L-FABP as a Potential Biomolecular Marker of Liver Graft Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol
2.2. Sample Processing
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
3.1. Patient Demographics
3.2. Preoperative Values of L-FABP
3.3. Graft Age
3.4. Duration of Surgery, Cold and Warm Ischaemia Duration
3.5. Intraoperative Blood Loss
3.6. Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starzl, T.E.; Groth, C.G.; Brettschneider, L.; Penn, I.; Fulginiti, V.A.; Moon, J.B.; Blanchard, H.; Martin, A.J.; Porter, K.A. Orthotopic homotransplantation of the human liver. Ann. Surg. 1968, 168, 392–415. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on liver transplantation. J. Hepatol. 2024, 81, 1040–1086. [Google Scholar] [CrossRef]
- Wan Song, A.T.; Avelino-Silva, V.; Arruda Pecora, R.; Pugliese, V.; Carniero D’Albuquerque, L.; Abdala, E. Liver transplantation: Fifty years of experience. World J. Gastroenterol. 2014, 20, 5363–5374. [Google Scholar] [CrossRef]
- Choi, W.J.; Ivanics, T.; Rajendran, L.; Li, Z.; Gavira, F.; Jones, O.; Gravely, A.; Claasen, M.; Yoon, P.D.; Ladak, F.; et al. Comparative analysis of treatment modalities for solitary, small (≤3 cm) hepatocellular carcinoma: A systematic review and network meta-analysis of oncologic outcomes. Surgery 2025, 180, 108917. [Google Scholar] [CrossRef]
- Ciria, R.; Ivanics, T.; Aliseda, D.; Claasen, M.; Alconchel, F.; Gaviria, F.; Briceño, J.; Berardi, G.; Rotellar, F.; Sapisochin, G. Liver transplantation for primary and secondary liver tumors: Patient-level meta-analyses compared to UNOS conventional indications. Hepatology 2025, 81, 1700–1713. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Azoulay, D.; Sutcliffe, R.P.; Roberts, K.J. Split versus living-related adult liver transplantation: A systematic review and meta-analysis. Langenbeck’s Arch. Surg. 2019, 404, 285–292. [Google Scholar] [CrossRef]
- Ter Burg, H.W.; Chorley Rn, A.J.; Polak, W.G.; Kranenburg, L.W.; Boehnert, M.U.; Minnee, R.C. Older living liver donors can enlarge the donor pool: A systematic review and meta-analysis. Int. J. Surg. 2024, 110, 5022–5033. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Kakos, C.D.; Esagian, S.M.; Skarentzos, K.; Alexopoulos, S.P.; Shingina, A.; Montenovo, M.I. Liver transplant after donation from controlled circulatory death versus brain death: A UNOS database analysis and publication bias adjusted meta-analysis. Clin. Transplant. 2022, 36, e14521. [Google Scholar] [CrossRef]
- Jaber, F.; Abuelazm, M.; Soliman, Y.; Madi, M.; Abusuilik, H.; Amin, A.M.; Saeed, A.; Gowaily, I.; Abdelazeem, B.; Rana, A.; et al. Machine perfusion strategies in liver transplantation: A systematic review, pairwise, and network meta-analysis of randomized controlled trials. Liver Transplant. 2025, 31, 596–615. [Google Scholar] [CrossRef]
- Neri, I.; Pascale, M.M.; Bianco, G.; Frongillo, F.; Agnes, S.; Giovinazzo, F. Age and liver graft: A systematic review with meta-regression. Updates Surg. 2023, 75, 2075–2083. [Google Scholar] [CrossRef]
- Durand, F.; Levitsky, J.; Cauchy, F.; Gilgenkrantz, H.; Soubrane, O.; Francoz, C. Age and liver transplantation. J. Hepatol. 2019, 70, 745–758. [Google Scholar] [CrossRef]
- Lue, A.; Solanas, E.; Baptista, P.; Lorente, S.; Araiz, J.J.; Garcia-Gil, A.; Serrano, M.T. How important is donor age in liver transplantation? World J. Gastroenterol. 2016, 22, 4966–4976. [Google Scholar] [CrossRef]
- Bittermann, T.; Goldberg, D.S. Quantifying the effect of transplanting older donor livers into younger recipients: The need for donor-recipient age matching. Transplantation 2018, 102, 2033–2203. [Google Scholar] [CrossRef]
