hyperintensity of the retroorbital and periorbital fat, the lacrimal gland, the posterior sclera, and the optic nerve sheath (
Figure 2). B-scan ultrasonography showed fibrotic consolidations and organized vitreous hemorrhage, along with marked choroidal thickening displaying medium–low reflectivity and elevation of the retinal contour, suggestive of infiltrative material (
Figure 3). The associated optic disc distortion and perineural sheath edema, together with MRI findings, supported the diagnosis of multifocal leukemic infiltration with adnexal and retrobulbar extension, rather than hemorrhagic changes.
The hematological evaluation, including a complete blood count, showed hemoglobin of 8.9 g/dL, white blood cell count of 1010/mm3, neutrophil count of 430/mm3, and platelet count of 15,000/mm3. Due to these hematological abnormalities, periocular or transcleral tissue biopsy was contraindicated.
Unfortunately, the patient’s condition continued to deteriorate, and four weeks later, he died from a hemorrhagic stroke, most likely related to bleeding diathesis secondary to chemotherapy-induced bone marrow aplasia, rather than a direct leukemic manifestation.
3. Literature Review
3.1. Leukemic Ocular Manifestations
The ophthalmic manifestations of leukemia can be classified into two main categories: primary (direct) involvement, due to infiltration of neoplastic cells such as leukemic infiltrates; and secondary (indirect) involvement, from dysregulated hematopoiesis or chemotherapy, causing cytopenias (anemia, thrombocytopenia), hyperviscosity, and immunosuppression with opportunistic infections [
8]. These ocular findings occur across acute and chronic leukemias, both myeloid and lymphoblastic. Because they arise at different disease stages and via distinct mechanisms, we will discuss them separately.
3.1.1. Retina
Historically, fundus abnormalities were the most frequently reported ocular manifestation of leukemia [
8]; recent comparative and cohort analyses confirm that posterior segment involvement remains common, occurring in up to 64% of cases [
9,
10,
11].
Primary Involvement
Among the earliest findings are tortuous, dilated retinal veins with a “sausage-like” configuration and vascular sheathing, likely from perivascular leukemic infiltration. Hard exudates and cotton-wool spots may also occur, reflecting nerve fiber layer infarcts or focal leukemic deposits [
8,
9,
12].
Retinal leukemic infiltrates present as nodular cell accumulations of variable size, often with tissue destruction, necrosis, and hemorrhage; they are generally confined by the internal limiting membrane, though rare vitreous extension, likely from the optic nerve head, has been reported [
8,
13,
14,
15]. Large deposits can cause total retinal detachment, sometimes as an isolated relapse [
16], whereas smaller infiltrates are typically perivascular [
15]. Subretinal infiltration may occur, occasionally appearing as a subretinal hypopyon [
17]. Cotton-wool spots may accompany these findings, reflecting ischemia from anemia, hyperviscosity or direct leukemic involvement [
13]. Notably, nodular infiltrates correlate with marked leukocytosis with blast predominance and fulminant disease with poor survival; thus, retinal infiltration plus leukocytosis is a negative prognostic marker [
8].
Secondary Involvement
The term “leukemic retinopathy” generally refers to retinal changes secondary to anemia, thrombocytopenia, and hyperviscosity, rather than direct leukemic infiltration [
18]. It is the most common ocular manifestation, characterized by vessel tortuosity and retinal hemorrhages, typically intraretinal, round or flame-shaped, at the posterior pole. Subhyaloid “boat-shaped” and subretinal hemorrhages are less common; subhyaloid bleeds may rarely break into the vitreous [
9,
11,
19]. It is often seen at relapse, but not pathognomonic nor clearly prognostic and primarily related to severe anemia [
8]. In acute lymphoblastic leukemia (ALL), intraretinal hemorrhages correlate with thrombocytopenia and low hematocrit, whereas leukocyte count showed no association; profound anemia plus thrombocytopenia increases risk, implicating hematocrit as a key determinant [
20,
21,
22]. Changes are predominantly reversible, with OCTA showing improved both macular and peripapillary perfusion and increased vessel density in remission [
23,
24].
Roth spots are intraretinal hemorrhages characterized by a central white focus, which may represent cellular debris, capillary emboli, or leukemic aggregates [
8]. In acute leukemia they reflect capillary rupture with fibrin–platelet thrombi; proposed drivers include abnormal hematopoiesis, elevated intravascular pressure, ischemia, and increased capillary fragility, leading to endothelial injury and thrombosis [
8]. Patients with Roth spots show higher platelet counts than controls [
10]; in AML, Roth spots associate with marked leukocytosis or anemia [
20]. They are largely reversible with hematologic normalization after chemotherapy or transplantation, with VA improvement despite possible residual macular thinning; recovery varies by disease status and age [
10].
Peripheral microaneurysms occur in half of eyes in chronic leukemia and are uncommon in acute leukemia; they are more frequent in chronic myelogenous leukemia (CML) than chronic lymphocytic leukemia (CLL) [
8,
11,
25]. Prolonged leukocytosis can contribute, but findings are inconsistent [
26]. The prevailing mechanism is hyperviscosity from markedly increased leukocytes or platelets, causing flow impairment, peripheral capillary dropout, microaneurysm formation and, rarely, proliferative retinopathy resembling sickle-cell disease [
8,
13].
