Abstract
Introduction: Botulinum toxin-A (BTX-A) injections are regularly used to treat temporomandibular disorders (TMD). However, consensus regarding the long-term efficacy of BTX-A for TMD-related myalgia remains lacking. This pragmatic, practice-based clinical study aimed to evaluate the Patient-Reported Outcome Measures of pain, health status, quality of life, and function after BTX-A injections in patients with TMD-related myalgia. Methods: This prospective cohort study included 35 patients with TMD-related myalgia who received BTX-A injections in the masseter and temporalis muscles. The Visual Analogue Scale for pain, the EQ-5D-3L for health status, the Oral Health Impact Profile-14 for oral health-related quality of life, the Mandibular Function Impairment Questionnaire for function and the maximum interincisal opening were assessed before treatment and at one, three and six months follow-up. Results: Patients reported a statistically significant and clinically relevant reduction in pain (p < 0.001), improvement of health status (p ≤ 0.003), and oral health-related quality of life (p < 0.001) at one-month follow-up, which remained present at three and six months post-treatment. Self-reported mandibular function and active and passive mouth opening showed no significant change over all time points. Conclusions: In this pragmatic cohort, BTX-A injections in the masseter and temporalis muscles seem to improve pain and oral health-related quality of life in patients with TMD-related myalgia within one month and show effects lasting up to six months, while mandibular function did not improve.
1. Introduction
Temporomandibular disorders (TMD) describe a group of disorders involving the temporomandibular joint (TMJ), masticatory muscles, and associated structures [1] and are reported to affect 10% to 30% of the global population [2]. Etiological factors contributing to TMD can be psychological, biological, and biomechanical [3]. According to the worldwide-accepted diagnostic criteria for temporomandibular disorders (DC/TMD), TMD are divided into two subgroups: articular disorders with signs and symptoms related to the TMJ, and pain related disorders with signs and symptoms related to muscular pain (myalgia) and headache [4].
In most cases of TMD, myalgia of the masticatory muscles is observed, which is frequently accompanied by restricted jaw opening and impaired oral function [5,6]. Consequently, TMD-related myalgia is associated with a reduced quality of life, psychological disorders (e.g., depression and anxiety), and may result in chronicity [7]. Current management for TMD-related myalgia includes the avoidance of triggers, behavioural techniques, decreasing jaw muscle activity through a soft diet, physical therapy, dental review for occlusal splints, and simple analgesia [1]. However, the long-term efficacy of these therapeutic modalities remains unclear, resulting in the search for other therapeutic interventions [8]. When TMD-related myalgia is persistent and does not respond to these conservative treatment options, therapy with Botulinum toxin-A (BTX-A) may be considered [1,6].
There is a growing use of BTX-A injections in the masticatory muscles as an intervention for TMD-related myalgia [9]. BTX-A results in muscle relaxation by temporarily blocking the release of acetylcholine from presynaptic cholinergic nerve terminals. The injected muscle remains paralyzed until new synaptic connections are formed through sprouting [10]. Additionally, BTX-A is reported to have antinociceptive effects by blocking the release of inflammatory mediators, such as substance P and glutamate [10,11]. The effects of BTX-A are reported to last two to four months [12]. Due to these muscle-relaxing and possible analgesic effects, BTX-A has gained increased interest as a possible treatment for TMD-related myalgia [13].
Studies have shown short-term improvements in Patient-Reported Outcome Measures (PROM’s) (up to three months) in pain intensity, function, and quality of life after BTX-A injections in the masseter and temporalis muscles in patients with TMD-related myalgia [14,15,16]. A systematic review by Thambar et al. [1] reported mixed short-term results: four studies showed significant pain reduction up to one or two months [17,18,19,20], while two others found no effect at three months [21,22].
Long-term outcomes (>three months) remain unclear [1,23]. One randomized controlled trial (RCT) reported lower pain scores at 12 months compared to other treatments [24]. A meta-analysis by Machado et al. [25] found no difference between BTX-A and placebo at three and six months suggesting that placebo responses may contribute to the observed effects, complicating the interpretation of BTX-A efficacy. An umbrella review by De la Torre Canales et al. [26] concluded that BTX-A is more effective than placebo for pain, but not superior to standard care, and highlighted potential adverse effects such as muscle atrophy and jawbone changes. Only one included study assessed outcomes beyond six months [27]. Importantly, most studies focused on pain, neglecting oral health-related quality of life [25,26]. A study by Li et al. [28] reported a reduction in pain intensity at six months following BTX-A treatment for TMD-related pain. However, Li et al. [28] included a relatively small sample size and a heterogeneous population with myogenous and arthrogenic TMD as well as bruxism-related complaints, which may limit the generalizability specifically to TMD-related myalgia. Moreover, while Li et al. [28] primarily evaluated pain reduction, the impact on quality of life and self-reported functional outcomes was not addressed.
The recent literature has emphasized that current evidence remains fragmented and that the clinical application of BTX-A for TMD-related myalgia should primarily be regarded as an adjuvant therapy or as a last treatment alternative [26,29]. Nevertheless, consensus regarding the efficacy of BTX-A for TMD-related myalgia remains lacking due to the inconsistent diagnostic criteria, variable dosages, potential side effects, and limited follow-up data [1,10,13,26,28]. Therefore, studies with extended follow-up that evaluate multidimensional outcomes such as pain, quality of life, and mandibular function are needed to guide clinical decision-making regarding BTX-A use in TMD-related myalgia.
