Pharmacokinetics and Monitoring of Methotrexate in Adults with Acute Lymphoblastic Leukaemia: A 10-Year Follow-Up at an Italian Centre
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Analytical Determination of MTX Plasmatic Concentrations
2.3. Data Analysis
2.4. Ethical Statement
3. Results
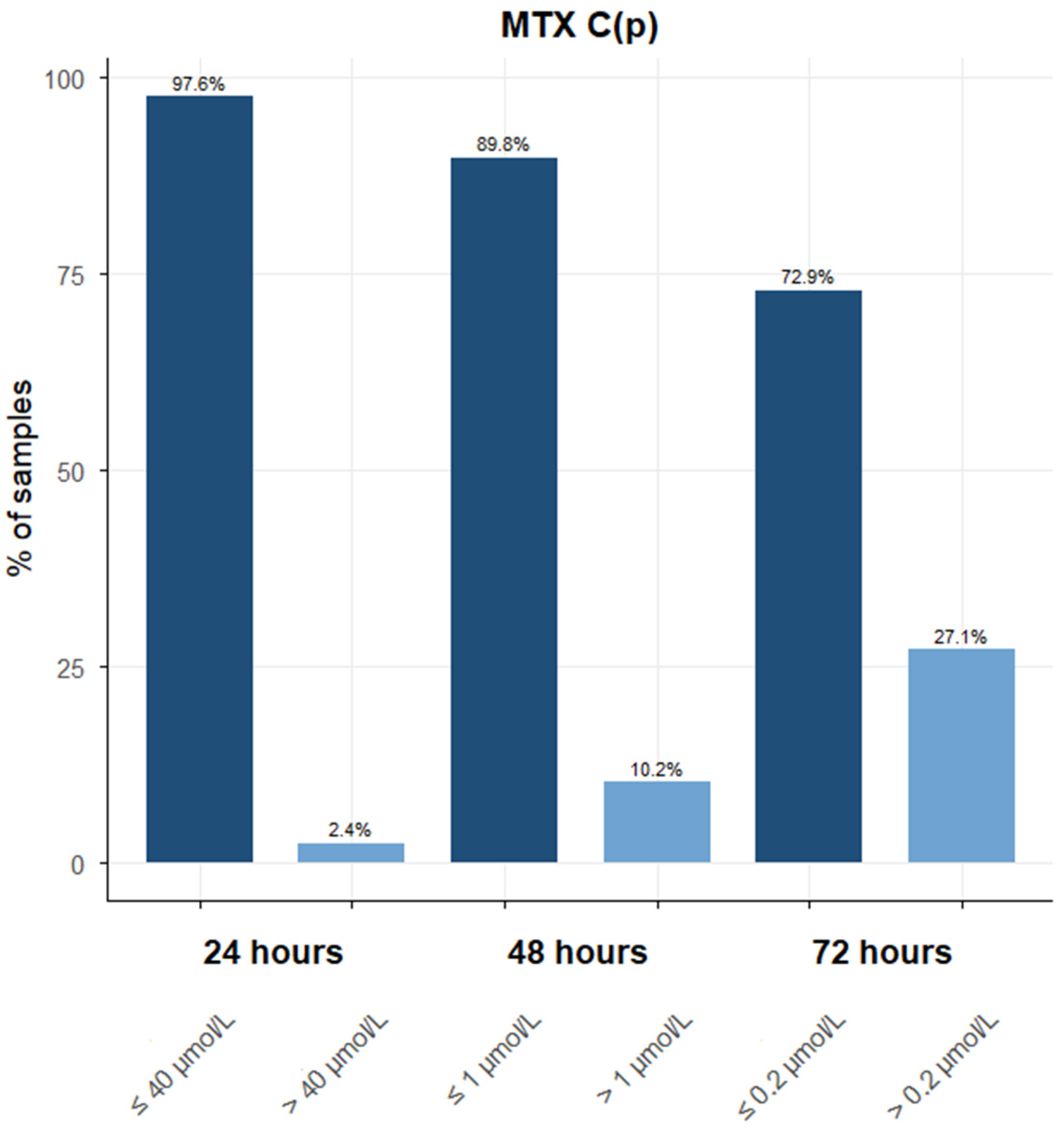
3.1. Descriptive Analyses of MTX Samples and Patients During the Period 2013–2022
3.2. PK Analysis
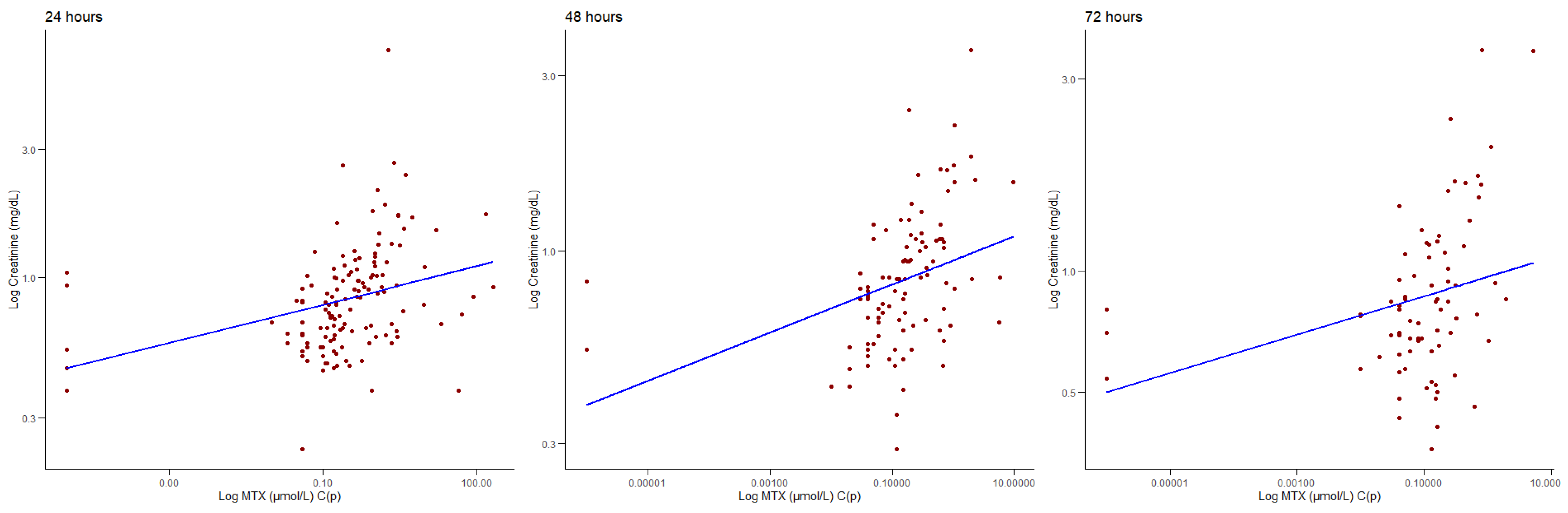
3.3. Correlations Between MTX C(p) and Biochemical Parameters
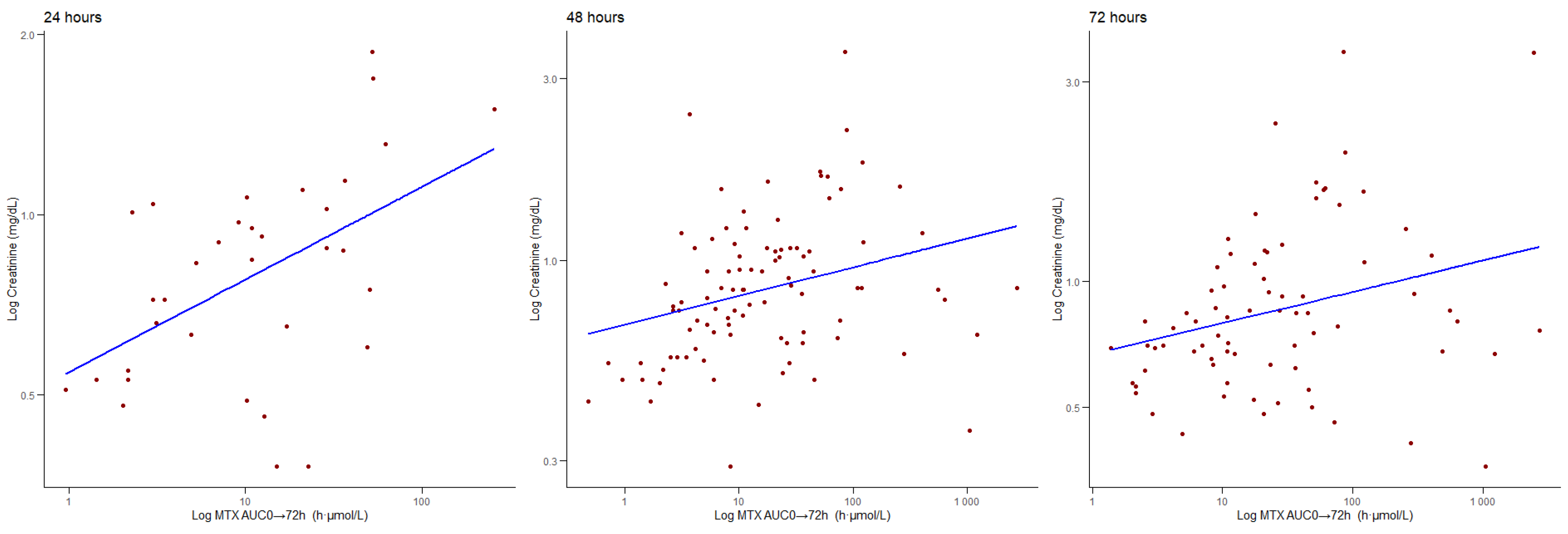
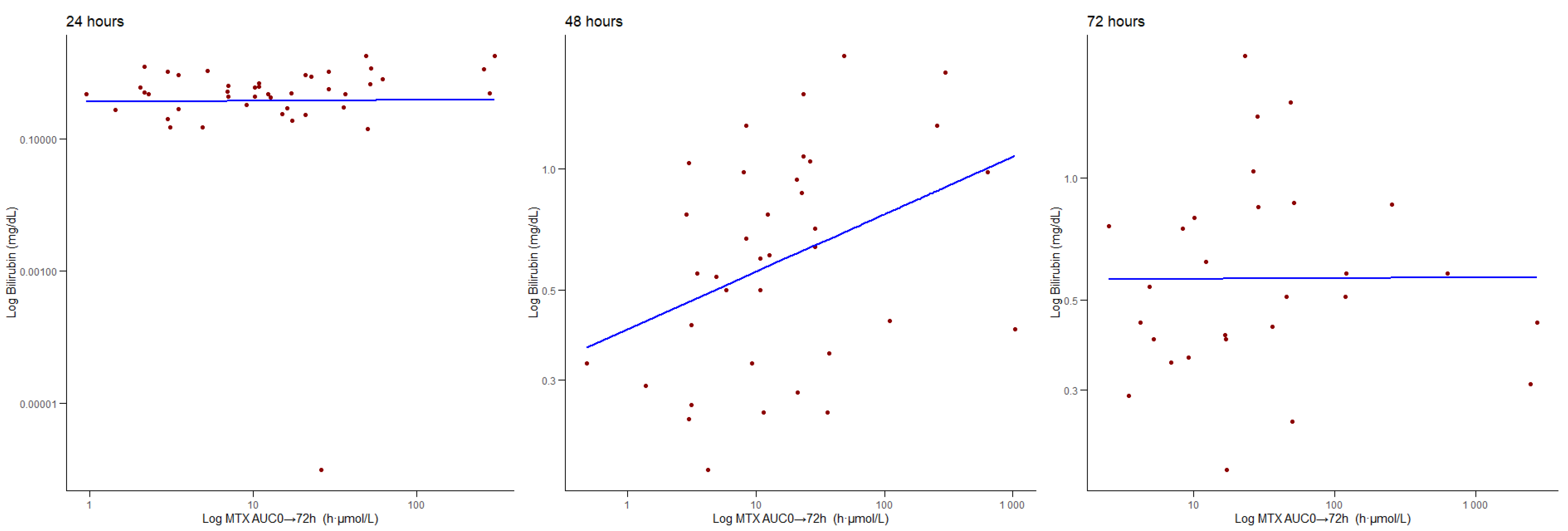
3.4. Correlations Between AUC0 → 72 h and Biochemical Parameters
3.5. Correlations Between t½ and Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTX | Methotrexate |
| HDMTX | High-dose methotrexate |
| ALL | Acute lymphoblastic leukaemia |
| AUC | Area under the curve |
| PK | Pharmacokinetics |
| TDM | Therapeutic drug monitoring |
| C(p) | Plasmatic concentration |
| t½ | Half-life |
References
- Mantadakis, E.; Cole, P.D.; Kamen, B.A. High-Dose Methotrexate in Acute Lymphoblastic Leukemia: Where Is the Evidence for Its Continued Use? Pharmacotherapy 2005, 25, 748–755. [Google Scholar] [CrossRef]