CRVO is an uncommon leukemic complication, usually linked to hyperviscosity, but it may also result from leukemic infiltration. Infiltration-related occlusions can present with or without disc edema or infiltrates and may even herald CNS involvement (e.g., relapsed AML). Accordingly, retinal vessels occlusions in leukemia warrant evaluation for CNS disease because of prognostic and therapeutic implications [
2].
Neutropenia (disease- or therapy-related) predisposes to opportunistic ocular infections of viral (CMV, HSV, VZV), fungal (
Candida,
Aspergillus), and bacterial origin. Clinical syndromes include necrotizing retinitis, keratitis, uveitis, and endophthalmitis; Candida retinitis may extend into the vitreous with “cotton-ball” opacities, although its incidence has declined with earlier candidemia detection and prompt antifungal therapy [
13].
3.1.2. Choroid
Primary Involvement
Primary choroidal involvement is clinically uncommon, yet histopathology shows choroidal infiltration in up to 65% of patients and in one-third of post-mortem eyes, making the choroid the most frequently affected ocular site, often at relapse and with concurrent CNS or systemic recurrence [
19,
27,
28]. Symptomatic cases usually show posterior serous retinal detachment in AML, driven by reduced choroidal perfusion, RPE ischemia, barrier dysfunction, and fluid-pump failure, with resolution after systemic chemotherapy [
10,
13,
29,
30,
31,
32]. Choroidal disease may also precipitate angle-closure glaucoma [
31,
33]. Only two cases have described choroidal infiltration as the first sign of relapse without CNS or systemic disease [
27,
34].
Secondary Involvement
Indirect choroidal involvement is rare. Retinochoroidal infarction has occasionally been observed during ALL therapy [
35].
3.1.3. Optic Nerve
Primary Involvement
Primary optic nerve involvement in leukemia most often presents with papilledema, typically in association with CNS disease (reported in 13–18%), while histopathology demonstrates optic nerve infiltration in up to one-third of ocular specimens, predominantly in acute leukemia [
13,
36,
37]. Two clinical patterns are described. Prelaminar infiltration appears as superficial, fluffy over the lamina cribrosa, with edema and hemorrhage; vision is often preserved unless there is macular extension. Retrolaminar disease presents as marked disc swelling and hemorrhage with severe vision loss [
8,
9]. Optic nerve disease is frequent during ALL, may herald relapse, and portends poor prognosis, especially when occurring during active treatment [
38,
39,
40]. Management is challenging because the nerve can serve as a sanctuary site; therapy typically combines intrathecal chemotherapy and radiotherapy [
38,
41].
Secondary Involvement
Chemotherapy, antibiotic or radiotherapy toxicity, ischemia due to anemia or hyperviscosity, and opportunistic infections in immunocompromised patients are among the main causes of secondary optic nerve involvement [
39,
42,
43].
3.1.4. Anterior Segment
Primary Involvement
Conjunctival disease may present with hyperemia and edema of the inferior tarsal conjunctiva, occasionally an early sign in ALL, and with perivascular infiltrates or mass-like lesions; rare subconjunctival tumors have been reported in both AML and ALL [
8,
13,
44,
45,
46]. Corneal infiltration is exceptionally uncommon given its avascularity, but when present it may appear as ring ulcers, subepithelial limbal infiltrates, or peripheral ulceration [
8,
13,
47]. Anterior chamber and iris disease may manifest as uveitis, pseudohypopyon, or spontaneous hyphema, most often in relapsed ALL, with occasional iris infiltration causing heterochromia, hypopyon, and secondary glaucoma; slit-lamp evaluation and, when indicated, anterior chamber paracentesis are critical because ocular or CNS involvement in leukemia signals poor prognosis and may necessitate radiotherapy [
8,
13,
48]. Scleral involvement in ALL often localizes around episcleral vessels, is frequently asymptomatic, and has been detected at autopsy; adult T-cell leukemia can mimic scleritis or episcleritis [
49,
50].
Secondary Involvement
Conjunctival changes due to blood disorders are uncommon, though hyperviscosity in chronic leukemias can produce comma-shaped veins [
51]. The most frequent conjunctival pathology is related to graft-versus-host disease (GVHD) after allogeneic transplantation for ALL, typically severe dry eye and keratoconjunctivitis sicca with adverse prognostic implications; chronic GVHD is linked to meibomian gland dysfunction, fibrotic tarsal changes, eyelid malposition, progressive keratinization, and recurrent corneal erosions or ulcers [
13,
52]. Additional conjunctival manifestations may result from antileukemic drugs, such as methotrexate-induced keratoconjunctivitis, or from opportunistic infections favored by immunosuppression [
13,
53]. Chemotherapy, particularly Cytarabine, can cause corneal toxicity by disrupting epithelial DNA synthesis. In GVHD, keratoconjunctivitis sicca is common and may progress to keratitis, ulcers, opacification, or corneal calcification. Immunosuppression further predisposes to infectious keratitis, which can lead to thinning, ulceration, or perforation [
13]. Extramedullary relapse may present with hypopyon uveitis, while anemia or hyperviscosity can induce anterior segment ischemia with edema, inflammation, hypertension, pain, and visual loss, and cataracts may develop from treatment or ischemia [
13,
48]. Opportunistic infectious scleritis may sometimes develop in immunocompromised patients [
13].