Furthermore, by adopting a design that reflects practice-based clinical treatment conditions, where BTX-A is typically used as an adjunctive rather than a standalone therapy, this study aims to provide clinically relevant insights.
The aim of this study was to evaluate the clinical course of patients with TMD-related myalgia treated with BTX-A injections over a six-month period, focusing on pain intensity, health status, quality of life, and mandibular function within a pragmatic context. The hypothesis tested was that patients receiving BTX-A due to TMD-related myalgia would report a reduction in pain and increase in quality of life and mandibular function after one, three, and six months.
2. Materials and Methods
The study design was a prospective observational cohort study. The Medical Research Ethics Committees United provided a non-WMO (Medical Research Involving Human Subject Act) waiver (W23.212) prior to commencement of this study. All participants were informed and gave their written consent prior to participating, according to the requirements of the Declaration of Helsinki (World Medical Association, 2013).
2.1. Participants
Participants were recruited between January and August 2024 from the Department of Oral and Maxillofacial Surgery (OMS) at the Diakonessenhuis, Utrecht, The Netherlands.
Inclusion criteria for participation were the following:
- Adults with TMD-related myalgia in accordance with the DC/TMD;
- Acceptance of BTX-A injections as treatment;
- Ability to understand and complete questionnaires in Dutch.
Exclusion criteria were the following:
- Systemic inflammatory and connective tissue diseases (e.g., rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis);
- Surgery in the TMJ area < 12 months ago;
- Pain of dental origin;
- Limited cognitive functioning (not allowed to make medical decisions);
- Use of muscle relaxants or aminoglycoside antibiotics;
- A history of allergic reactions to BTX;
- Pregnancy and lactation.
All measurements were conducted between January 2024 and February 2025.
Prior BTX-A treatment was not an exclusion criterion, which may have influenced expectations and treatment response.
2.2. Study Procedure
Participants who were willing to enrol in the study received an information letter. Those who agreed to participate provided written informed consent prior to inclusion in the study. During the first visit, participants completed a standardized form detailing their demographic information, health history, previous treatments for TMD-related myalgia, and current symptoms. The researcher then checked the inclusion and exclusion criteria. Prior to the BTX-A injection, participants rated their current pain on a Visual Analogue Scale (VAS) [30]. Next, they completed the European Quality of Life 5 Dimensions 3 Level Version (EQ-5D-3L) [31], the Oral Health Impact Profile-14 (OHIP-14) [32], and the Mandibular Function Impairment Questionnaire (MFIQ) [33]. Then, the researcher measured the maximum active and passive mouth opening according to the DC/TMD.
2.3. Description of Measuring Instruments and Questionnaires
The VAS, which measures pain intensity, consists of a 100 mm line with two set points: 0 (indicating no pain) and 10 (indicating worst imaginable pain). The participants had to indicate their current level of pain by placing a mark on the line [30]. The minimal clinical difference in patients with chronic TMD-related pain is 19.5 mm [34].
The EQ-5D-3L questionnaire was used to assess the participants health status [31]. The participants rated their health on a 3-point Likert scale across five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQ-5D index, which ranges from less than 0 (worse health state) to 1 (perfect health), was calculated based on the Dutch population. Participants were also asked to rate their current general health status on a VAS-scale, ranging from 0, the worst possible health, to 100, the best possible health [31].
The OHIP-14 questionnaire assesses the oral health-related quality of life with answer options on a 5-point Likert scale, ranging from 0 (never) to 4 (very often). The total OHIP-14 score ranges from 0 to 56, with higher scores indicating more severe outcomes [32]. The smallest detectable difference (SDD) is 5 points [32,35].
Mandibular function was assessed by the sum score of the 17-item Dutch translation of the MFIQ [33]. Each item is scored on a 5-point Likert scale with score 0 indicating no difficulty and score 4 being extremely difficult to perform a mandibular task, resulting in a total score ranging from 0 to 68. Higher scores are indicative of increased levels of functional impairment. The SDD of the MFIQ is 10 points [33].
Maximal mouth opening was measured as the distance between the incisal edges of the central incisors in millimetres. This measurement was conducted using a metal ruler for both the active (AMO) and passive (PMO) mouth opening according to the DC/TMD [4]. The mean of three measurements was used [36]. The SDD in patients with painful restriction of the TMJ is 3 mm [36].
All measures were assessed prior to the BTX-A injection (T0) and at one (T1), three months (T2), and six months (T3) post-treatment by the same researcher (M.A.). The researcher was not involved in any treatment session. All measurements were conducted without the presence of the healthcare practitioner providing the injections to avoid observer bias due to the patient–doctor relationship. At all post-treatment measurements, the exclusion criteria were re-evaluated by asking the participants if they had other treatments for their TMD-related myalgia.
2.4. BTX-A Injections
All participants received a solution of BTX-A (Xeomin, Merz Pharma GmbH, Frankfurt am Main, Germany) in their masseter and temporalis muscles (total of 100 units reconstituted with 4cc unpreserved 0.9% sodium chloride). The injection was administered by the same maxillofacial surgeon (M.d.R.). The participants were asked to clench their teeth to determine the anatomical position of the masseter and temporal muscles with palpation. Intramuscular BTX-A injections were administered on both sides at the location of the masseter muscle (80 units), distributed in three different locations, and temporalis muscle (20 units), distributed in one location (Figure 1).