- Meyer, L.M.; Miller, F.R.; Rowen, M.J.; Bock, G.; Rutzky, J. Treatment of Acute Leukemia with Amethopterin (4-Amino, 10-Methyl Pteroyl Glutamic Acid). Acta Haematol. 1950, 4, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Hashkes, P.J.; Becker, M.L.; Cabral, D.A.; Laxer, R.M.; Paller, A.S.; Rabinovich, C.E.; Turner, D.; Zulian, F. Methotrexate: New Uses for an Old Drug. J. Pediatr. 2014, 164, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Ravindran, V. Low-Dose Methotrexate in Rheumatology: A Reinvented Drug. J. R. Coll. Physicians Edinb. 2025, 55, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Aickara, D.; Bashyam, A.M.; Pichardo, R.O.; Feldman, S.R. Topical Methotrexate in Dermatology: A Review of the Literature. J. Dermatol. Treat. 2022, 33, 512–517. [Google Scholar] [CrossRef]
- Solangon, S.A.; Van Wely, M.; Van Mello, N.; Mol, B.W.; Ross, J.A.; Jurkovic, D. Methotrexate vs Expectant Management for Treatment of Tubal Ectopic Pregnancy: An Individual Participant Data Meta-analysis. Acta Obstet. Gynecol. Scand. 2023, 102, 1159–1175. [Google Scholar] [CrossRef]
- Larsen, E.C.; Devidas, M.; Chen, S.; Salzer, W.L.; Raetz, E.A.; Loh, M.L.; Mattano, L.A.; Cole, C.; Eicher, A.; Haugan, M.; et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults with High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J. Clin. Oncol. 2016, 34, 2380–2388. [Google Scholar] [CrossRef]