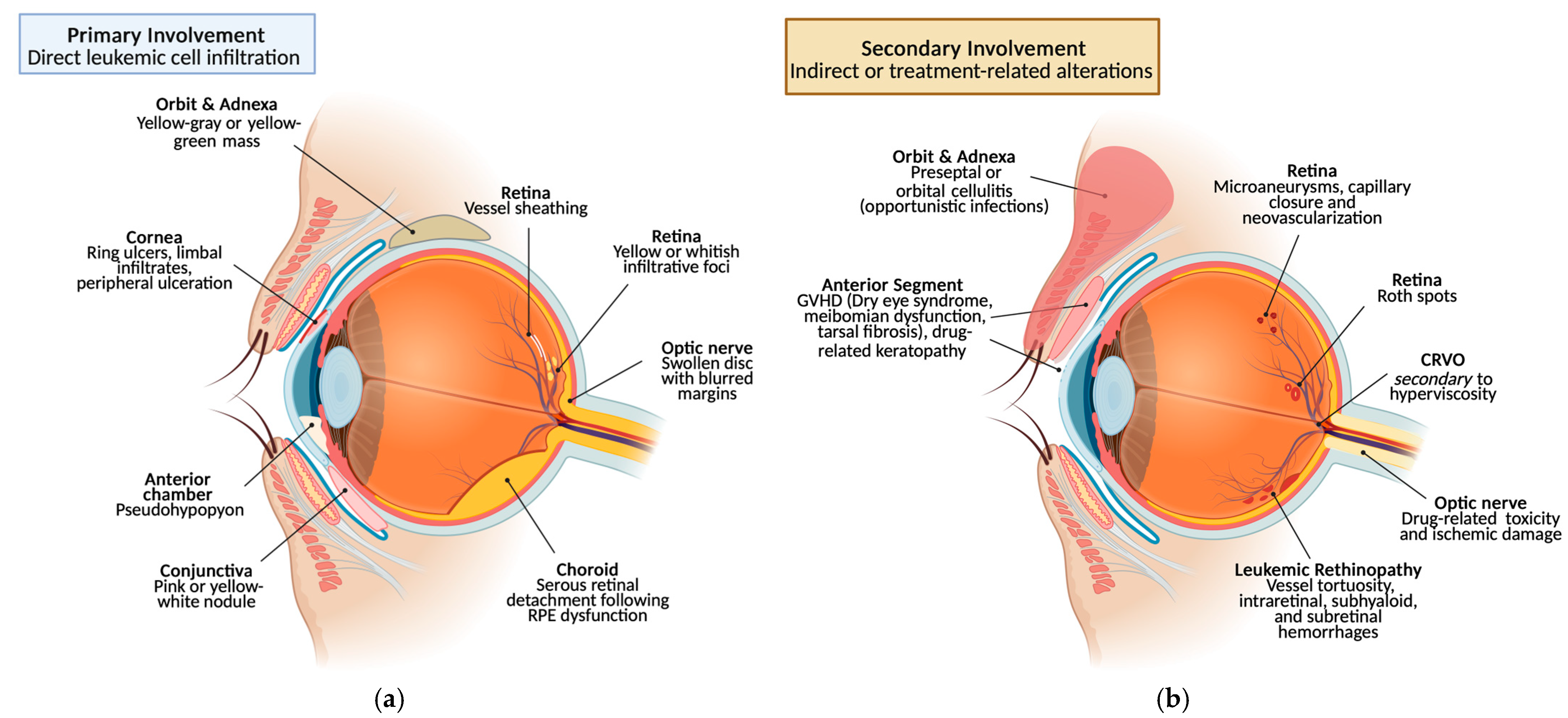
3.1.5. Orbit and Ocular Adnexa
Primary Involvement
In ALL, infiltration of lacrimal glands, eyelids, orbital soft tissues, and extraocular muscles can cause exophthalmos or diplopia; the presentation mirrors other orbital masses and diagnosis requires biopsy. Eyelid involvement may manifest with edema, inflammation, chemosis, and pain [
13]. Myeloid sarcoma is a rare extramedullary tumor occurring in 2.5–9.1% of AML, more frequent in children; it may precede, coincide with, or follow systemic disease, including post-transplant relapse. Imaging is nonspecific and lesions may be poorly differentiated; systemic disease often develops within one year. Proptosis is most common, with ptosis, eyelid swelling, diplopia, or vision loss also reported; lesions are usually unilateral and superior or superotemporal, whereas bilateral disease strongly suggests myeloid sarcoma [
2]. The prognostic impact is debated [
54,
55,
56,
57]; chemotherapy is the mainstay while radiotherapy and early chemotherapy for isolated orbital chloroma show no prognostic benefit [
2].
Secondary Involvement
Orbital involvement may arise post-remission or because of therapy or GVHD. Immunocompromised patients are prone to opportunistic infections. Lacrimal gland infiltration by leukemia, GVHD, or radiation commonly causes severe dry eye with potential corneal complications [
49,
58]. Infectious processes such as cellulitis or dacryocystitis require prompt antibiotic and chemotherapy management [
59] (
Figure 4).