- Mourad, M.; Algarni, A.; Liossis, C.; Bramhall, S. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J. Gastroenterol. 2014, 20, 6159–6169. [Google Scholar] [CrossRef]
- de Vries, Y.; von Meijenfeldt, F.; Porteb, R. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. BBA—Mol. Basis Dis. 2018, 1864, 1507–1515. [Google Scholar] [CrossRef]
- Magro, B.; Tacelli, M.; Mazzola, A.; Conti, F.; Celsa, C. Biliary complications after liver transplantation: Current perspectives and future strategies. Hepatobiliary Surg. Nutr. 2021, 10, 76–92. [Google Scholar] [CrossRef]
- Buros, C.; Dave, A.A.; Furlan, A. Immediate and Late Complications After Liver Transplantation. Radiol. Clin. N. Am. 2023, 61, 785–795. [Google Scholar] [CrossRef]
- Bardhi, E.; McDaniels, J.; Rousselle, T.; Maluf, D.G.; Mas, V.R. Nucleic acid biomarkers to assess graft injury after liver transplantation. JHEP Rep. 2022, 4, 100439. [Google Scholar] [CrossRef]
- Thomaides-Brears, H.B.; Alkhouri, N.; Allende, D.; Harisinghani, M.; Noureddin, M.; Reau, N.S.; French, M.; Pantoja, C.; Mouchti, S.; Cryer, D.R.H. Incidence of Complications from Percutaneous Biopsy in Chronic Liver Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2022, 67, 3366–3394. [Google Scholar] [CrossRef]
- Pelsers, M.; Hermens, W.; Glatz, J. Fatty acid-binding proteins as plasma markers of tissue injury. Clin. Chim. Acta 2005, 352, 15–35. [Google Scholar] [CrossRef]
- Chmurzyńska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef]
- Gajda, A.; Storch, J. Enterocyte fatty acid-binding proteins (FABPs): Different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot. Essent. Fat. Acids 2015, 93, 9–16. [Google Scholar] [CrossRef]
- Lawrie, R.C.; Dundas, S.R.; Curran, S.; Murray, G.I. Liver fatty acid binding protein expression in colorectal neoplasia. Br. J. Cancer 2004, 90, 1955–1960. [Google Scholar] [CrossRef]
- Chang, C.; Hsu, C.; Yu, T.; Hung, W.; Kuo, S.; Chen, C.; Wu, C.; Chung, F.; Lee, Y.; Wei, C. Plasma levels and tissue expression of liver-type fatty acid-binding protein in patients with breast cancer. World J. Surg. Oncol. 2023, 21, 52. [Google Scholar] [CrossRef]
- Okuda, H.; Obata, Y.; Kamijo- Ikemori, A.; Inoue, S. Quantitative and qualitative analyses of urinary L-FABP for predicting acute kidney injury after emergency laparotomy. J. Anesth. 2022, 36, 38–45. [Google Scholar] [CrossRef]
- Lipiec, K.; Adamczyk, P.; Świętochowska, E.; Ziora, K.; Szczepańska, M. L-FABP and IL-6 as markers of chronic kidney damage in children after hemolytic uremic syndrome. Adv. Clin. Exp. Med. 2018, 27, 955–962. [Google Scholar] [CrossRef]
- Karvellas, C.J.; Speiser, J.L.; Tremblay, M.; Lee, W.M.; Rose, C.F. Elevated FABP1 serum levels are associated with poorer survival in acetaminophen-induced acute liver failure. Hepatology 2017, 65, 938–949. [Google Scholar] [CrossRef]
- Coufal, S.; Kokesova, A.; Tlaskalova-Hogenova, H.; Frybova, B.; Snajdauf, J.; Rygl, M.; Kverka, M. Urinary I-FABP, L-FABP, TFF-3, and SAA Can Diagnose and Predict the Disease Course in Necrotizing Enterocolitis at the Early Stage of Disease. J. Immunol. Res. 2020, 2020, 3074313. [Google Scholar] [CrossRef]
- Juanola, A.; Graupera, I.; Elia, C.; Piano, S.; Solé, C.; Carol, M.; Pérez-Guasch, M.; Bassegoda, O.; Escudé, L.; Rubio, A.-B.; et al. Urinary L-FABP is a promising prognostic biomarker of ACLF and mortality in patients with decompensated cirrhosis. J. Hepatol. 2022, 76, 107–114. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef]
- Cheng, X.; Zhu, J.; Li, Y.; Luo, W.; Xiang, H.; Zhang, Q.; Peng, W. Serum biomarkers of isoniazid-induced liver injury: Aminotransferases are insufficient, and OPN, L-FABP and HMGB1 can be promising novel biomarkers. J. Appl. Toxicol. 2022, 42, 516–528. [Google Scholar] [CrossRef]