- Aldoss, I.; Forman, S.J.; Pullarkat, V. Acute Lymphoblastic Leukemia in the Older Adult. J. Oncol. Pract. 2019, 15, 67–75. [Google Scholar] [CrossRef]
- Gökbuget, N.; Boissel, N.; Chiaretti, S.; Dombret, H.; Doubek, M.; Fielding, A.; Foà, R.; Giebel, S.; Hoelzer, D.; Hunault, M.; et al. Management of ALL in Adults: 2024 ELN Recommendations from a European Expert Panel. Blood 2024, 143, 1903–1930. [Google Scholar] [CrossRef]
- Howard, S.C.; McCormick, J.; Pui, C.-H.; Buddington, R.K.; Harvey, R.D. Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef]
- Giletti, A.; Esperon, P. Genetic Markers in Methotrexate Treatments. Pharmacogenom. J. 2018, 18, 689–703. [Google Scholar] [CrossRef]
- Toksvang, L.N.; Brigitha, L.J.; van der Sluis, I.M.; Brivio, E.; Raja, R.; Pontoppidan, P.; Buhl Rasmussen, A.S.; Andres-Jensen, L.; Uhlving, H.H.; Kielsen, K.; et al. Therapeutic Drug Monitoring in Acute Lymphoblastic Leukemia–a Deep Dive into Pharmacokinetics, -Dynamics, and -Genetics of Antileukemic Drugs. Expert. Rev. Clin. Pharmacol. 2025, 18, 131–149. [Google Scholar] [CrossRef]
- Song, Z.; Hu, Y.; Liu, S.; Wang, G.; Zhai, S.; Zhang, X.; Li, Y.; Du, G.; Shi, Y.; Chen, Y.; et al. Medication Therapy of High-dose Methotrexate: An Evidence-based Practice Guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Br. J. Clin. Pharmacol. 2022, 88, 2456–2472. [Google Scholar] [CrossRef]