3.2. Ocular Immune Privilege and Leukemia-Related Vascular Involvement
The eye, historically regarded as an immune-privileged organ, preserves this status through local mechanisms that actively suppress inflammation, including reduced MHC expression, anti-inflammatory cytokine release, and T-cell regulation. Immune privilege does not abolish immunity, but establishes a tightly regulated niche that safeguards ocular tissues and supports recovery after injury. Specialized vascular barriers, the blood–aqueous and blood–retinal barriers, preserve ocular homeostasis and antigen isolation but may be disrupted by inflammation or vascular occlusion, facilitating leukemic infiltration [
61,
62,
63]. OCTA studies in acute leukemia show reduced retinal vessel density and capillary dilation, partially reversible after remission, suggesting a link between retinal microcirculation, cytopenia, and bone marrow function. Capillary dilation and disruption of the normal branching architecture in acute leukemia may reflect anemia, decreased perfusion pressure, blast-derived cytokines, or their combined effects [
23]. Moreover, evidence suggests active interactions between circulating leukemic cells and vascular endothelium across multiple organs, contributing to local disease invasion even within protected niches [
64].
3.3. Multiple Signs Association
In clinical practice, ophthalmic presentation may be more complex than the involvement of a single anatomical structure, as primary ocular involvement can coexist with secondary manifestations. This pattern is mainly observed in children, either at onset or relapse, and can present with complex findings such as conjunctival, uveal, choroidal, and orbital infiltration, often associated with proptosis, subconjunctival hemorrhage, or corneal complications. Reported cases include children with multifocal infiltration without systemic disease [
65], bilateral orbital and choroidal lesions with proptosis [
66,
67], and retinal detachment with secondary glaucoma signaling relapse after remission [
68].
3.4. Laterality of Ocular Involvement
In a recent comparative cohort of 244 patients with leukemic ophthalmopathy, ocular involvement at presentation was unilateral in 45% and bilateral in 55%, across all subtypes (ALL, AML, CML, CLL). This distribution supports that direct leukemic infiltration may present unilaterally, whereas bilateral disease remains common overall due to systemic vascular and choroidal involvement. Patterns also differed by subtype, with myeloid leukemias showing more hemorrhagic posterior findings and lymphoid leukemias more non-hemorrhagic anterior or posterior changes [
9].
3.5. Ocular Signs and Prognosis
Ocular involvement in leukemia, and particularly posterior segment infiltration (excluding leukemic retinopathy), is associated with poorer prognosis and a higher risk of bone marrow relapse and CNS involvement [
66,
69]. Indeed, more than 50% of patients with intraocular leukemia develop CNS involvement, with even higher rates observed in those with posterior segment infiltrates [
27].
Leukemic retinopathy is associated with more aggressive disease and poorer outcomes, with survival significantly reduced in patients showing retinal lesions, particularly cotton wool spots [
6,
70,
71]. In pediatric AML and ALL, ocular or orbital involvement correlates with higher rates of bone marrow relapse and CNS infiltration, underscoring its prognostic relevance and impact on overall survival [
72].
4. Discussion
Leukemic ocular involvement is a well-recognized manifestation of the disease, reported with variable frequency in both adult and pediatric populations. Virtually any ocular structure may be affected, either through direct leukemic infiltration or secondary complications. Importantly, ocular manifestations may occur as the initial presenting sign of leukemia or emerge during relapse, and in both settings they have been consistently associated with more aggressive disease and poorer prognosis.
To the best of our knowledge, this is one of the few reported adult cases showing simultaneous orbital, adnexal, choroidal, and retinal involvement, accompanied by hemorrhagic changes in both anterior and posterior segments. This multifocal presentation, involving several ocular structures concurrently, is highly suggestive of leukemic relapse and reflects extensive ocular dissemination rather than isolated infiltration. Previous reports have predominantly described similar concomitant ocular signs in pediatric patients [
65,
66,
67], whereas adult presentations remain exceptionally rare. Chawla et al. [
73] reported a 24-year-old patient with ALL presenting with bilateral blindness due to multiple simultaneous ocular abnormalities, including sclerokeratitis, retinal hemorrhages, and optic nerve thickening. Together with our case, these findings underscore the potential severity and complexity of leukemic ocular involvement beyond childhood.
Several mechanisms may have contributed to the ocular presentation observed in our case. Endothelial injury during the leukocytosis phase may have disrupted the vascular barriers, facilitating leukemic cell migration and localization within ocular tissues. Once sequestered in this sanctuary site, these cells may have been less responsive to systemic chemotherapy. Furthermore, the pancytopenia that followed intensive treatment may have exacerbated vascular fragility and ischemic damage, thereby aggravating the clinical presentation and contributing to the overlap of direct leukemic infiltration with secondary, treatment-related ocular alterations [
23,
64].
Establishing a diagnosis that can effectively guide therapy is particularly challenging when direct and indirect signs overlap. Systemic disease severity, the toxic effects of chemotherapy, and profound immunosuppression may mask or mimic ocular manifestations [
49], while biopsy, often the gold standard, may be precluded due to severe thrombocytopenia and bleeding risk. In this setting, careful ophthalmic examination combined with imaging modalities such as B-scan ultrasonography and MRI plays a pivotal role in raising clinical suspicion and supporting timely recognition of relapse.