- Voth, M.; Verboket, R.; Henrich, D.; Marzi, I. L-FABP and NGAL are novel biomarkers for detection of abdominal injury and hemorrhagic shock. Injury 2023, 54, 1246–1256. [Google Scholar] [CrossRef]
- Relja, B.; Szermutzky, M.; Henrich, D.; Maier, M.; de Haan, J.; Lubbers, T.; Buurman, W.A.; Marzi, I. Intestinal-FABP and Liver-FABP: Novel Markers for Severe Abdominal Injury. Acad. Emerg. Med. 2010, 17, 729–735. [Google Scholar] [CrossRef]
- Monbaliu, D.; de Vries, B.; Crabbé, T.; van Heurn, E.; Verwaest, C.; Roskams, T.; Fevery, J.; Pirenne, J.; Buurman, W. Liver fatty acid-binding protein: An early and sensitive plasma marker of hepatocellular damage and a reliable predictor of graft viability after liver transplantation from non heart-beating donors. Transplant. Proc. 2005, 37, 413–416. [Google Scholar] [CrossRef]
- Ye, X.; He, Y.; Wang, S.; Wong, G.T.; Irwin, M.G.; Xia, Z. Heart-type fatty acid binding protein (H-FABP) as a biomarker for acute myocardial injury and long-term post-ischemic prognosis. Acta Pharmacol. Sin. 2018, 39, 1155–1163. [Google Scholar] [CrossRef]
- Guaman-Pilco, D.; Chocano, E.; Palà, E.; Lamana-Vallverdu, M.; Penalba, A.; Garcia-Rodriguez, P.; Rubiera, M.; Bustamante, A.; Rovira, À.; Pérez-Sánchez, S.; et al. H-FABP as a Biomarker in Transient Ischemic Attack. J. Cardiovasc. Transl. Res. 2025, 18, 40–47. [Google Scholar] [CrossRef]
- Greisenegger, S.; Segal, H.C.; Burgess, A.I.; Poole, D.L.; Mehta, Z.; Rothwell, P.M. Biomarkers and mortality after TIA and minor ischemic stroke: Population-based study. Stroke 2015, 46, 659–666. [Google Scholar] [CrossRef]
- Uitterdijk, A.; Sneep, S.; van Duin, R.W.; Krabbendam-Peters, I.; Gorsse-Bakker, C.; Duncker, D.J.; van der Giessen, W.J.; van Beusekom, H.M.M. Serial measurement of hFABP and high-sensitivity troponin I post-PCI in STEMI: How fast and accurate can myocardial infarct size and no-reflow be predicted? Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1104–H1110. [Google Scholar] [CrossRef][Green Version]
- Bjurman, C.; Petzold, M.; Venge, P.; Farbemo, J.; Fu, M.; Hammarsten, O. High-sensitive cardiac troponin, NT-proBNP, hFABP and copeptin levels in relation to glomerular filtration rates and a medical record of cardiovascular disease. Clin. Biochem. 2015, 48, 302–307. [Google Scholar] [CrossRef]
- Ritter, A.; Lötterle, L.; Han, J.; Kalbitz, M.; Henrich, D.; Marzi, I.; Leppik, L.; Weber, B. Evaluation of New Cardiac Damage Biomarkers in Polytrauma: GDF-15, HFABP and uPAR for Predicting Patient Outcomes. J. Clin. Med. 2024, 13, 961. [Google Scholar] [CrossRef]
- Roth, J.A.; Chrobak, C.; Schädelin, S.; Hug, B.L. MELD score as a predictor of mortality, length of hospital stay, and disease burden: A single-center retrospective study in 39,323 inpatients. Medicine 2017, 96, e7155. [Google Scholar] [CrossRef]
- Toyoda, H.; Johnson, P.J. The ALBI score: From liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022, 4, 100557. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Vij, M.; Rammohan, A.; Rela, M. Long-term liver allograft fibrosis: A review with emphasis on idiopathic post-transplant hepatitis and chronic antibody mediated rejection. World J. Hepatol. 2022, 14, 1541–1549. [Google Scholar] [CrossRef]
- Polyak, A.; Kuo, A.; Sundaram, V. Evolution of liver transplant organ allocation policy: Current limitations and future directions. World J. Hepatol. 2021, 13, 830–839. [Google Scholar] [CrossRef]
- Safi, K.; Pawlicka, A.J.; Pradhan, B.; Sobieraj, J.; Zhylko, A.; Struga, M.; Grąt, M.; Chrzanowska, A. Perspectives and Tools in Liver Graft Assessment: A Transformative Era in Liver Transplantation. Biomedicines 2025, 13, 494. [Google Scholar] [CrossRef]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482489/ (accessed on 1 September 2025).
