Impact of Preoperative Comprehensive Body Composition on Postoperative Outcomes in Patients with Esophageal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment and Follow-Up
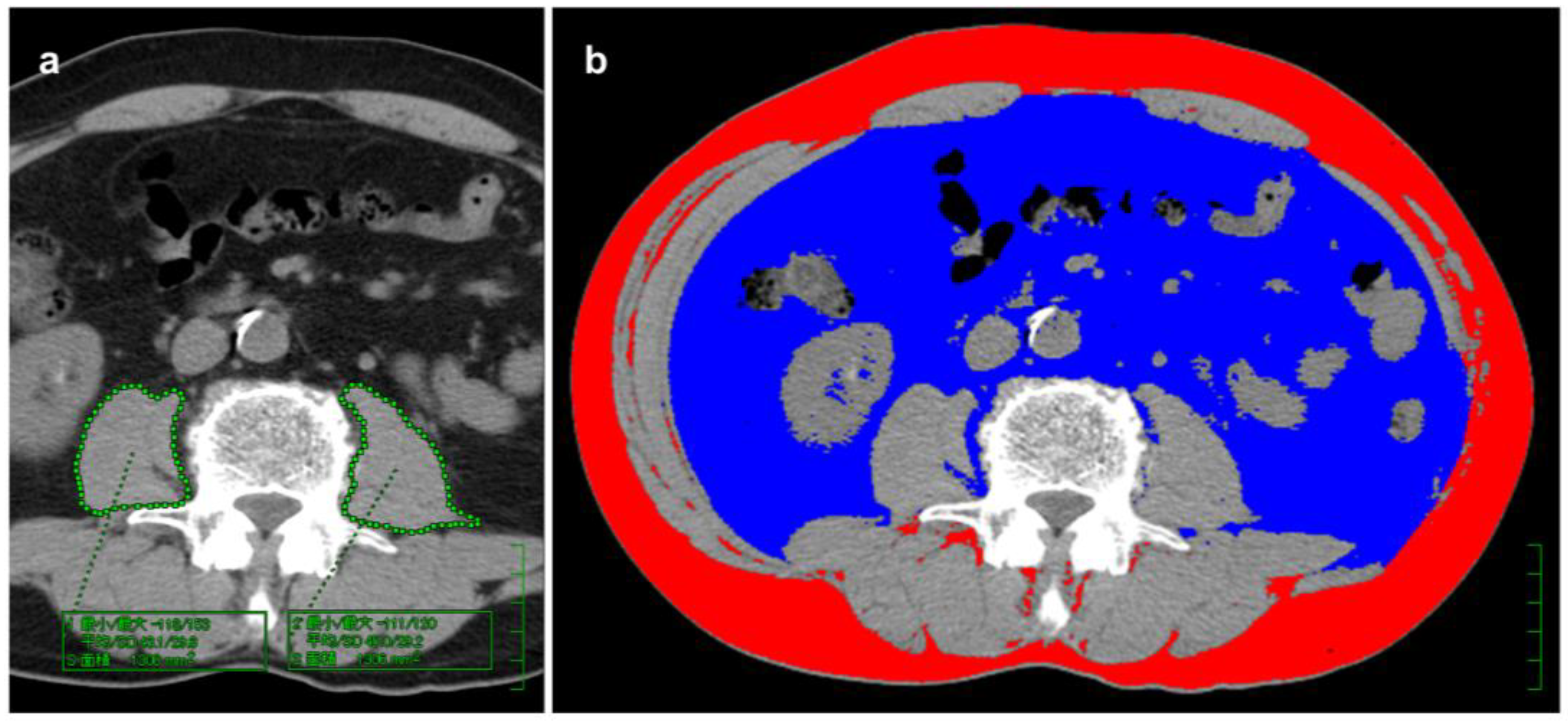
2.3. Measurement of BC Components
2.4. Cutoff Values for BC Components and BC Scoring
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
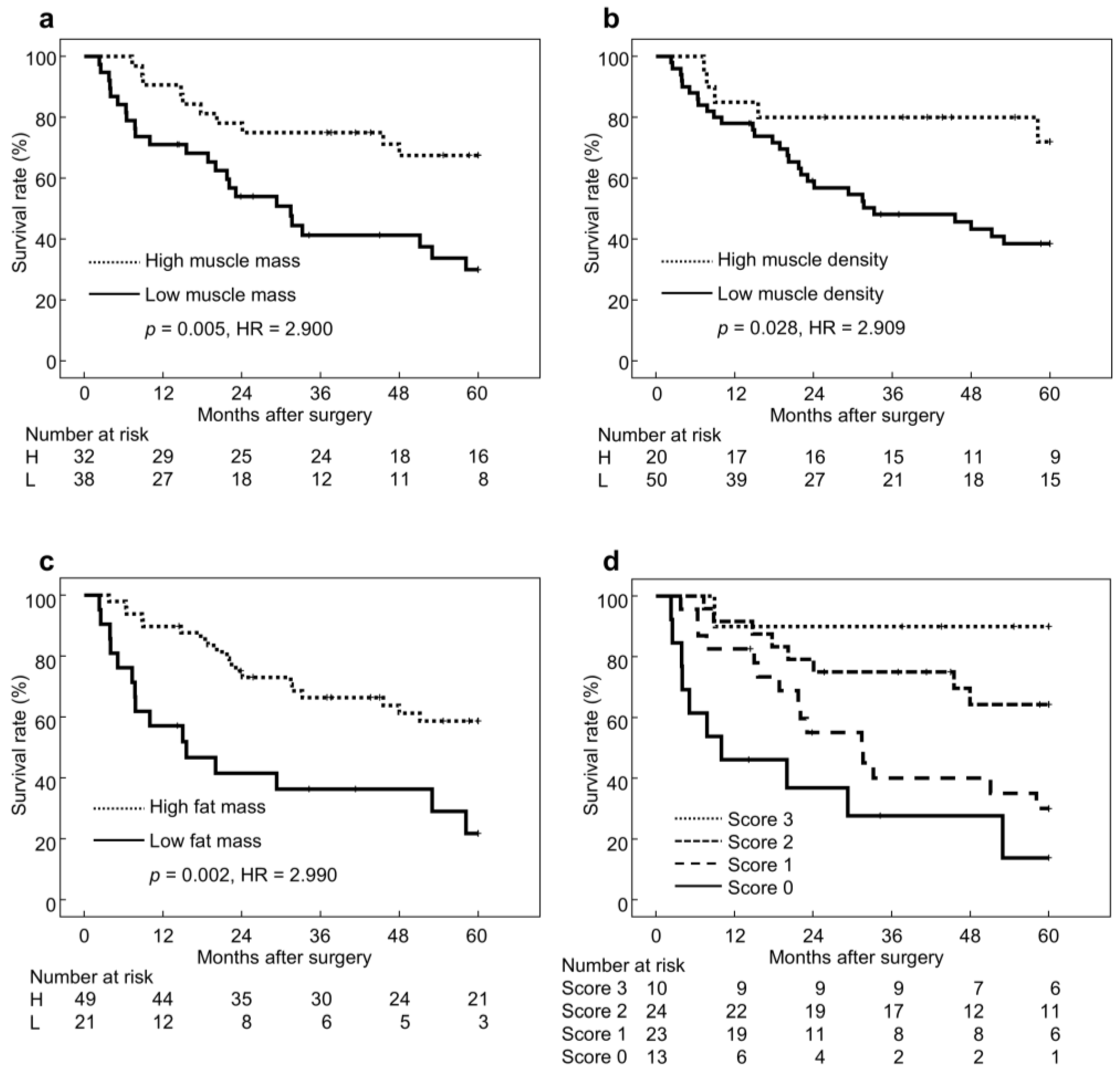
3.2. Long-Term Outcomes According to BC Status
3.3. Patient Characteristics According to BC Status
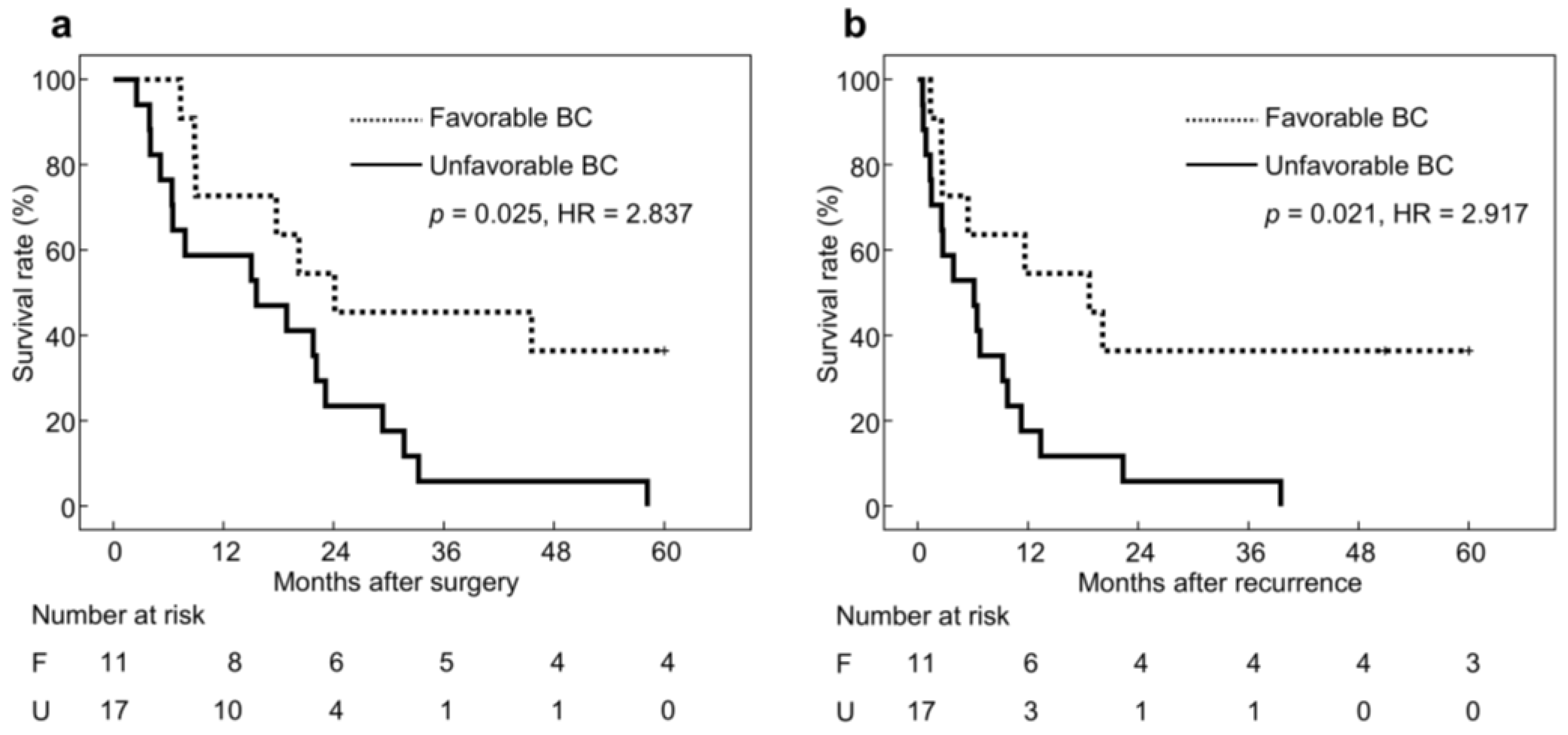
3.4. Long-Term Outcomes in Patients with Recurrence
3.5. Short-Term Outcomes According to BC Status
3.6. Prognostic Factor Analysis Including BC Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Body composition |
| EC | Esophageal cancer |
| SCC | Squamous cell carcinoma |
| OS | Overall survival |
| UICC | Union for International Cancer Control |
| 5-FU | 5-Fluorouracil |
| CDDP | Cisplatin |
| L-OHP | Oxaliplatin |
| DTX | Docetaxel |
| CT | Computed tomography |
| HU | Hounsfield unit |
| ROC | Receiver operating characteristic |
| IQR | Interquartile range |
| HR | Hazard ratio |
| CI | Confidence intervals |
| BMI | Body mass index |
| Alb | Albumin |
| CRP | C-reactive protein |
| TC | Total cholesterol |
| NLR | Neutrophil-to-lymphocyte ratio |
| CCI | Charlson comorbidity index |
References
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lundberg, E.; Mattsson, F.; Gottlieb-Vedi, E.; Lagergren, J. Time trends in survival after surgery for esophageal cancer in a national population-based study in Sweden. Ann. Surg. Oncol. 2025, 32, 3167–3174. [Google Scholar] [CrossRef]
- Martin, L.; Lagergren, J.; Lindblad, M.; Rouvelas, I.; Lagergren, P. Malnutrition after oesophageal cancer surgery in Sweden. Br. J. Surg. 2007, 94, 1496–1500. [Google Scholar] [CrossRef]
- Wang, Z.M.; Pierson, R.N., Jr.; Heymsfield, S.B. The five-level model: A new approach to organizing body-composition research. Am. J. Clin. Nutr. 1992, 56, 19–28. [Google Scholar] [CrossRef]