The prognostic implications of ocular involvement are considerable. Posterior segment infiltration, in particular, has been linked to higher rates of bone marrow relapse and CNS disease [
27,
66,
69]. Leukemic retinopathy and retinal infiltration have also been correlated with more aggressive disease, reduced survival, and worse outcomes in both adult and pediatric cohorts [
6,
71,
72]. Thus, the detection of simultaneous and multifocal ocular signs should prompt urgent systemic reassessment and therapeutic re-evaluation. It should be noted, however, that most studies on ocular involvement and prognosis in leukemia are retrospective and involve small, heterogeneous cohorts, limiting comparability and generalizability. Thus, while ocular findings seem to correlate with poorer outcomes, these associations should be interpreted with caution until confirmed by larger prospective studies.
In conclusion, this case emphasizes the importance of early recognition of ocular manifestations as a potential sign of systemic relapse. A multidisciplinary approach involving ophthalmologists, hematologists, and oncologists is essential for prompt diagnosis and treatment planning. Ocular involvement, particularly when multifocal, should be regarded as a red flag for faster disease progression, and vigilance is warranted even in patients apparently in hematologic remission.
Author Contributions
Conceptualization, E.M. and M.C.S.; methodology, E.M.; validation, M.M., C.M. and M.A.; investigation: E.M. and M.C.S.; writing—original draft preparation, E.M.; writing—review and editing, M.C.S.; visualization, E.M.; supervision, M.M.; project administration: M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as it describes a single anonymized clinical case.
Informed Consent Statement
Written informed consent was obtained from the patient’s family after the patient’s death, ensuring the anonymous publication of this clinical case and the related images included in this review.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lowenberg, B.; Downing, J.R.; Burnett, A. Acute Myeloid Leukemia. N. Engl. J. Med. 1999, 341, 1051–1062. [Google Scholar] [CrossRef]
- El Salloukh, N.A.; Hage, D.G.; Bashshur, A.Z.; Kheir, W.J. Early Ophthalmological Manifestations of Acute Myeloid Leukemia: Current Perspectives. Clin. Ophthalmol. 2022, 16, 2119–2127. [Google Scholar] [CrossRef]
- Skarsgård, L.S.; Andersson, M.K.; Persson, M.; Larsen, A.-C.; Coupland, S.E.; Stenman, G.; Heegaard, S. Clinical and genomic features of adult and paediatric acute leukaemias with ophthalmic manifestations. BMJ Open Ophthalmol. 2019, 4, e000362. [Google Scholar] [CrossRef]
- Soman, S.; Kasturi, N.; Srinivasan, R.; Vinod, K.V. Ocular Manifestations in Leukemias and Their Correlation with Hematologic Parameters at a Tertiary Care Setting in South India. Ophthalmol. Retin. 2018, 2, 17–23. [Google Scholar] [CrossRef]
- Margulies, L.J. Ocular manifestations of cardiovascular and hematologic disorders. Curr. Opin. Ophthalmol. 1994, 5, 99–104. [Google Scholar] [CrossRef]
- Ohkoshi, K.; Tsiaras, W.G. Prognostic importance of ophthalmic manifestations in childhood leukaemia. Br. J. Ophthalmol. 1992, 76, 651–655. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Rosenthal, A.R. Ocular manifestations of leukemia. A review. Ophthalmology 1983, 90, 899–905. [Google Scholar] [CrossRef]
- Agarwal, D.; Tyagi, M.; Pappuru, R.K.R.; Raval, V. Ophthalmic manifestations and treatment outcomes in leukaemia: A comparative analysis of clinical spectrum, visual prognosis and predictors of disease remission across subtypes. Br. J. Ophthalmol. 2025. [Google Scholar] [CrossRef]
- Yan, B.-J.; Luo, L.-H.; Shen, J.; Huang, Y.-X. Secondary Ocular Manifestations in Acute Leukemia: Clinical Patterns and Hematologic Correlates. J. Multidiscip. Healthc. 2025, 18, 3289–3297. [Google Scholar] [CrossRef]
- Yassin, M.A.; Ata, F.; Mohamed, S.F.; Alkhateeb, A.; Naeem, U.; Al-Qatami, A.I.; Nashwan, A.J.; Fernyhough, L.J. Ophthalmologic manifestations as the initial presentation of chronic myeloid leukemia: A review. Surv. Ophthalmol. 2022, 67, 530–543. [Google Scholar] [CrossRef]
- Ballantyne, A.J.; Michaelson, I.C. Textbook of the Fundus of the Eye; Elsevier Health Sciences: Amsterdam, The Netherlands, 1970. [Google Scholar]