- Moriles, K.E.; Zubair, M.; Azer, S.A. Alanine Aminotransferase (ALT) Test. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559278/ (accessed on 1 September 2025).
- Panteghini, M. Aspartate aminotransferase isoenzymes. Clin. Biochem. 1990, 23, 311–319. [Google Scholar] [CrossRef]
- Koenig, G.; Seneff, S. Gamma-Glutamyltransferase: A Predictive Biomarker of Cellular Antioxidant Inadequacy and Disease Risk. Dis. Markers 2015, 2015, 818570. [Google Scholar] [CrossRef]
- Green, R.M.; Flamm, S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002, 123, 1367–1384. [Google Scholar] [CrossRef]
- Ahn, K.S.; Yoon, Y.S.; Han, H.S.; Cho, J.Y. Use of Liver Function Tests as First-line Diagnostic Tools for Predicting Common Bile Duct Stones in Acute Cholecystitis Patients. World J. Surg. 2016, 40, 1925–1931. [Google Scholar] [CrossRef]
- Moman, R.N.; Gupta, N.; Varacallo, M.A. Physiology, Albumin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Guerra Ruiz, A.R.; Crespo, J.; López Martínez, R.M.; Mercadal, G.C.; Garcés, M.L.; Lavin, B.; Ruiz, M.M. Measurement and clinical usefulness of bilirubin in liver disease. Adv. Lab. Med. 2021, 2, 352–372. [Google Scholar] [CrossRef]
- Thachil, J. Relevance of clotting tests in liver disease. Postgrad. Med. J. 2008, 84, 177–181. [Google Scholar] [CrossRef]
- Sherlock, S. Diseases of the Liver and Biliary System: Needle Biopsy of the Liver, 8th ed.; Blackwell Scientific Publications: Boston, MA, USA; Melbourne, Australia, 1989; pp. 36–48. [Google Scholar]
- Valori, R.; Elias, E. How to perform a percutaneous liver biopsy. Br. J. Hosp. Med. 1989, 42, 408–410. [Google Scholar]
- Gilmore, I.T.; Burroughs, A.; Murray-Lyon, I.M.; Williams, R.; Jenkins, D.; Hopkins, A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: An audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut 1995, 36, 437–441. [Google Scholar] [CrossRef]