- Lieffers, J.R.; Bathe, O.F.; Fassbender, K.; Winget, M.; Baracos, V.E. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br. J. Cancer 2012, 107, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Ashida, R.; Yamamoto, Y.; Aramaki, T.; Sugiura, T.; Okamura, Y.; Ito, T.; Ohgi, K.; Uesaka, K. Preoperative skeletal muscle fat infiltration is a strong predictor of poorer survival in gallbladder cancer underwent surgery. Clin. Nutr. ESPEN 2022, 52, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Juez, L.D.; Priego, P.; Cuadrado, M.; Blázquez, L.A.; Sánchez-Picot, S.; Gil, P.; Longo, F.; Galindo, J.; Fernández-Cebrián, J.M.; Botella-Carretero, J.I. Impact of neoadjuvant treatment on body composition in patients with locally advanced gastric cancer. Cancers 2024, 16, 2408. [Google Scholar] [CrossRef]
- Shuto, K.; Nabeya, Y.; Mori, M.; Yamazaki, M.; Kosugi, C.; Narushima, K.; Usui, A.; Nojima, H.; Shimizu, H.; Koda, K. Postoperative changes in body composition predict long-term prognosis in patients with gastric cancer. Cancers 2025, 17, 738. [Google Scholar] [CrossRef]
- Qian, J.; Si, Y.; Zhou, K.; Tian, Y.; Guo, Q.; Zhao, K.; Yu, J. Sarcopenia is associated with prognosis in patients with esophageal squamous cell cancer after radiotherapy or chemoradiotherapy. BMC Gastroenterol. 2022, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Tan, B.H.; Cui, H.; Bhalla, A.; Fearon, K.C.H.; Parsons, S.L.; Catton, J.A.; Lobo, D.N. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin. Nutr. 2012, 31, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Tajika, M.; Tanaka, T.; Yamada, K.; Kamiya, T.; Abe, T.; Higaki, E.; Fujieda, H.; Nagao, T.; Inaba, Y.; et al. Effect of body composition change during neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J. Clin. Med. 2022, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Diallo, T.D.; Scheef, T.; Reisert, M.; Rau, A.; Russe, M.F.; Bamberg, F.; Fichtner-Feigl, S.; Quante, M.; Weiss, J. Association between body composition and survival in patients with gastroesophageal adenocarcinoma: An automated deep learning approach. JCO Clin. Cancer Inform. 2024, 8, e2300231. [Google Scholar] [CrossRef]