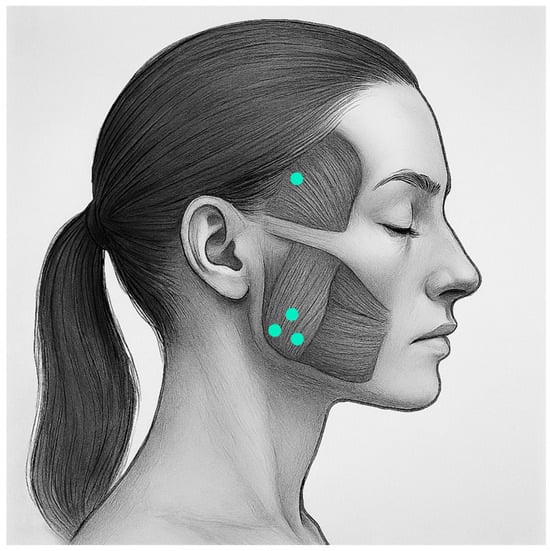
Figure 1.
Location of BTX-A injections.
This technique was similar to other studies using BTX-A for the masseter and temporal muscles [17,20,37].
2.5. Statistical Analyses
All data were considered as metric values and were tested for normality of distribution using the Shapiro–Wilk Test and visual inspection of the histograms. Normally distributed data (p > 0.05) were presented as mean ± standard deviation, while non-normally distributed data were presented as median and (interquartile range 25–75%). Differences between T0, T1, T2, and T3 were analyzed using a repeated-measures ANOVA model for normally distributed data, and the Friedman test was applied in case of non-normally distributed data. In cases where significant outcomes were found, post hoc tests with Bonferroni corrections for multiple comparisons were applied.
A priori power analysis (G*Power 3.1) determined that a total of 30 participants were needed to achieve sufficient power (>90%) for detecting a pre–post difference on the VAS (Cohen’s d = 0.73) at α = 0.05. This power analysis assumes a known population mean of 65 mm with a standard deviation of 11 mm on the VAS [38]. The anticipated mean VAS for the study population was projected at 57 mm. This power calculation allows for an attrition rate of 10%. The alpha level for statistical significance was set at 0.05. All statistical analysis were performed using SPSS Statistics version 28.0.1.0 (IBM, Armonk, NY, USA).
3. Results
A total of 52 patients with TMD-related myalgia were invited to participate. Of these, 44 (85%) participated in this study. Nine participants were excluded during the study. Three patients were lost to follow-up, three patients received a new BTX-A treatment before the final follow-up, two participants became pregnant, and one participant was diagnosed with dental-related pain, which prevented them from further participation according to the exclusion criteria (Figure 2).
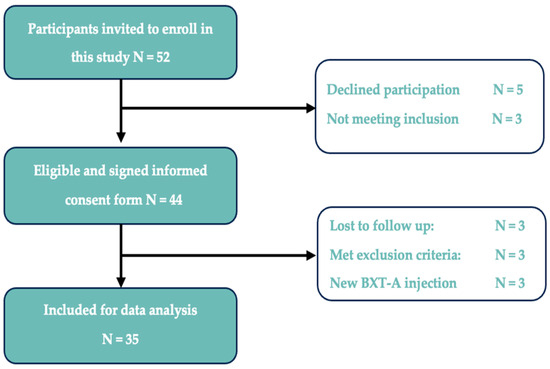
Figure 2.
Flow chart of study inclusion/exclusion.
The 35 remaining participants (32 female, 3 male) completed all measurements. The characteristics of the participants are summarized in Table 1. All participants had previously received treatments for their TMD-related myalgia, including oral splint therapy, physical therapy, medication, or previous BTX-A injections.

Table 1.
Participants characteristics.
Concurrent conservative therapies were recorded at baseline and monitored throughout follow-up. Minor individual changes were observed over time (Appendix B, Table A1), but these did not affect the primary outcome results.
Outcomes for the VAS, EQ-5D-3L, OHIP-14, MFIQ, AMO, and PMO at T0, T1, T2, and T3 are presented in Table 2.

Table 2.
Outcomes at baseline (T0), 1 month (T1), 3 months (T2), and 6 months (T3) (n = 35).
The median VAS scores revealed a statistically significant difference (χ2 = 25.435, p < 0.001) over time. Post hoc analysis (Table 3a) revealed significant reductions in median pain at T1 (Z = −4.38, p < 0.001), T2 (Z = −3.58, p < 0.001), and T3 (Z = −4.13, p < 0.001), compared with T0. No significant differences in median pain were observed between T1, T2, and T3.

Table 3.
(a) Pairwise comparisons of outcome measures between T0, T1, T2, and T3 a. (b) Pairwise comparisons of outcome measures between T0, T1, T2, and T3.
The median health status scores, as measured by the EQ-5D index scores, showed a statistically significant difference (χ2 = 14.598, p = 0.002) over time. Post hoc analysis (Table 3a) revealed a significant increase in health status at T1 (Z = −2.94, p = 0.003), T2 (Z = −3.43, p < 0.001), and T3 (Z = −3.01, p = 0.003), compared with T0. No significant differences in health status were observed between T1, T2, and T3. There were no significant mean differences in the EQ VAS over time (F (3, 102) = 1.751, p = 0.161).