- Lin, F.; Juan, Y.; Zheng, S.-E.; Shen, Z.; Tang, L.-N.; Zhao, H.; Yao, Y. Relationship of Serum Methotrexate Concentration in High-Dose Methotrexate Chemotherapy to Prognosis and Tolerability: A Prospective Cohort Study in Chinese Adults with Osteosarcoma. Curr. Ther. Res. 2009, 70, 150–160. [Google Scholar] [CrossRef]
- Widemann, B.C.; Adamson, P.C. Understanding and Managing Methotrexate Nephrotoxicity. Oncologist 2006, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Stotts, M.J.; Horton, B.; Schiff, D. Hepatotoxicity from High-Dose Methotrexate in Primary Central Nervous System Lymphoma. Neuro-Oncol. Pract. 2023, 10, 291–300. [Google Scholar] [CrossRef]
- Florin, L.; Lemahieu, C.; Stove, V. Evaluation of the New Methotrexate CMIA Assay on the Architect I2000SR. Clin. Chem. Lab. Med. (CCLM) 2016, 54, e15–e17. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Wu, Y.; Jin, X.; Zeng, Z.; Gu, X.; Li, T.; Chen, X.; Li, G.; Liu, J. The Incidence Rate and Influence Factors of Hemolysis, Lipemia, Icterus in Fasting Serum Biochemistry Specimens. PLoS One 2022, 17, e0262748. [Google Scholar] [CrossRef] [PubMed]
- ISO 9001:2015; Quality Management Systems—Requirements. ISO: Geneva, Switzerland, 2015.