- Eghtedari, M.; McKenzie, C.; Tang, L.C.Y.; Majumdar, A.; Kench, J.G. Banff 2016 Global Assessment and Quantitative Scoring for T Cell-Mediated Liver Transplant Rejection are Interchangeable. J. Transplant. 2023, 2023, 3103335. [Google Scholar] [CrossRef]
- Ozcan, B.; Aydemir, O.; Isik, D.U.; Bas, A.Y.; Demirel, N. Severe Anemia Is Associated with Intestinal Injury in Preterm Neonates. Am. J. Perinatol. 2020, 37, 603–606. [Google Scholar] [CrossRef]
- Bardallo, R.Q.; Da Silva, R.T.; Carbonell, T.; Palmeira, C.; Folch-Puy, E.; Rosello-Catafau, J.; Adam, R.; Panisello-Rosello, A. Liver Graft Hypothermic Static and Oxygenated Perfusion (HOPE) Strategies: A Mitochondrial Crossroads. Int. J. Mol. Sci. 2022, 23, 5742. [Google Scholar] [CrossRef]
- Liu, J.; Man, K. Mechanistic Insight and Clinical Implications of Ischemia/Reperfusion Injury Post Liver Transplantation. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 1463–1474. [Google Scholar] [CrossRef]
- Kietzmann, T. Liver Zonation in Health and Disease: Hypoxia and Hypoxia-Inducible Transcription Factors as Concert Masters. Int. J. Mol. Sci. 2019, 20, 2347. [Google Scholar] [CrossRef]
- Bhogal, R.H.; Weston, C.J.; Curbishley, S.M.; Bhatt, A.N.; Adams, D.H.; Afford, S.C. Variable responses of small and large human hepatocytes to hypoxia and hypoxia/reoxygenation (H-R). FEBS Lett. 2011, 585, 935–941. [Google Scholar] [CrossRef]
- Connor, K.L.; O’Sullivan, E.D.; Marson, L.P.; Wigmore, S.J.; Harrison, E.M. The Future Role of Machine Learning in Clinical Transplantation. Transplantation 2021, 105, 723–735. [Google Scholar] [CrossRef]
- Schmuck, R.B.; Reutzel-Selke, A.; Raschzok, N.; Morgul, H.M.; Struecker, B.; Lippert, S.; Fischer, C.d.C.; Schmelzle, M.; Boas-Knoop, S.; Bahra, M.; et al. Bile: miRNA pattern and protein-based biomarkers may predict acute cellular rejection after liver transplantation. Biomarkers 2017, 22, 19–27. [Google Scholar] [CrossRef]