- Fang, P.; Zhou, J.; Xiao, X.; Yang, Y.; Luan, S.; Liang, Z.; Li, X.; Zhang, H.; Shang, Q.; Zeng, X.; et al. The prognostic value of sarcopenia in oesophageal cancer: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 3–16. [Google Scholar] [CrossRef]
- Srpcic, M.; Jordan, T.; Popuri, K.; Sok, M. Sarcopenia and myosteatosis at presentation adversely affect survival after esophagectomy for esophageal cancer. Radiol. Oncol. 2020, 54, 237–246. [Google Scholar] [CrossRef]
- Ichinohe, D.; Muroya, T.; Akasaka, H.; Hakamada, K. Skeletal muscle mass and quality before preoperative chemotherapy influence postoperative long-term outcomes in esophageal squamous cell carcinoma patients. World J. Gastrointest. Surg. 2023, 15, 621–633. [Google Scholar] [CrossRef]
- Mine, S.; Tanaka, K.; Kawachi, H.; Shirakawa, Y.; Kitagawa, Y.; Toh, Y.; Yasuda, T.; Watanabe, M.; Kamei, T.; Oyama, T.; et al. Japanese Classification of Esophageal Cancer, 12th Edition: Part I. Esophagus 2024, 21, 179–215. [Google Scholar] [CrossRef]
- Doki, Y.; Tanaka, K.; Kawachi, H.; Shirakawa, Y.; Kitagawa, Y.; Toh, Y.; Yasuda, T.; Watanabe, M.; Kamei, T.; Oyama, T.; et al. Japanese Classification of Esophageal Cancer, 12th Edition: Part II. Esophagus 2024, 21, 216–269. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; Kuribayashi, S.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 1. Esophagus 2023, 20, 343–372. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; Kuribayashi, S.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 2. Esophagus 2023, 20, 373–389. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: New York, NY, USA, 2017; pp. 63–66. [Google Scholar]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann. Surg. Oncol. 2012, 19, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Machida, R.; Ito, Y.; Daiko, H.; Ozawa, S.; Ogata, T.; Hara, H.; Kojima, T.; Abe, T.; Bamba, T.; et al. Doublet chemotherapy, triplet chemotherapy, or doublet chemotherapy combined with radiotherapy as neoadjuvant treatment for locally advanced oesophageal cancer (JCOG1109 NExT): A randomised, controlled, open-label, phase 3 trial. Lancet 2024, 404, 55–66. [Google Scholar] [CrossRef]