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An Add-in Program for Pharmacokinetic and Pharmacodynamic Data Analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2 Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Takeda, M.; Khamdang, S.; Narikawa, S.; Kimura, H.; Hosoyamada, M.; Cha, S.H.; Sekine, T.; Endou, H. Characterization of Methotrexate Transport and Its Drug Interactions with Human Organic Anion Transporters. J. Pharmacol. Exp. Ther. 2002, 302, 666–671. [Google Scholar] [CrossRef]
- Kakkadath, M.; Naidu, D.; Kanthlal, S.K.; Sharun, K. Combating Methotrexate Resistance in Cancer Treatment: A Review on Navigating Pathways and Enhancing Its Efficacy With Fat-Soluble Vitamins. Scientifica 2025, 2025, 8259470. [Google Scholar] [CrossRef]
- Seydoux, C.; Briki, M.; Wagner, A.D.; Choong, E.; Guidi, M.; Carrara, S.; Thoma, Y.; Livio, F.; Girardin, F.R.; Marzolini, C.; et al. Importance of Sex-Dependent Differences for Dosing Selection and Optimization of Chemotherapeutic Drugs. Chemotherapy 2024, 70, 92–101. [Google Scholar] [CrossRef]
- Kawakatsu, S.; Nikanjam, M.; Lin, M.; Le, S.; Saunders, I.; Kuo, D.J.; Capparelli, E.V. Population Pharmacokinetic Analysis of High-Dose Methotrexate in Pediatric and Adult Oncology Patients. Cancer Chemother. Pharmacol. 2019, 84, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Balis, F.M.; Holcenberg, J.S.; Poplack, D.G.; Ge, J.; Sather, H.N.; Murphy, R.F.; Ames, M.M.; Waskerwitz, M.J.; Tubergen, D.G.; Zimm, S.; et al. Pharmacokinetics and Pharmacodynamics of Oral Methotrexate and Mercaptopurine in Children with Lower Risk Acute Lymphoblastic Leukemia: A Joint Children’s Cancer Group and Pediatric Oncology Branch Study. Blood 1998, 92, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Braidotti, S.; Zudeh, G.; Franca, R.; Kiren, V.; Colombini, A.; Bettini, L.R.; Brivio, E.; Locatelli, F.; Vinti, L.; Bertorello, N.; et al. The Role of Candidate Polymorphisms in Drug Transporter Genes on High-Dose Methotrexate in the Consolidation Phase of the AIEOP-BFM ALL 2009 Protocol. Clin. Transl. Sci. 2025, 18, e70136. [Google Scholar] [CrossRef]
- Holmboe, L.; Andersen, A.M.; Mørkrid, L.; Slørdal, L.; Hall, K.S. High Dose Methotrexate Chemotherapy: Pharmacokinetics, Folate and Toxicity in Osteosarcoma Patients. Br. J. Clin. Pharmacol. 2012, 73, 106–114. [Google Scholar] [CrossRef]
- Lin, C.; Ma, R.; Zeng, X.; Zhang, B.; Cao, T.; Jiao, S.; Chen, H.; He, Y.; Liu, M.; Cai, H. Integration of Genomics, Clinical Characteristics and Baseline Biological Profiles to Predict the Risk of Liver Injury Induced by High-Dose Methotrexate. Front. Pharmacol. 2024, 15, 1423214. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Yu, L.; Hu, W.; Cai, J.; Wang, Z.; Chen, C.; Zhang, X.; Xie, Y.; Wu, K.; et al. High-dose Methotrexate Pharmacokinetics and Its Impact on Prognosis of Paediatric Acute Lymphoblastic Leukaemia Patients: A Population Pharmacokinetic Study. Br. J. Haematol. 2024, 204, 1354–1366. [Google Scholar] [CrossRef]