- Kim, N.; Yoon, Y.I.; Yoo, H.J.; Tak, E.; Ahn, C.-S.; Song, G.-W.; Lee, S.-G.; Hwang, S. Combined Detection of Serum IL-10, IL-17, and CXCL10 Predicts Acute Rejection Following Adult Liver Transplantation. Mol. Cells 2016, 39, 639–644. [Google Scholar] [CrossRef]
- Ren, Z.; Jiang, J.; Lu, H.; Chen, X.; He, Y.; Zhang, H.; Xie, H.; Wang, W.; Zheng, S.; Zhou, L. Intestinal microbial variation may predict early acute rejection after liver transplantation in rats. Transplantation 2014, 98, 844–852. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jang, J.W. Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score. World J. Gastroenterol. 2015, 21, 7637–7647. [Google Scholar] [CrossRef]
- Francoz, C.; Prié, D.; Abdelrazek, W.; Moreau, R.; Mandot, A.; Belghiti, J.; Valla, D.; Durand, F. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: Impact on the model for end-stage liver disease score. Liver Transpl. 2010, 16, 1169–1177. [Google Scholar] [CrossRef]
- Chedid, M.F.; Picon, R.V.; Chedid, A.D. ALBI and PALBI: Novel scores for outcome prediction of cirrhotic outpatients awaiting liver transplantation. Ann. Hepatol. 2018, 17, 906–907. [Google Scholar] [CrossRef]
- Bernardi, N.; Chedid, M.F.; Grezzana-Filho, T.J.M.; Chedid, A.D.; Pinto, M.A.; Leipnitz, I.; Prediger, J.E.; Prediger, C.; Backes, A.N.; Hammes, T.O.; et al. Pre-transplant ALBI grade 3 is associated with increased mortality after liver transplantation. Dig. Dis. Sci. 2019, 64, 1695–1704. [Google Scholar] [CrossRef]
- Schlegel, A.; Linecker, M.; Kron, P.; Müllhaupt, B.; Clavien, P.; Dutkowski, P. Risk assessment in High-and Low-MELD Liver Transplantation. Am. J. Transplant. 2017, 17, 1050–1063. [Google Scholar] [CrossRef]
- Ganser, G.H.; Hewett, P. An accurate substitution method for analyzing censored data. J. Occup. Environ. Hyg. 2010, 7, 233–244. [Google Scholar] [CrossRef]
- Wood, M.D.; Beresford, N.A.; Copplestone, D. Limit of detection values in data analysis: Do they matter? Radioprotection 2011, 46, S85–S90. [Google Scholar] [CrossRef]

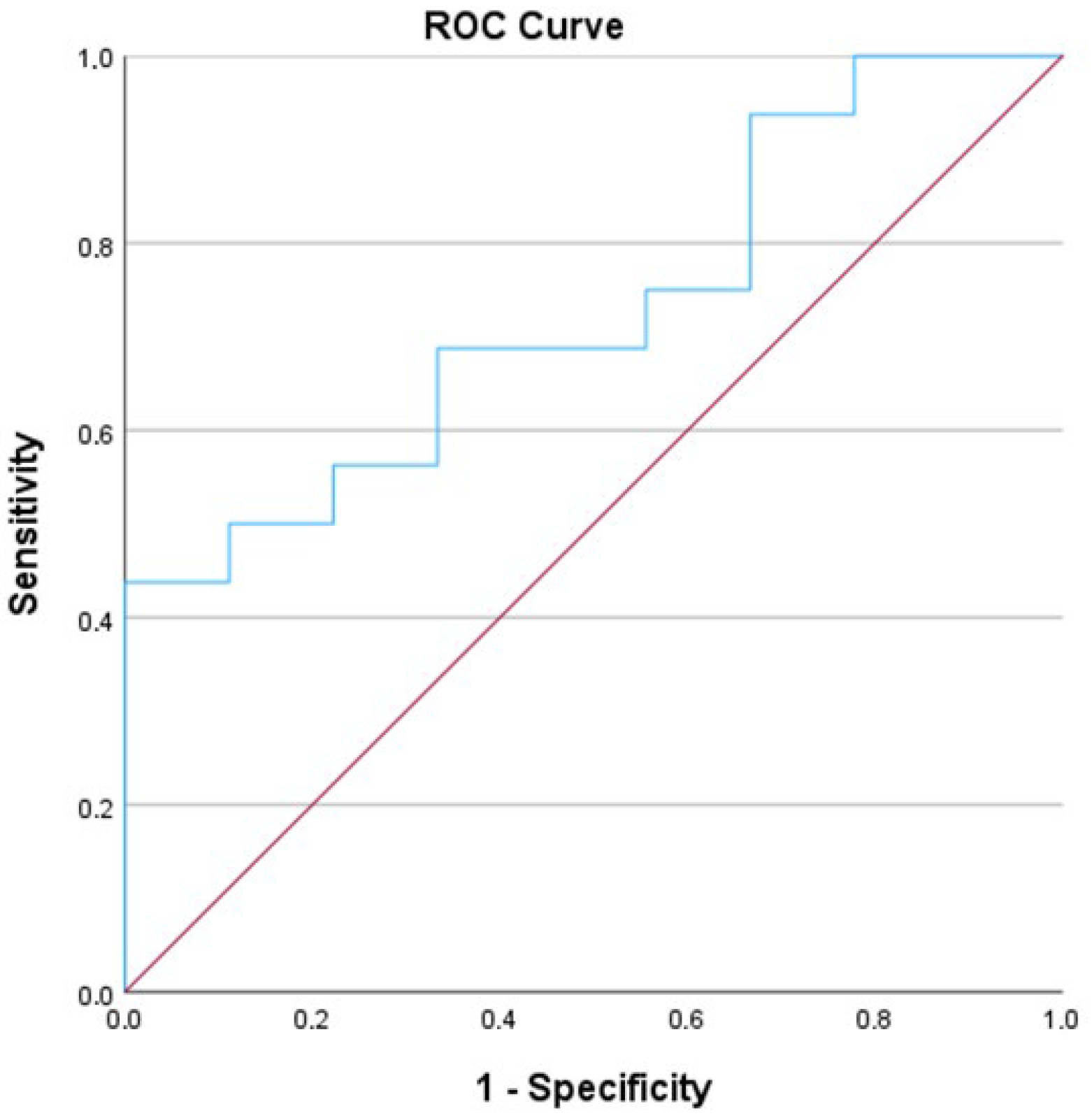