- Yamada, Y.; Higuchi, K.; Nishikawa, K.; Gotoh, M.; Fuse, N.; Sugimoto, N.; Nishina, T.; Amagai, K.; Chin, K.; Niwa, Y.; et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann. Oncol. 2015, 26, 141–148. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Leão Mendes, J.; Ferreira, R.Q.; Mata, I.; Vasco Barreira, J.; Rodrigues, Y.C.; Silva Dias, D.; Capelasm, M.L.; Mäkitie, A.; Guerreiro, I.; Pimenta, N.M.; et al. Body composition analysis in metastatic non-small-cell lung cancer: Depicting sarcopenia in Portuguese tertiary care. Cancers 2025, 17, 539. [Google Scholar] [CrossRef] [PubMed]
- Weigl, M.P.; Feurstein, B.; Clemens, P.; Attenberger, C.; Jäger, T.; Emmanuel, K.; Königsrainer, I.; Tschann, P. Alterations in body composition lead to changes in postoperative outcome and oncologic survival in patients with non-metastatic colon cancer. J. Clin. Med. 2025, 14, 3438. [Google Scholar] [CrossRef]
- Klassen, P.N.; Baracos, V.; Ghosh, S.; Martin, L.; Sawyer, M.B.; Mazurak, V.C. Muscle and adipose wasting despite disease control: Unaddressed side effects of palliative chemotherapy for pancreatic cancer. Cancers 2023, 15, 4368. [Google Scholar] [CrossRef]
- Song, G.J.; Ahn, H.; Son, M.W.; Yun, J.H.; Lee, M.S.; Lee, S.M. Adipose tissue quantification improves the prognostic value of GLIM criteria in advanced gastric cancer patients. Nutrients 2024, 16, 728. [Google Scholar] [CrossRef]
- Cheng, E.; Kirley, J.; Cespedes Feliciano, E.M.; Caan, B.J. Adiposity and cancer survival: A systematic review and meta-analysis. Cancer Causes Control 2022, 33, 1219–1246. [Google Scholar] [CrossRef]
- Rasmussen, S.R.; Nielsen, R.V.; Fenger, A.S.; Siemsen, M.; Ravn, H.B. Postoperative complications and survival after surgical resection of esophageal squamous cell carcinoma. J. Thorac. Dis. 2018, 10, 4052–4060. [Google Scholar] [CrossRef]
- Booka, E.; Kikuchi, H.; Hiramatsu, Y.; Takeuchi, H. The Impact of infectious complications after esophagectomy for esophageal cancer on cancer prognosis and treatment strategy. J. Clin. Med. 2021, 10, 4614. [Google Scholar] [CrossRef] [PubMed]