- Gao, X.; Qian, X.-W.; Zhu, X.-H.; Yu, Y.; Miao, H.; Meng, J.-H.; Jiang, J.-Y.; Wang, H.-S.; Zhai, X.-W. Population Pharmacokinetics of High-Dose Methotrexate in Chinese Pediatric Patients with Acute Lymphoblastic Leukemia. Front. Pharmacol. 2021, 12, 701452. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Tian, J.; Wang, Z. Renal Function and Plasma Methotrexate Concentrations Predict Toxicities in Adults Receiving High-Dose Methotrexate. Med. Sci. Monit. 2018, 24, 7719–7726. [Google Scholar] [CrossRef]
- Tsurusawa, M.; Gosho, M.; Mori, T.; Mitsui, T.; Sunami, S.; Kobayashi, R.; Fukano, R.; Tanaka, F.; Fujita, N.; Inada, H.; et al. Statistical Analysis of Relation between Plasma Methotrexate Concentration and Toxicity in High-dose Methotrexate Therapy of Childhood NonHodgkin Lymphoma. Pediatr. Blood Cancer 2015, 62, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Song, Y.; Fang, Q.; Lin, Y.; Chen, L.; Wang, X.; Zhang, P.; Wang, Z.; Gong, X.; Liu, K.; et al. Effects of Genetic Polymorphisms on Methotrexate Levels and Toxicity in Chinese Patients with Acute Lymphoblastic Leukemia. Blood Sci. 2023, 5, 32–38. [Google Scholar] [CrossRef]
- Thompson, P.A.; Murry, D.J.; Rosner, G.L.; Lunagomez, S.; Blaney, S.M.; Berg, S.L.; Camitta, B.M.; Dreyer, Z.E.; Bomgaars, L.R. Methotrexate Pharmacokinetics in Infants with Acute Lymphoblastic Leukemia. Cancer Chemother. Pharmacol. 2007, 59, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.Ø.; Weismann, K.; Hutters, L. Renal Function and the Rate of Disappearance of Methotrexate from Serum. Eur. J. Clin. Pharmacol. 1975, 8, 439–444. [Google Scholar] [CrossRef]
- McTaggart, M.P.; Bluett, J.; Keevil, B.G. A Sensitive LC-MS/MS Methotrexate Assay Capable of Assessing Adherence to Methotrexate Therapy in Rheumatoid Arthritis. Clin. Chem. Lab. Med. (CCLM) 2024, 62, 111–117. [Google Scholar] [CrossRef]
- Li, J.; Rietschlin, J.; Miller, I.; Weber, C.; Scheidegger, M.; Barringer, S.; Kerlin, R.; Williams, J. Evaluation of the Preanalytical Interference of Hemoglobin, Bilirubin, or Lipids in Therapeutic Drug Monitoring Assays on Beckman Coulter AU Analyzers. Lab. Med. 2022, 53, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Bouquié, R.; Grégoire, M.; Hernando, H.; Azoulay, C.; Dailly, E.; Monteil-Ganière, C.; Pineau, A.; Deslandes, G.; Jolliet, P. Evaluation of a Methotrexate Chemiluminescent Microparticle Immunoassay. Am. J. Clin. Pathol. 2016, 146, 119–124. [Google Scholar] [CrossRef]
- Abe, K.; Maeda-Minami, A.; Ishizu, T.; Iwata, S.; Kobayashi, E.; Shimoi, T.; Kawano, Y.; Hashimoto, H.; Yamaguchi, M.; Furukawa, T.; et al. Risk Factors for Hepatic Toxicity of High-Dose Methotrexate in Patients with Osteosarcoma. Anticancer Res. 2022, 42, 1043–1050. [Google Scholar] [CrossRef]
- Moghadam, A.R.; Tutunchi, S.; Namvaran-Abbas-Abad, A.; Yazdi, M.; Bonyadi, F.; Mohajeri, D.; Mazani, M.; Marzban, H.; Łos, M.J.; Ghavami, S. Pre-Administration of Turmeric Prevents Methotrexate-Induced Liver Toxicity and Oxidative Stress. BMC Complement. Altern. Med. 2015, 15, 246. [Google Scholar] [CrossRef]