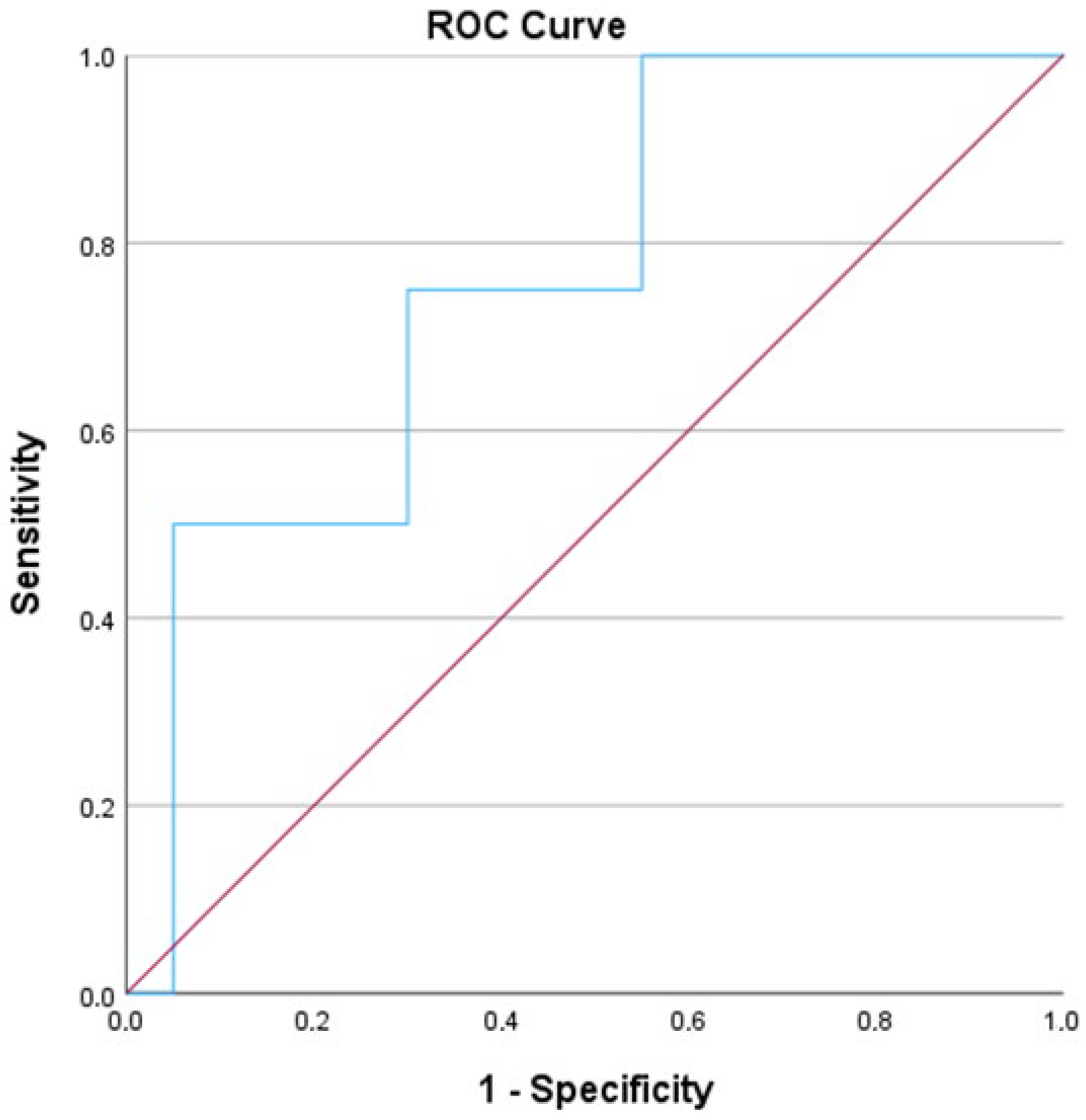
| Patient Demographics | N = 29 |
|---|---|
| Age, years—mean (SD) | 55.21 (SD = 11.88) |
| Gender—n (%) | |
| Male | 18 (62.1%) |
| Female | 11 (37.9%) |
| Comorbidities—n (%) | |
| Pulmonary | 5 (17.2%) |
| Cardiovascular | 8 (27.6%) |
| Renal | 8 (27.6%) |
| Diabetes mellitus | 7 (24.1%) |
| Other | 10 (34.5%) |
| Indication for transplantation—n (%) | |
| Alcohol-related liver disease | 11 (37.9%) |
| Liver malignancy in cirrhotic liver | 6 (20.7%) |
| Autoimmune | 6 (20.7%) |
| Genetic/metabolic | 3 (10.3) |
| Cryptogenic | 2 (6.9%) |
| Other | 5 (17.2%) |
| Intraoperative details | |
| Biliary anastomosis type—n (%) | |
| Ducto-ductal anastomosis | 24 (82.8%) |
| Hepatico-jejunostomy | 5 (17.2%) |
| Portocaval shunt—n (%) | 14 (48.3%) |
| Warm ischemia, minutes—mean (SD) | 44.2 (SD = 14.5) |
| Cold ischemia, minutes—mean (SD) | 487.1 (SD = 96.4) |
| Variable | FABP-L Before Surgery | MELD Score | |
|---|---|---|---|
| FABP-L before surgery | Spearman’s rho | 1.000 | −0.344 |
| Sig. | / | 0.085 | |
| MELD score | Spearman’s rho | −0.344 | 1.000 |
| Sig. | 0.085 | / | |
| Variable | FABP-L Before Surgery | ALBI Score | |
|---|---|---|---|
| FABP-L before surgery | Spearman’s rho | 1.000 | −0.397 |
| Sig. | / | 0.045 | |
| ALBI score | Spearman’s rho | −0.397 | 1.000 |
| Sig. | 0.045 | / | |
| Variable | n | Mean | Median | SD | U | Z-Value | r | Sig. | |
|---|---|---|---|---|---|---|---|---|---|
| FABP-L POD 1 | Under 65 | 9 | 8 | 37.2 | 126.1 | 62.0 | −0.122 | 0.03 | 0.928 |
| 65 and older | 16 | 16 | 39.2 | 34.3 | |||||
| FABP-L POD 3 | Under 65 | 10 | 10 | 18.4 | 19.8 | 43.0 | −1.950 | 0.46 | 0.053 |
| 65 and older | 16 | 16 | 38.8 | 47.5 | |||||
| FABP-L POD 5 | Under 65 | 9 | 9 | 29.4 | 35.9 | 49.0 | −0.882 | 0.22 | 0.403 |
| 65 and older | 15 | 14 | 41.4 | 99.6 | |||||
| FABP-L POD 7 | Under 65 | 9 | 9 | 38.6 | 29.2 | 32.0 | −2.117 | 0.52 | 0.035 |
| 65 and older | 16 | 15 | 80.5 | 67.9 | |||||