The median OHIP-14 scores showed a significant difference over time (χ2 = 43.659, p = < 0.001). Post hoc analysis (Table 3a) revealed a significant median improvement of oral health-related quality of life at T1 (Z = −4.88, p < 0.001), T2 (Z = −4.47, p < 0.001), and T3 (Z = −4.27, p < 0.001), compared with T0. No significant differences in oral health-related quality of life were observed between T1, T2 and T3.
The median MFIQ scores showed a significant difference over time (χ2 = 8.672, p = 0.034). However, post hoc analysis with Bonferroni corrections (Table 3a) revealed no significant pairwise differences (p > 0.008). Similarly, the mean AMO showed a significant increase over time (F (2.156, 73.298) = 3.476, p = 0.033) but no significant pairwise differences during post hoc analysis (p > 0.008) (Table 3b). The mean PMO showed no significant increase over time (F (2.167, 73.507) = 2.334, p = 0.1).
One adverse effect has been reported during this study. A participant experienced mild asymmetry when smiling two days after receiving the BTX-A injections, which resolved completely within 12 weeks. No other adverse effects were reported.
4. Discussion
This prospective study evaluated the outcomes of BTX-A injections on pain, health status, oral health-related quality of life, and mandibular function in patients with TMD-related myalgia. We observed a significant and clinically relevant reduction in pain, as well as an improvement in health status and oral health-related quality of life at one month post-treatment, which was sustained until six months. No significant improvement was found in mandibular function or active and passive mouth opening. These findings support the positive outcomes of BTX-A injections for TMD-related myalgia with regard to pain and oral health-related quality of life up to six months, while mandibular function outcomes remain ambiguous.
These findings align with previous research demonstrating short-term improvements in pain and quality of life after BTX-A treatment [16,17,19,21,39,40] and extend these results to six months. A systematic review by Li et al. [28] showed similar results with a significant reduction in pain six months after BTX-A compared to placebo. However, the placebo-controlled, cross-over trial by Sitnikova et al. [40] showed that both placebo and BTX-A injections significantly reduced pain intensity and pain related disability at three and four months post-treatment. The discrepancy between the study of Li et al. [28] and Sitnikova et al. [40] may be explained by the lower dosage of BTX-A (50 units) but also reflects broader methodological issues such as heterogeneity in diagnostic criteria, variation in BTX-A dosage protocols, and the influence of placebo effects [29,40]. These findings highlight the need for standardized treatment protocols and further research comparing BTX-A with other therapeutic modalities and placebo.
No significant difference in mandibular function was observed across MFIQ, AMO, and PMO outcomes, consistent with previous studies [21,22]. While De la Torre Canales et al. [27] reported significant improvements in AMO and PMO after six months post BTX-A treatment, they found no significant improvements after one month. The delayed improvement in mouth opening suggests that these changes may be due to the normalization of mandibular function over time rather than directly being induced by the effects of BTX-A. Additionally, the assessment of the mandibular function may have been affected by the paralyzing effects of BTX-A, potentially resulting in higher impairment scores on the MFIQ due to reduced maximal biting forces when consuming tough and hard foods [41,42]. In light of the conflicting evidence on the efficacy of BTX-A in improving mandibular function, inclusion criteria for BTX-A injections in TMD-related myalgia should consider factors such as pain severity and quality of life.
All participants in this study had previously undergone conservative treatments such as physical therapy, oral splint use, or medication before receiving BTX-A, consistent with current recommendations [26]. However, there is no clear consensus on when conservative treatments should be considered insufficient, or when treatment with BTX-A should be initiated. While concurrent therapies were monitored, occlusal and prosthetic factors were not assessed. Although occlusal abnormalities have been associated with TMD, evidence for a causal relationship and specifically for their role in TMD-related myalgia remains unclear [43].
The treatment dosage of BTX-A varies widely in the literature [1,13,28]. Li et al. [28] showed that a bilateral dose of 60–100 units might be an optimal choice for treating pain in TMD-related myalgia. This variability underscores the need for standardized clinical guidelines and treatment steps defining dosage and injection protocols to ensure both efficacy and safety.
Repeated BTX-A injections are common due to their limited duration of effect (two to four months) [10,12,44], yet evidence on the efficacy and safety of repeated treatments of BTX-A for TMD-related myalgia is scarce. In this study, 37% of the participants had previously received BTX-A treatments, and three participants required repeated BTX-A due to an increase in pain levels. Future studies should evaluate the effects of repeated BTX-A treatments on pain, quality of life, and mandibular function, while also considering potential cumulative risks such as jawbone changes [26]. Long-term data on repeated injections are needed to determine whether treatment efficacy is maintained or diminishes over time. Cost-effectiveness should also be considered, particularly when repeated treatments offer limited additional benefit, and the cumulative costs may outweigh the clinical gains.
We acknowledge several limitations in our study. First, 37% of the participants had received prior BTX-A treatment. This may have introduced bias through altered expectations of efficacy or potential physiological carry-over effects, which could have influenced the outcomes and limited the generalizability. Moreover, TMD-related myalgia has a higher prevalence in women than in men [2]. Given that 91% of our study population was female, the findings may have limited external validity for the male population. Participants were not asked to discontinue concurrent conservative therapies such as splint use, physical therapy, or medication, reflecting a pragmatic design but introducing potential confounding. This approach mirrors routine clinical practice, where BTX-A is commonly used as an adjunctive rather than a standalone therapy, but it limits attribution of outcomes solely to BTX-A. Three patients were excluded as they required additional BTX-A treatment for severe pain, which may have led to the exclusion of high pain scores. Furthermore, the total scores of the OHIP-14 and MFIQ were treated as continuous variables in the analysis, consistent with previous research [16,45,46], although the individual items of these instruments are ordinal. Lastly, as this was a prospective observational cohort study, no causal conclusions can be drawn regarding the effects of BTX-A for TMD-related myalgia.