- Altea-Manzano, P.; Decker-Farrell, A.; Janowitz, T.; Erez, A. Metabolic interplays between the tumour and the host shape the tumour macroenvironment. Nat. Rev. Cancer 2025, 25, 274–292. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Lee, S.; Kang, D.H.; Ahn, T.S.; Kim, S.S.; Yun, J.H.; Kim, H.J.; Seo, S.H.; Kim, T.W.; Kong, H.J.; Baek, M.J. The impact of pre-chemotherapy body composition and immunonutritional markers on chemotherapy adherence in stage III colorectal cancer patients. J. Clin. Med. 2023, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Zhou, X.L.; Yu, C.H.; Wang, W.W.; Ji, F.Z.; He, D.C.; Zhu, W.G.; Tong, Y.S. Association of sarcopenia with toxicity and survival in postoperative recurrent esophageal squamous cell carcinoma patients receiving chemoradiotherapy. Front. Oncol. 2021, 11, 655071. [Google Scholar] [CrossRef]
- Rice, T.W.; Rusch, V.W.; Ishwaran, H.; Blackstone, E.H. Cancer of the esophagus and esophagogastric junction: Data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Worldwide esophageal cancer collaboration. Cancer 2010, 116, 3763–3773. [Google Scholar] [CrossRef]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Katz, A.; Nevo, Y.; Ramírez García Luna, J.L.; Anchouche, S.; Tankel, J.; Caminsky, N.; Mueller, C.; Spicer, J.; Cools-Lartigue, J.; Ferri, L. Long-Term Quality of Life After Esophagectomy for Esophageal Cancer. Ann. Thorac. Surg. 2023, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Skořepa, P.; Ford, K.L.; Alsuwaylihi, A.; O’Connor, D.; Prado, C.M.; Gomez, D.; Lobo, D.N. The impact of prehabilitation on outcomes in frail and high-risk patients undergoing major abdominal surgery: A systematic review and meta-analysis. Clin. Nutr. 2024, 43, 629–648. [Google Scholar] [CrossRef] [PubMed]
- Baimas-George, M.; Watson, M.; Elhage, S.; Parala-Metz, A.; Vrochides, D.; Davis, B.R. Prehabilitation in Frail Surgical Patients: A Systematic Review. World J. Surg. 2020, 44, 3668–3678. [Google Scholar] [CrossRef]