- Mateo, J.; Ascaso, F.J.; Núñez, E.; Peiro, C.; González, G.; Cristóbal, J.A. Ophthalmological manifestations in acute lymphoblastic leukemia. In Novel Aspects in Acute Lymphoblastic Leukemia; IntechOpen: London, UK, 2011. [Google Scholar]
- Hoyos, A.; Lugo, J.C.; Pérez-Vergara, V. Ophthalmic findings leading to diagnose chronic myeloid leukemia: A multimodal image case report. Eur. J. Ophthalmol. 2025, 35, NP58–NP62. [Google Scholar] [CrossRef]
- Vicini, G.; Nicolosi, C.; Malandrino, D.; Tozzetti, C.; Rizzo, S.; Sodi, A. Leukostasis retinopathy with leukemic infiltrates as onset manifestation of chronic myeloid leukemia: A case report. Eur. J. Ophthalmol. 2021, 31, NP116–NP121. [Google Scholar] [CrossRef]
- Primack, J.D.; Smith, M.E.; Tychsen, L. Retinal detachment in a child as the first sign of leukemic relapse: Histopathology, MRI findings, treatment, and tumor-free follow up. J. Pediatr. Ophthalmol. Strabismus 1995, 32, 253–256. [Google Scholar] [CrossRef]
- Schworm, H.D.; Nasemann, J.E.; Schriever, S. Subretinal hypopyon in prolymphocytic leukaemia. Br. J. Ophthalmol. 1995, 79, 863–864. [Google Scholar] [CrossRef]
- Talcott, K.E.; Garg, R.J.; Garg, S.J. Ophthalmic manifestations of leukemia. Curr. Opin. Ophthalmol. 2016, 27, 545–551. [Google Scholar] [CrossRef]
- Green, W.; Rao, P.K.; Harocopos, G.J. Extramedullary Relapse of Acute Myelogenous Leukemia Presenting as a Large Serous Retinal Detachment. Ocul. Oncol. Pathol. 2017, 3, 95–100. [Google Scholar] [CrossRef]
- Guyer, D.R.; Schachat, A.P.; Vitale, S.; Markowitz, J.A.; Braine, H.; Burke, P.J.; Karp, J.E.; Graham, M. Leukemic retinopathy. Relationship between fundus lesions and hematologic parameters at diagnosis. Ophthalmology 1989, 96, 860–864. [Google Scholar] [CrossRef]
- Özdogan, S.; Çelik, A. Başcı S. Evaluation of Factors Associated with Retinal Hemorrhage in Patients with Acute Leukemia. Acta Oncol. Turc. 2022, 55, 174–179. [Google Scholar] [CrossRef]
- Rubenstein, R.A.; Yanoff, M.; Albert, D.M. Thrombocytopenia, Anemia, and Retinal Hemorrhage. Am. J. Ophthalmol. 1968, 65, 435–439. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Mastaglio, S.; Menean, M.; Marchese, A.; Miserocchi, E.; Modorati, G.; Bernardi, M.; Ciceri, F.; Bandello, F. Retinal Microvascular Changes in Patients with Acute Leukemia. Retina 2022, 42, 1762–1771. [Google Scholar] [CrossRef]
- Mastrogiuseppe, E.; Visioli, G.; Albanese, G.M.; Iannetti, L.; Romano, E.; Guillot, A.; Lucchino, L.; Gharbiya, M. Peripapillary and Macular Optical Coherence Tomography Angiography Predictors of Visual Improvement in Patients Treated with Vitrectomy for Idiopathic Epiretinal Membrane. Ophthalmologica 2025, 248, 54–66. [Google Scholar] [CrossRef]
- Almeida, D.R.P.; Chin, E.K.; Grant, L.W. Chronic myelogenous leukemia presenting with bilateral optic disc neovascularization. Can. J. Ophthalmol. 2014, 49, e68–e70. [Google Scholar] [CrossRef]
- Jampol, L.M.; Goldberg, M.F.; Busse, B. Peripheral retinal microaneurysms in chronic leukemia. Am. J. Ophthalmol. 1975, 80, 242–248. [Google Scholar] [CrossRef]
- Kassam, F.; Gale, J.S.; Sheidow, T.G. Intraocular leukemia as the primary manifestation of relapsing acute myelogenous leukemia. Can. J. Ophthalmol. 2003, 38, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Schwartz, R.; Fiedler, W.; Linke, S. Bilateral retinal pigment epithelial detachment as the presenting symptom of acute myeloid leukaemia. Eye 2006, 20, 851–852. [Google Scholar] [CrossRef][Green Version]
- Sharma, H.; Majumder, P.D.; Rao, C.; Biswas, J. A case of Acute Myeloid Leukemia masquerading as unilateral exudative detachment. Am. J. Ophthalmol. Case Rep. 2016, 4, 47–49. [Google Scholar] [CrossRef]
- Naseripour, M.; Abdolalizadeh, P.; Abdi, F.; Mehrvar, A.; Tashvighi, M. Serous retinal detachment as an initial presentation of childhood acute myeloid leukemia. Can. J. Ophthalmol. 2019, 54, e170–e173. [Google Scholar] [CrossRef]
- Patel, A.V.; Miller, J.B.; Nath, R.; Shih, H.A.; Yoon, M.K.; Freitag, S.K.; Papaliodis, G.; Chen, T.C.; Eliott, D.; Kim, I.K. Unilateral Eye Findings: A Rare Herald of Acute Leukemia. Ocul. Oncol. Pathol. 2016, 2, 166–170. [Google Scholar] [CrossRef]
- Wu, L.; Calderón, M.; Hernández, G.; Marbis, J.; Ramírez, V. Bilateral exudative retinal detachment as the first sign of relapsing acute myelogenous leukaemia. Clin. Exp. Ophthalmol. 2006, 34, 623–625. [Google Scholar] [CrossRef]