Despite these limitations, the study has several strengths. The pragmatic design reflects routine clinical practice, enhancing the clinical relevance of the results. Moreover, the six-month follow-up period contributes to understanding the long-term outcomes of BTX-A treatment for TMD-related myalgia. Furthermore, the sample size (n = 35) met the required threshold based on the power calculation, enhancing the reliability of the findings. Additionally, by including quality of life, the results broaden the scope of outcomes beyond pain and functional impairment in TMD-related myalgia. Given the limited knowledge on the clinical outcomes of quality of life after BTX-A, these findings may provide valuable insights into its clinical application in treating TMD-related myalgia.
Future research should prioritize high-quality randomized controlled trials with follow-up periods exceeding three months and a more balanced gender distribution to better assess BTX-A effectiveness. Comparative studies evaluating BTX-A against other treatment modalities, such as physical therapy, oral appliances, or pharmacological interventions, are needed to establish a more comprehensive treatment approach for TMD-related myalgia. Moreover, future studies with sufficient power should also consider stratifying outcomes based on prior BTX-A treatment and concurrent conservative therapies to enhance clinical transferability and guide the development of more personalized treatment approaches for patients with TMD-related myalgia. The lack of data on the cost-effectiveness of BTX-A for TMD-related myalgia highlights the need for further investigation in this area in clinical decision-making.
Given the current findings, BTX-A injections could be a valuable treatment option for reducing pain and improving health status and oral health-related quality of life in patients with TMD-related myalgia up to six months post-treatment. The lack of improvement in mandibular function should be taken into consideration when evaluating BTX-A treatment for TMD-related myalgia.
Author Contributions
Conceptualization, M.v.S., L.R., and M.d.R.; methodology, M.v.S., L.R., and M.d.R.; software, M.v.S.; validation, M.v.S., M.d.R. and I.T.; formal analysis, M.v.S. and L.R.; investigation, M.v.S.; resources, M.v.S., L.R., and M.d.R.; data curation, M.v.S. and M.d.R.; writing—original draft preparation, M.v.S.; writing—review and editing, L.R., I.T., L.K., and E.v.d.H.; visualization, M.v.S.; supervision, M.d.R.; project administration, M.v.S.; funding acquisition, M.d.R. and M.v.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Diakonessenhuis research grant 2024, grant number 32.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of The Medical Research Ethics Committees United waiver (W23.212) for studies involving humans on 22 October 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BTX-A | Botulinum toxin-A |
| TMD | Temporomandibular disorders |
| TMJ | Temporomandibular joint |
| DC/TMD | Diagnostic criteria for temporomandibular disorders |
| RCT | Randomized controlled trial |
| OMS | Oral and maxillofacial surgery |
| EQ-5D-3L | European Quality of Life 5 Dimensions 3 Level Version |
| VAS | Visual Analogue Scale |
| OHIP-14 | Oral Health Impact Profile 14 |
| MFIQ | Mandibular Function Impact Questionnaire |
| AMO | Active mouth opening |
| PMO | Passive mouth opening |
Appendix A
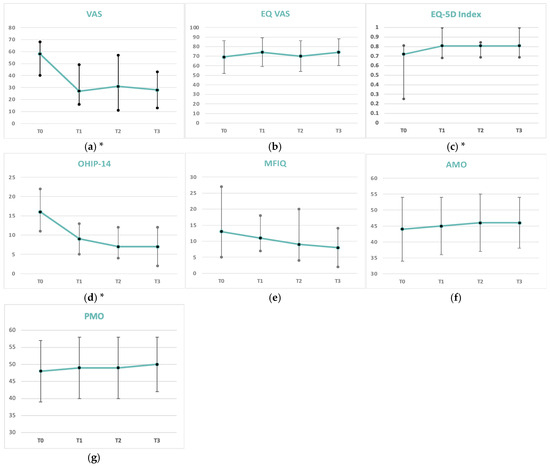
Figure A1.
Graphical outcomes at baseline (T0), 1 month (T1), 3 months (T2), and 6 months (T3) (N = 35) for (a) VAS, (b) EQ-5D Index, (c) EQ. VAS, (d) OHIP-14, (e) MFIQ, (f) AMO, (g) PMO. (*) Significant differences at one-month follow-up, which remained present at three and six months post-treatment, were observed for (a) VAS (p < 0.001), (b) EQ-5D Index (p < 0.003), and (d) OHIP-14 (p < 0.001). Data are presented as median (IQR 25–75%) or as mean ± SD.
Appendix B

Table A1.
Overview of individual VAS scores, concurrent conservative therapies, and changes throughout the study period.
Table A1.