- Shanmugasundaram Prema, S.; Ganapathy, D.; Shanmugamprema, D. Prehabilitation strategies: Enhancing surgical resilience with a focus on nutritional optimization and multimodal interventions. Adv. Nutr. 2025, 16, 100392. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Malerba, S.; Nardone, V.; Salvestrini, V.; Calomino, N.; Testini, M.; Boccardi, V.; Desideri, I.; Gentili, C.; De Luca, R.; et al. Progress and challenges in integrating nutritional care into oncology practice: Results from a national survey on behalf of the NutriOnc Research Group. Nutrients 2025, 17, 188. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kaido, T.; Hamaguchi, Y.; Okumura, S.; Taura, K.; Hatano, E.; Okajima, H.; Uemoto, S. Impact of postoperative changes in sarcopenic factors on outcomes after hepatectomy for hepatocellular carcinoma. J. Hepato-Biliary-Pancreat. Sci. 2016, 23, 57–64. [Google Scholar] [CrossRef]
- Kudou, K.; Saeki, H.; Nakashima, Y.; Kimura, K.; Ando, K.; Oki, E.; Ikeda, T.; Maehara, Y. Postoperative skeletal muscle loss predicts poor prognosis of adenocarcinoma of upper stomach and esophagogastric junction. World J. Surg. 2019, 43, 1068–1075. [Google Scholar] [CrossRef]
- Marasco, G.; Serenari, M.; Renzulli, M.; Alemanni, L.V.; Rossini, B.; Pettinari, I.; Dajti, E.; Ravaioli, F.; Golfieri, R.; Cescon, M.; et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J. Gastroenterol. 2020, 55, 927–943. [Google Scholar] [CrossRef]
| Characteristics | |
|---|---|
| Age, years, median [IQR] | 70 [63–73] |
| Sex | |
| Male/Female | 62/8 |
| Histological type | |
| Squamous cell carcinoma/Adenocarcinoma | 47/23 |
| Main tumor location | |
| Upper thoracic/Middle thoracic/Lower thoracic | 2/24/44 |
| Clinical stage | |
| 0/I/II/III/IV | 1/15/11/37/6 |
| Neoadjuvant chemotherapy | |
| Yes/No | 30/40 |
| Thoracic approach | |
| Thoracoscopy/Thoracotomy | 54/16 |
| Esophagectomy | |
| McKeown/Ivor-Lewis | 43/27 |
| Lymphadenectomy | |
| Three-field/Two-field | 31/39 |
| Reconstructed organ | |
| Gastric conduit/Jejunum/Colon | 54/12/4 |
| Reconstruction route | |
| Posterior mediastinal/Retrosternal | 65/5 |
| Pathological stage | |
| 0/I/II/III/IV | 7/16/14/27/6 |
| Postoperative adjuvant chemotherapy | |
| Yes/No | 47/23 |
| Home nutritional supplementation ≥ 3 months | |
| Yes/No | 24/46 |
| Characteristics | Favorable BC | Unfavorable BC | p-Value |