- Tumuluri, K.; Woo, T.; Crowston, J.; Healey, P.R.; Gottlieb, D.; Maloof, A.J. Bilateral Leukemic Orbital Infiltration Presenting as Proptosis and Narrow-Angle Glaucoma. Ophthalmic Plast. Reconstr. Surg. 2004, 20, 248–250. [Google Scholar] [CrossRef]
- Uozumi, K.; Takatsuka, Y.; Ohno, N.; Hanada, S.; Tabata, Y.; Arimura, H.; Nakao, K.; Arima, T. Isolated choroidal leukemic infiltration during complete remission. Am. J. Hematol. 1997, 55, 164–165. [Google Scholar] [CrossRef]
- Kato, Y.; Takano, Y.; Kobayashi, M.; Ito, F.; Hara, T.; Yanagisawa, T.; Hoshi, Y.; Eto, Y. Retinochoroidal infarction during the treatment of acute lymphoblastic leukemia. Pediatr. Int. 2006, 48, 495–497. [Google Scholar] [CrossRef]
- Allen, R.A.; Straatsma, B.R. Ocular Involvement in Leukemia and Allied Disorders. Arch. Ophthalmol. 1961, 66, 490–508. [Google Scholar] [CrossRef]
- Rahman, E.Z.; Ellis, M.; Pardee, T.; Shah, R. Non-Blast Phase Chronic Myelogenous Leukemia Presenting as Bilateral Infiltration of the Optic Nerve. Ophthalmic Surg. Lasers Imaging Retin. 2025, 56, 102–107. [Google Scholar] [CrossRef]
- Curto, M.L.; D’Angelo, P.; Jankovic, M.; Fugardi, M.G.; Ziino, O.; Casale, F. Isolated ocular relapse in childhood acute lymphoblastic leukemia during continuing complete remission. Haematologica 1996, 81, 47–50. [Google Scholar]
- Bhatt, U.K.; Gregory, M.E.; Madi, M.S.; Fraser, M.; Woodruff, G.H.A. Sequential leukemic infiltration and human herpesvirus optic neuropathy in acute lymphoblastic leukemia. J. AAPOS 2008, 12, 200–202. [Google Scholar] [CrossRef]
- Schocket, L.S.; Massaro-Giordano, M.; Volpe, N.J.; Galetta, S.L. Bilateral optic nerve infiltration in central nervous system leukemia. Am. J. Ophthalmol. 2003, 135, 94–96. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Das, D.; Das, G.; Gayen, S. Unilateral optic nerve infiltration as an initial site of relapse of acute lymphoblastic leukemia in remission. Oman J. Ophthalmol. 2010, 3, 153–154. [Google Scholar] [CrossRef]
- Joshi, L.; Taylor, S.R.J.; Large, O.; Yacoub, S.; Lightman, S. A case of optic neuropathy after short-term linezolid use in a patient with acute lymphocytic leukemia. Clin. Infect. Dis. 2009, 48, e73–e74. [Google Scholar] [CrossRef][Green Version]
- Cleveland, K.O.; Gelfand, M.S. Optic neuropathy following linezolid use in a patient with acute lymphocytic leukemia. Clin. Infect. Dis. 2009, 49, 645–646. [Google Scholar] [CrossRef]
- Cook, B.E.; Bartley, G.B. Acute lymphoblastic leukemia manifesting in an adult as a conjunctival mass. Am. J. Ophthalmol. 1997, 124, 104–105. [Google Scholar] [CrossRef]
- Nau, J.A.; Shields, C.L.; Shields, J.A.; Eagle, R.C.; Rice, E. Clinicopathologic reports, case reports, and small case series: Acute myeloid leukemia manifesting initially as a conjunctival mass in a patient with acquired immunodeficiency syndrome. Arch. Ophthalmol. 2002, 120, 1741–1742. [Google Scholar] [CrossRef]
- Lei, K.I.; Liew, C.T.; Lam, D.S.; Chan, A.T.; Wickham, N.W. Acute monoblastic leukaemia with conjunctival tumours. Clin. Oncol. R. Coll. Radiol. 1995, 7, 405–406. [Google Scholar] [CrossRef]
- Eiferman, R.A.; Levartovsky, S.; Schulz, J.C. Leukemic corneal infiltrates. Am. J. Ophthalmol. 1988, 105, 319–320. [Google Scholar] [CrossRef]
- Yu, A.M.; Chan, S.C.; Iordanous, Y.; Padmore, R.F.; O’Connor, M.D. Anterior segment infiltration of acute lymphoblastic leukemia: Case report and systematic review. Can. J. Ophthalmol. 2019, 54, 20–26. [Google Scholar] [CrossRef]
- Sharma, T.; Grewal, J.; Gupta, S.; Murray, P.I. Ophthalmic manifestations of acute leukaemias: The ophthalmologist’s role. Eye 2004, 18, 663–672. [Google Scholar] [CrossRef]
- Burton, B.J.L.; Cunningham, E.T.; Cree, I.A.; Pavesio, C.E. Eye involvement mimicking scleritis in a patient with chronic lymphocytic leukaemia. Br. J. Ophthalmol. 2005, 89, 775–776. [Google Scholar] [CrossRef]
- Swartz, M.; Jampol, L.M. Comma-shaped venular segments of conjunctiva in chronic granulocytic leukemia. Can. J. Ophthalmol. 1975, 10, 458–461. [Google Scholar]
- Ogawa, Y.; Kuwana, M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea 2003, 22, S19–S27. [Google Scholar] [CrossRef]
- Cogan, D.G. Immunosuppression and eye disease. First Vail lecture. Am. J. Ophthalmol. 1977, 83, 777–788. [Google Scholar] [CrossRef]