Overview of individual VAS scores, concurrent conservative therapies, and changes throughout the study period.
| VAS-Scores | Concurrent Therapies at T0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age | T0 | T1 | T2 | T3 | Medication | Physical Therapy | Oral Splint Use | Changes in Therapy |
| 1 | Female | 26 | 70 | 60 | 50 | 40 | No | Yes | No | |
| 2 | Male | 42 | 45 | 41 | 18 | 40 | No | No | No | |
| 3 | Female | 25 | 52 | 20 | 16 | 3 | No | Yes | No | |
| 4 | Male | 56 | 61 | 50 | 15 | 28 | No | Yes | Yes | |
| 5 | Male | 62 | 50 | 25 | 5 | 17 | No | Yes | No | |
| 6 | Female | 50 | 15 | 16 | 38 | 40 | No | Yes | Yes | Discontinued splint use at T3 |
| 7 | Female | 39 | 55 | 12 | 0 | 0 | No | No | Yes | |
| 8 | Female | 28 | 82 | 86 | 84 | 91 | No | No | Yes | Initiated physical therapy at T1 |
| 9 | Female | 65 | 68 | 30 | 10 | 10 | No | Yes | Yes | |
| 10 | Female | 62 | 58 | 37 | 24 | 54 | No | Yes | No | Discontinued physical therapy at T3 |
| 11 | Female | 30 | 25 | 43 | 57 | 13 | No | Yes | Yes | |
| 12 | Female | 40 | 83 | 40 | 50 | 48 | No | Yes | No | |
| 13 | Female | 56 | 65 | 10 | 60 | 0 | No | Yes | Yes | |
| 14 | Female | 25 | 62 | 40 | 62 | 65 | No | Yes | No | |
| 15 | Female | 56 | 68 | 65 | 65 | 60 | No | Yes | No | Initiated splint use at T1 |
| 16 | Female | 42 | 79 | 19 | 71 | 72 | No | Yes | Yes | |
| 17 | Female | 38 | 63 | 18 | 6 | 3 | No | Yes | Yes | |
| 18 | Female | 24 | 18 | 42 | 51 | 34 | No | Yes | Yes | |
| 19 | Female | 56 | 36 | 50 | 41 | 25 | No | Yes | Yes | Discontinued physical therapy at T3 |
| 20 | Female | 27 | 76 | 59 | 72 | 85 | No | Yes | Yes | |
| 21 | Female | 31 | 73 | 27 | 19 | 9 | No | Yes | No | |
| 22 | Female | 29 | 58 | 16 | 11 | 16 | No | Yes | Yes | |
| 23 | Female | 31 | 87 | 67 | 37 | 53 | No | Yes | Yes | |
| 24 | Female | 29 | 31 | 4 | 20 | 15 | No | Yes | Yes | |
| 25 | Female | 28 | 29 | 26 | 9 | 28 | No | Yes | Yes | |
| 26 | Female | 60 | 73 | 5 | 31 | 63 | No | Yes | No | |
| 27 | Female | 44 | 60 | 7 | 6 | 4 | No | Yes | No | |
| 28 | Female | 30 | 46 | 10 | 11 | 35 | No | Yes | Yes | |
| 29 | Female | 23 | 34 | 4 | 10 | 23 | No | Yes | No | |
| 30 | Female | 29 | 25 | 0 | 18 | 15 | No | No | No | |
| 31 | Female | 69 | 55 | 33 | 6 | 3 | No | Yes | Yes | |
| 32 | Female | 79 | 63 | 50 | 52 | 54 | No | No | Yes | Initiated physical therapy at T3 |
| 33 | Female | 31 | 40 | 23 | 64 | 28 | No | Yes | No | |
| 34 | Female | 34 | 45 | 26 | 52 | 50 | No | Yes | Yes | |
| 35 | Female | 40 | 61 | 49 | 71 | 31 | No | Yes | No | |
Overview of concurrent conservative therapies and Visual Analogue Scale (VAS) pain scores throughout the study period. Concurrent therapies, including oral splint use, physical therapy, and prescribed medication, were assessed at baseline (T0) and at one (T1), three (T2), and six (T3) months. “Changes in therapy” indicate when participants initiated, discontinued, or modified ongoing treatments during follow-up. Age is reported in years.