|---|---|---|---|
| Age, years | 69 [62–73] | 70 [63–73] | 0.097 |
| Sex, male, n (%) | 28 (82) | 34 (94) | 0.112 |
| BMI, kg/m2 | 23.0 [21.2–24.1] | 22.1 [20.2–23.9] | 0.039 |
| Alb, g/dL | 4.0 [3.6–4.2] | 4.0 [3.6–4.2] | 0.273 |
| CRP, mg/dL | 0.3 [0.3–0.3] | 0.30 [0.30–0.30] | 0.330 |
| TC, mg/dL | 187 [152–232] | 190 [158–226] | 0.481 |
| NLR | 1.94 [1.26–2.90] | 1.96 [1.28–3.18] | 0.296 |
| Visceral obesity *, yes, n (%) | 19 (56) | 17 (47) | 0.469 |
| Age-adjusted CCI | 6 (18) | 18 (50) | 0.004 |
| Histology, SCC, n (%) | 23 (68) | 24 (67) | 0.930 |
| Tumor location, lower, n (%) | 13 (38) | 13 (36) | 0.854 |
| Clinical stage, ≥II, n (%) | 20 (59) | 23 (64) | 0.663 |
| Neoadjuvant chemotherapy, yes, n (%) | 13 (38) | 17 (47) | 0.448 |
| Postoperative Complication | Favorable BC | Unfavorable BC | p-Value |
|---|---|---|---|
| Grade classification | |||
| All grade, n (%) | 29 (85) | 33 (92) | 0.402 |
| Grade II ≥, n (%) | 19 (56) | 24 (67) | 0.354 |
| Grade III ≥, n (%) | 6 (18) | 15 (42) | 0.028 |
| Infectious complication * | |||
| All grade, n (%) | 15 (44) | 22 (61) | 0.155 |
| Grade II ≥, n (%) | 14 (41) | 18 (50) | 0.459 |
| Grade III ≥, n (%) | 5 (15) | 14 (39) | 0.023 |
| Factor | Category | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR [95% CI] | p-Value | HR [95% CI] | p-Value | ||
| Age | ≥72 years | 2.166 [1.103–4.254] | 0.025 | 0.882 [0.357–2.718] | 0.785 |
| Sex | Male | 1.012 [0.356–2.874] | 0.982 | ||
| BMI | ≥20.5 kg/m2 | 1.497 [0.729–3.073] | 0.272 | ||
| Alb | <4.1 g/dL | 2.514 [1.220–5.179] | 0.012 | 0.952 [0.358–2.535] | 0.922 |
| CRP | <0.5 mg/dL | 2.110 [1.025–4.344] | 0.043 | 0.681 [0.274–1.689] | 0.407 |
| TC | <190 mg/dL | 1.754 [0.878–3.505] | 0.112 | ||
| NLR | ≥2.71 | 2.797 [1.402–5.463] | 0.003 | 1.693 [0.722–3.971] | 0.226 |
| Visceral obesity * | Yes | 0.807 [0.412–1.583] | 0.534 | ||
| Age-adjusted CCI | ≥score 5 | 3.222 [1.633–6.356] | <0.001 | 2.093 [0.791–5.541] | 0.137 |
| Histology | SCC | 1.092 [0.532–2.240] | 0.811 | ||
| Tumor location | Lower | 1.236 [0.602–2.536] | 0.564 | ||
| Neoadjuvant chemotherapy | Yes | 1.697 [0.865–3.329] | 0.124 | ||