- He, X.; Yao, P.; Li, M.; Liang, H.; Liu, Y.; Du, S.; Zhang, M.; Sun, W.; Wang, Z.; Hao, X.; et al. A Risk Scoring Model for High-Dose Methotrexate-Induced Liver Injury in Children with Acute Lymphoblastic Leukemia Based on Gene Polymorphism Study. Front. Pharmacol. 2021, 12, 726229. [Google Scholar] [CrossRef]

| PK Parameters | All Patients | Females | Males |
|---|---|---|---|
| Age (mean, range) | 48.57 (19–76) | 49.11 (19–72) | 47.58 (19–76) |
| MTX C(p) 24 h (μmol/L; mean ± SEM) | 36.09 (±15.53) | 18.00 (±12.02) | 51.93 (±26.82) |
| MTX C(p) 48 h | 0.93 (±0.43) * | 0.39 (±0.09) * | 1.24 (±0.67) * |
| MTX C(p) 72 h | 0.30 (±0.07) ** | 0.28 (±0.07) ** | 0.3 (±0.09) ** |
| Overall AUC0 → 72 h (h·μmol/L; mean ± SEM) | 112.85 (±34.09) | 68.77 (±26.12) | 136.43 (±50.37) |
| AUC0 → 72 h first cycle | 98.48 (±34.83) | 77.18 (±36.98) | 110.48 (±50.50) |
| AUC0 → 72 h second cycle | 126.86 (±89.10) | 46.33 (±28.42) | 165.21 (±131.07) |
| AUC0 → 72 h third cycle | 237.92 (±200.64) | 75.87 (±68.47) | 344.74 (±336.72) |
| t½ (h; mean ± SEM) | 17.15 (±2.40) | 22.20 (±6.54) | 14.45 (±1.15) |
| t½ first cycle | 17.96 (±3.60) | 26.20 (±9.57) | 13.32 (±1.48) |
| t½ second cycle | 16.09 (±2.04) | 16.46 (±2.04) | 15.91 (±2.46) §§ |
| t½ third cycle | 12.88 (±2.48) | 10.44 (±5.23) | 14.50 (±2.49) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrò, P.F.; Biso, L.; Lastella, M.; Bandini, A.; Banchi, M.; Tacchi, C.; Tang, D.; Carli, M.; Fogli, S.; Paolicchi, A.; et al. Pharmacokinetics and Monitoring of Methotrexate in Adults with Acute Lymphoblastic Leukaemia: A 10-Year Follow-Up at an Italian Centre. J. Clin. Med. 2025, 14, 7400. https://doi.org/10.3390/jcm14207400
Calabrò PF, Biso L, Lastella M, Bandini A, Banchi M, Tacchi C, Tang D, Carli M, Fogli S, Paolicchi A, et al. Pharmacokinetics and Monitoring of Methotrexate in Adults with Acute Lymphoblastic Leukaemia: A 10-Year Follow-Up at an Italian Centre. Journal of Clinical Medicine. 2025; 14(20):7400. https://doi.org/10.3390/jcm14207400
Chicago/Turabian StyleCalabrò, Pasquale Fabio, Letizia Biso, Marianna Lastella, Arianna Bandini, Marta Banchi, Costanza Tacchi, Donghao Tang, Marco Carli, Stefano Fogli, Aldo Paolicchi, and et al. 2025. "Pharmacokinetics and Monitoring of Methotrexate in Adults with Acute Lymphoblastic Leukaemia: A 10-Year Follow-Up at an Italian Centre" Journal of Clinical Medicine 14, no. 20: 7400. https://doi.org/10.3390/jcm14207400
APA StyleCalabrò, P. F., Biso, L., Lastella, M., Bandini, A., Banchi, M., Tacchi, C., Tang, D., Carli, M., Fogli, S., Paolicchi, A., Scarselli, M., Di Paolo, A., & Bocci, G. (2025). Pharmacokinetics and Monitoring of Methotrexate in Adults with Acute Lymphoblastic Leukaemia: A 10-Year Follow-Up at an Italian Centre. Journal of Clinical Medicine, 14(20), 7400. https://doi.org/10.3390/jcm14207400