References
- Thambar, S.; Kulkarni, S.; Armstrong, S.; Nikolarakos, D. Botulinum toxin in the management of temporomandibular disorders: A systematic review. Br. J. Oral Maxillofac. Surg. 2020, 58, 508–519. [Google Scholar] [CrossRef]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral. Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Chisnoiu, A.M.; Picos, A.M.; Popa, S.; Chisnoiu, P.D.; Lascu, L.; Picos, A.; Chisnoiu, R. Factors involved in the etiology of temporomandibular disorders-a literature review. Clujul Med. 2015, 88, 473–478. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Golanska, P.; Saczuk, K.; Domarecka, M.; Kuć, J.; Lukomska-Szymanska, M. Temporomandibular myofascial pain syndrome-aetiology and biopsychosocial modulation. A narrative review. Int. J. Environ. Res. Public Health 2021, 18, 7807. [Google Scholar] [CrossRef] [PubMed]
- Kalladka, M.; Young, A.; Khan, J. Myofascial pain in temporomandibular disorders: Updates on etiopathogenesis and management. J. Bodyw. Mov. Ther. 2021, 28, 104–113. [Google Scholar] [CrossRef]
- Pigozzi, L.B.; Pereira, D.D.; Pattussi, M.P.; Moret-Tatay, C.; Irigaray, T.Q.; Weber, J.B.B.; Grossi, P.K.; Grossi, M.L. Quality of life in young and middle age adult temporomandibular disorders patients and asymptomatic subjects: A systematic review and meta-analysis. Health Qual. Life Outcomes 2021, 19, 83. [Google Scholar] [CrossRef]
- Gil-Martínez, A.; Paris-Alemany, A.; López-de-Uralde-Villanueva, I.; La Touche, R. Management of pain in patients with temporomandibular disorder (TMD): Challenges and solutions. J. Pain Res. 2018, 11, 571–587. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Câmara-Souza, M.B.; Do Amaral, C.F.; Garcia, R.C.; Manfredini, D. Is there enough evidence to use botulinum toxin injections for bruxism management? A systematic literature review. Clin. Oral Investig. 2017, 21, 727–734. [Google Scholar] [CrossRef]
- Patel, J.; Cardoso, J.A.; Mehta, S. A systematic review of botulinum toxin in the management of patients with temporomandibular disorders and bruxism. Br. Dent. J. 2019, 226, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Sunil Dutt, C.; Ramnani, P.; Thakur, D.; Pandit, M. Botulinum toxin in the treatment of muscle specific Oro-facial pain: A literature review. J. Maxillofac. Oral Surg. 2015, 14, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Sipahi Calis, A.; Colakoglu, Z.; Gunbay, S. The use of botulinum toxin-A in the treatment of muscular temporomandibular joint disorders. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, F.; Lanza, A.; Di Blasio, M.; Vaienti, B.; Cafferata, E.A.; Cervino, G.; Cicciù, M.; Minervini, G. Application of Botulinum Toxin in Temporomandibular Disorders: A Systematic Review of Randomized Controlled Trials (RCTs). Appl. Sci. 2022, 12, 12409. [Google Scholar] [CrossRef]
- Chaurand, J.; Pacheco-Ruíz, L.; Orozco-Saldívar, H.; López-Valdés, J. Efficacy of botulinum toxin therapy in treatment of myofascial pain. J. Oral Sci. 2017, 59, 351–356. [Google Scholar] [CrossRef]
- Miotto, E.; Freitas, K.M.S.; Mori, A.A.; Valarelli, F.P.; Gobbi de Oliveira, R.C.; Oliveira, R.C. Effect of botulinum toxin on quality of life of patients with chronic myofascial pain. Pain Manag. 2021, 11, 583–593. [Google Scholar] [CrossRef]
- Villa, S.; Raoul, G.; Machuron, F.; Ferri, J.; Nicot, R. Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 2–6. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Stecco, A.; Stecco, C.; Masiero, S.; Manfredini, D. Myofascial pain of the jaw muscles: Comparison of short-term effectiveness of botulinum toxin injections and Fascial Manipulation technique. Cranio–J. Craniomandib. Pract. 2012, 30, 95–102. [Google Scholar] [CrossRef]
- Kurtoglu, C.; Gur, O.H.; Kurkcu, M.; Sertdemir, Y.; Guler-Uysal, F.; Uysal, H. Effect of botulinum toxin-A in myofascial pain patients with or without functional disc displacement. J. Oral Maxillofac. Surg. 2008, 66, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Lerner, M.Z.; Blitzer, A. IncobotulinumtoxinA Injection for Temporomandibular Joint Disorder. Ann. Otol. Rhinol. Laryngol. 2017, 126, 328–333. [Google Scholar] [CrossRef]
- Von Lindern, J.J.; Niederhagen, B.; Bergé, S.; Appel, T. Type A Botulinum Toxin in the Treatment of Chronic Facial Pain Associated with Masticatory Hyperactivity. J. Oral Maxillofac. Surg. 2003, 61, 774–778. [Google Scholar] [CrossRef]
- Ernberg, M.; Hedenberg-Magnusson, B.; List, T.; Svensson, P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: A randomized, controlled, double-blind multicenter study. Pain 2011, 152, 1988–1996. [Google Scholar] [CrossRef]