- Cavdar, A.O.; Arcasoy, A.; Babacan, E.; Gözdaşoğlu, S.; Topuz, U.; Fraumeni, J.F. Ocular granulocytic sarcoma (chloroma) with acute myelomonocytic leukemia in Turkish children. Cancer 1978, 41, 1606–1609. [Google Scholar] [CrossRef]
- Yaghouti, F.; Nouri, M.; Mannor, G.E. Ocular adnexal granulocytic sarcoma as the first sign of acute myelogenous leukemia relapse. Am. J. Ophthalmol. 1999, 127, 361–363. [Google Scholar] [CrossRef]
- Zimmerman, L.E.; Font, R.L. Ophthalmologic manifestations of granulocytic sarcoma (myeloid sarcoma or chloroma). The third Pan American Association of Ophthalmology and American Journal of Ophthalmology Lecture. Am. J. Ophthalmol. 1975, 80, 975–990. [Google Scholar] [CrossRef]
- Bidar, M.; Wilson, M.W.; Laquis, S.J.; Wilson, T.D.; Fleming, J.C.; Wesley, R.E.; Ribeiro, R.C.; Haik, B.G. Clinical and imaging characteristics of orbital leukemic tumors. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 87–93. [Google Scholar] [CrossRef]
- Im, S.-K.; Yoon, K.-C. Corneal perforation with preseptal cellulitis in a patient with acute lymphocytic leukemia. J. Korean Med. Sci. 2010, 25, 1251–1252. [Google Scholar] [CrossRef]
- Wirostko, W.J.; Garcia, G.H.; Cory, S.; Harris, G.J. Acute dacryocystitis as a presenting sign of pediatric leukemia. Am. J. Ophthalmol. 1999, 127, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiuseppe, E. 2025. Available online: https://BioRender.com (accessed on 1 September 2025).
- Benhar, I.; London, A.; Schwartz, M. The privileged immunity of immune privileged organs: The case of the eye. Front. Immunol. 2012, 3, 296. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W. Ocular immune privilege: The eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 2003, 74, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Katamay, R.; Nussenblatt, R.B. Blood–retinal barrier, immune privilege, and autoimmunity. Retina 2012, 579–589. [Google Scholar]
- Fodil, S.; Arnaud, M.; Vaganay, C.; Puissant, A.; Lengline, E.; Mooney, N.; Itzykson, R.; Zafrani, L. Endothelial cells: Major players in acute myeloid leukaemia. Blood Rev. 2022, 54, 100932. [Google Scholar] [CrossRef]
- Kiratli, H.; Bilgiç, S.; Emeç, S. Simultaneous Conjunctival, Uveal, and Orbital Involvement as the Initial Sign of Acute Lymphoblastic Leukemia. Jpn. J. Ophthalmol. 2007, 51, 139–141. [Google Scholar] [CrossRef]
- Khaja, W.A.; Pogrebniak, A.E.; Bolling, J.P. Combined orbital proptosis and exudative retinal detachment as initial manifestations of acute myeloid leukemia. J. AAPOS 2015, 19, 479–482. [Google Scholar] [CrossRef]
- Susiyanti, M.; Mawarasti, B.; Kumalasari, S.R. Bilateral Orbital Proptosis and Corneal Ulcer in a Child with Acute Myeloid Leukemia. Case Rep. Ophthalmol. 2022, 13, 191–195. [Google Scholar] [CrossRef]
- Reddy, S.C. Unilateral bullous retinal detachment in a child with acute lymphoblastic leukaemia, and hypopyon as the first sign of leukaemic relapse. Int. J. Ophthalmol. 2018, 11, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.-Y.; Chen, Y.-C.; Lin, Y.-Y.; Chu, S.-J.; Tsai, S.-H. Simultaneous bilateral central retinal vein occlusion as the initial presentation of acute myeloid leukemia. Am. J. Med. Sci. 2010, 339, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.L.; Zingone, A.; Acquaviva, A.; Bagnulo, S.; Calculli, L.; Cristiani, L.; Dini, G.; Di Tullio, M.T.; Guazzelli, C.; Jancovic, M.; et al. Leukemic infiltration of the eye: Results of therapy in a retrospective multicentric study. Med. Pediatr. Oncol. 1989, 17, 134–139. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Al-Momen, A.K.; Kangave, D.; Harakati, M.S. Prognostic importance of retinopathy in acute leukemia. Doc. Ophthalmol. 1995, 91, 273–281. [Google Scholar] [CrossRef]
- Russo, V.; Scott, I.U.; Querques, G.; Stella, A.; Barone, A.; Delle Noci, N. Orbital and Ocular Manifestations of Acute Childhood Leukemia: Clinical and Statistical Analysis of 180 Patients. Eur. J. Ophthalmol. 2008, 18, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Chawla, B.; Agarwal, P.; Tandon, R.; Titiyal, J.S. Peripheral ulcerative keratitis with bilateral optic nerve involvement as an initial presentation of acute lymphocytic leukemia in an adult. Int. Ophthalmol. 2009, 29, 53–55. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).