| Thoracic approach | thoracotomy | 1.378 [0.642–2.958] | 0.411 | ||
| Esophagectomy | McKeown | 1.736 [0.810–3.720] | 0.156 | ||
| Lymphadenectomy | Three-field † | 1.411 [0.734–2.828] | 0.288 | ||
| Reconstructed organ | Gastric conduit | 1.193 [0.519–2.740] | 0.677 | ||
| Reconstruction route | Retrosternal | 1.182 [0.361–3.872] | 0.782 | ||
| Intraoperative blood loss | ≥7.31 g/kg | 2.224 [1.132–4.368] | 0.020 | 1.658 [0.753–3.648] | 0.209 |
| Postoperative complication | ≥Grade III | 2.144 [1.071–4.292] | 0.031 | 1.825 [0.817–4.076] | 0.142 |
| Pathological stage | ≥stage II | 5.314 [1.869–15.110] | 0.002 | 6.028 [1.717–21.155] | 0.005 |
| Postoperative adjuvant chemotherapy | Yes | 2.038 [0.887–4.684] | 0.093 | ||
| Home nutritional supplementation | <3 months | 1.874 [0.848–4.141] | 0.121 | ||
| Body composition status | Unfavorable (≤score 1) | 4.029 [1.871–8.672] | <0.001 | 3.940 [1.620–9.581] | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuto, K.; Nabeya, Y.; Mori, M.; Kosugi, C.; Usui, A.; Narushima, K.; Shimizu, H. Impact of Preoperative Comprehensive Body Composition on Postoperative Outcomes in Patients with Esophageal Cancer. J. Clin. Med. 2025, 14, 7392. https://doi.org/10.3390/jcm14207392
Shuto K, Nabeya Y, Mori M, Kosugi C, Usui A, Narushima K, Shimizu H. Impact of Preoperative Comprehensive Body Composition on Postoperative Outcomes in Patients with Esophageal Cancer. Journal of Clinical Medicine. 2025; 14(20):7392. https://doi.org/10.3390/jcm14207392
Chicago/Turabian StyleShuto, Kiyohiko, Yoshihiro Nabeya, Mikito Mori, Chihiro Kosugi, Akihiro Usui, Kazuo Narushima, and Hiroaki Shimizu. 2025. "Impact of Preoperative Comprehensive Body Composition on Postoperative Outcomes in Patients with Esophageal Cancer" Journal of Clinical Medicine 14, no. 20: 7392. https://doi.org/10.3390/jcm14207392
APA StyleShuto, K., Nabeya, Y., Mori, M., Kosugi, C., Usui, A., Narushima, K., & Shimizu, H. (2025). Impact of Preoperative Comprehensive Body Composition on Postoperative Outcomes in Patients with Esophageal Cancer. Journal of Clinical Medicine, 14(20), 7392. https://doi.org/10.3390/jcm14207392