- Nixdorf, D.R.; Heo, G.; Major, P.W. Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain 2002, 99, 465–473. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chiu, Y.W.; Chen, C.Y.; Chuang, S.K. Botulinum toxin therapy for temporomandibular joint disorders: A systematic review of randomized controlled trials. Int. J. Oral Maxillofac. Surg. 2015, 44, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Al-Wayli, H. Treatment of chronic pain associated with nocturnal bruxism with botulinum toxin. A prospective and randomized clinical study. J. Clin. Exp. Dent. 2017, 9, e112–e117. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Martimbianco, A.L.C.; Bussadori, S.K.; Pacheco, R.L.; Riera, R.; Santos, E.M. Botulinum Toxin Type A for Painful Temporomandibular Disorders: Systematic Review and Meta-Analysis. J. Pain 2020, 21, 281–293. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Câmara-Souza, M.B.; Ernberg, M.; Al-Moraissi, E.A.; Grigoriadis, A.; Poluha, R.L.; Christidis, M.; Jasim, H.; Lövgren, A.; Christidis, N. Botulinum Toxin-A for the Treatment of Myogenous Temporomandibular Disorders: An Umbrella Review of Systematic Reviews. Drugs 2024, 84, 779–809. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Câmara-Souza, M.B.; Poluha, R.L.; de Figueredo, O.M.C.; Nobre, B.B.S.; Ernberg, M.; Conti, P.C.R.; Rizzatti-Barbosa, C.M. Long-Term Effects of a Single Application of Botulinum Toxin Type A in Temporomandibular Myofascial Pain Patients: A Controlled Clinical Trial. Toxins 2022, 14, 741. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tan, K.; Yacovelli, A.; Bi, W.G. Effect of Botulinum Toxin Type A on Muscular Temporomandibular Disorder: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Oral Rehabil. 2024, 51, 886–897. [Google Scholar] [CrossRef]
- Val, M.; Manfredini, D.; Guarda Nardini, L. Is botulinum toxin the future of orofacial pain management? Evidence and perspectives. Dent. Med. Probl. 2025, 62, 405–407. [Google Scholar] [CrossRef]
- Kahl, C.; Cleland, J.A. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: An overview of psychometric properties. Phys. Ther. Rev. 2005, 10, 123–128. [Google Scholar] [CrossRef]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, M.J.; John, M.T.; Naeije, M.; Lobbezoo, F. Developing abbreviated OHIP versions for use with TMD patients. J. Oral Rehabil. 2012, 39, 18–27. [Google Scholar] [CrossRef]
- Kropmans, T.J.; Dijkstra, P.U.; van Veen, A.; Stegenga, B.; de Bont, L.G. The smallest detectable difference of mandibular function impairment in patients with a painfully restricted temporomandibular joint. J. Dent. Res. 1999, 78, 1445–1449. [Google Scholar] [CrossRef]
- Emshoff, R.; Emshoff, I.; Bertram, S. Estimation of clinically important change for visual analog scales measuring chronic temporomandibular disorder pain. J. Orofac. Pain 2010, 24, 262–269. [Google Scholar] [PubMed]
- Locker, D.; Jokovic, A.; Clarke, M. Assessing the responsiveness of measures of oral health-related quality of life. Community Dent. Oral Epidemiol. 2004, 32, 10–18. [Google Scholar] [CrossRef]
- Walker, N.; Bohannon, R.W.; Cameron, D. Discriminant validity of temporomandibular joint range of motion measurements obtained with a ruler. J. Orthop. Sports Phys. Ther. 2000, 30, 484–492. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Canales, G.; Lorenzi Poluha, R.; Alvarez Pinzon, Y.N.; Rodrigues Conti, P.C.; Manfredini, D.; Sánchez-Ayala, A.; Rizzatti-Barbosa, C.M. Effects of Botulinum Toxin Type A on the Psychosocial Features of Myofascial Pain TMD Subjects: A Randomized Controlled Trial. J. Oral Facial Pain Headache 2021, 35, 288–296. [Google Scholar] [CrossRef]
- Gonzalez-Perez, L.M.; Vera-Martin, R.; Montes-Latorre, E.; Torres-Carranza, E.; Infante-Cossio, P. Botulinum Toxin and Percutaneous Needle Electrolysis for the Treatment of Chronic Masticatory Myalgia. Toxins 2023, 15, 278. [Google Scholar] [CrossRef]
- De Carli, B.M.; Magro, A.K.; Souza-Silva, B.N.; Matos, F.S.; De Carli, J.P.; Paranhos, L.R.; Magro, E.D. The Effect of Laser and Botulinum Toxin in the Treatment of Myofascial Pain and Mouth Opening: A Randomized Clinical Trial. J. Photochem. Photobiol. B 2016, 159, 120–123. [Google Scholar] [CrossRef]
- Sitnikova, V.; Kämppi, A.; Kämppi, L.; Alvesalo, E.; Burakova, M.; Kemppainen, P.; Teronen, O. Clinical benefit of botulinum toxin for treatment of persistent TMD-related myofascial pain: A randomized, placebo-controlled, cross-over trial. Pain Pract. 2024, 24, 1014–1023. [Google Scholar] [CrossRef]
- Jadhao, V.A.; Lokhande, N.; Habbu, S.G.; Sewane, S.; Dongare, S.; Goyal, N. Efficacy of botulinum toxin in treating myofascial pain and occlusal force characteristics of masticatory muscles in bruxism. Indian J. Dent. Res. 2017, 28, 493–497. [Google Scholar] [CrossRef]
- Kaya, D.I.; Ataoğlu, H. Botulinum toxin treatment of temporomandibular joint pain in patients with bruxism: A prospective and randomized clinical study. Niger. J. Clin. Pract. 2021, 24, 412–417. [Google Scholar] [CrossRef]
- Pascu, L.; Haiduc, R.S.; Almășan, O.; Leucuța, D.C. Occlusion and Temporomandibular Disorders: A Scoping Review. Medicina 2025, 61, 791. [Google Scholar] [CrossRef] [PubMed]
- Kahn, A.; Bertin, H.; Corre, P.; Praud, M.; Paré, A.; Kün-Darbois, J.D. Assessing the effectiveness of botulinum toxin injections into masticatory muscles in the treatment of temporomandibular disorders. J. Oral Med. Oral Surg. 2018, 24, 107–111. [Google Scholar] [CrossRef]
- Lemos, G.A.; Paulino, M.R.; Forte, F.D.S.; Beltrão, R.T.S.; Batista, A.U.D. Influence of temporomandibular disorder presence and severity on oral health-related quality of life. Rev. Dor. 2015, 16, 10–14. [Google Scholar] [CrossRef]
- Weinberg, F.M.; Rosenberg, A.J.W.P.; Withagen, K.P.A.; Gilijamse, M.; Forouzanfar, T.; Speksnijder, C.M. Oral functioning after open versus closed treatment of unilateral condylar neck or base fractures: A two-centre controlled clinical trial. J. Oral Rehabil. 2023, 50, 194–202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).