| FABP-L POD 14 | Under 65 | 10 | 10 | 135.9 | 130.8 | 65.0 | −0.791 | 0.18 | 0.452 |
| 65 and older | 15 | 16 | 102.3 | 112.4 | |||||
| Variable | Blood Transfusion During Surgery (mL) | |
|---|---|---|
| FABP-L POD 1 | Spearman’s rho | −0.316 |
| Sig. | 0.132 | |
| FABP-L POD 3 | Spearman’s rho | −0.362 |
| Sig. | 0.069 | |
| FABP-L POD 5 | Spearman’s rho | −0.677 |
| Sig. | <0.001 | |
| FABP-L POD 7 | Spearman’s rho | −0.455 |
| Sig. | 0.025 | |
| FABP-L POD 14 | Spearman’s rho | −0.256 |
| Sig. | 0.206 | |
| Subgroup | L-FABP Trends |
|---|---|
| Graft rejection | No statistical differences observed on PODs 1,3,5,7,14 |
| Vascular complications | No statistical differences observed on PODs 1,3,5,7,14 |
| Biliary complications | No statistical differences observed on PODs 1,3,5,7,14 |
| Postoperative hemorrhage | Statistically significantly lower values for L-FABP on PODs 5 and 7 |
| Grafts > 65 years | Statistically significantly higher values for L-FABP on POD 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalamutova, A.; Plevel, D.; Djokic, M.; Jerin, A.; Trotovšek, B.; Petric, M. L-FABP as a Potential Biomolecular Marker of Liver Graft Injury. J. Clin. Med. 2025, 14, 7404. https://doi.org/10.3390/jcm14207404
Kalamutova A, Plevel D, Djokic M, Jerin A, Trotovšek B, Petric M. L-FABP as a Potential Biomolecular Marker of Liver Graft Injury. Journal of Clinical Medicine. 2025; 14(20):7404. https://doi.org/10.3390/jcm14207404
Chicago/Turabian StyleKalamutova, Ana, Danaja Plevel, Mihajlo Djokic, Ales Jerin, Blaž Trotovšek, and Miha Petric. 2025. "L-FABP as a Potential Biomolecular Marker of Liver Graft Injury" Journal of Clinical Medicine 14, no. 20: 7404. https://doi.org/10.3390/jcm14207404
APA StyleKalamutova, A., Plevel, D., Djokic, M., Jerin, A., Trotovšek, B., & Petric, M. (2025). L-FABP as a Potential Biomolecular Marker of Liver Graft Injury. Journal of Clinical Medicine, 14(20), 7404. https://doi.org/10.3390/jcm14207404







