Osteoporosis in Severe Asthmatic Patients: Data from the Severe Asthma Network in Italy (SANI) Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Ethical Issues
2.3. Statistical Analysis
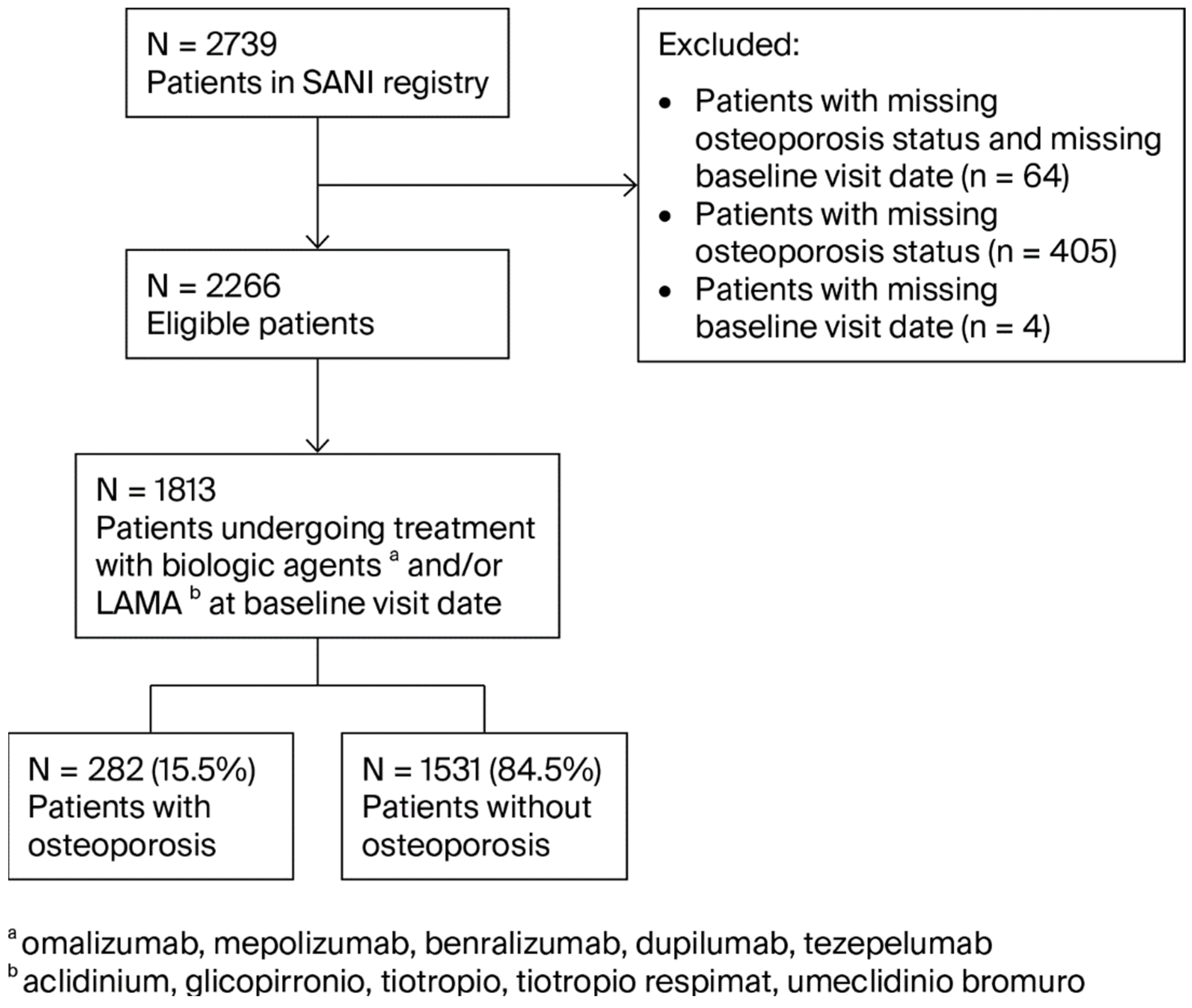
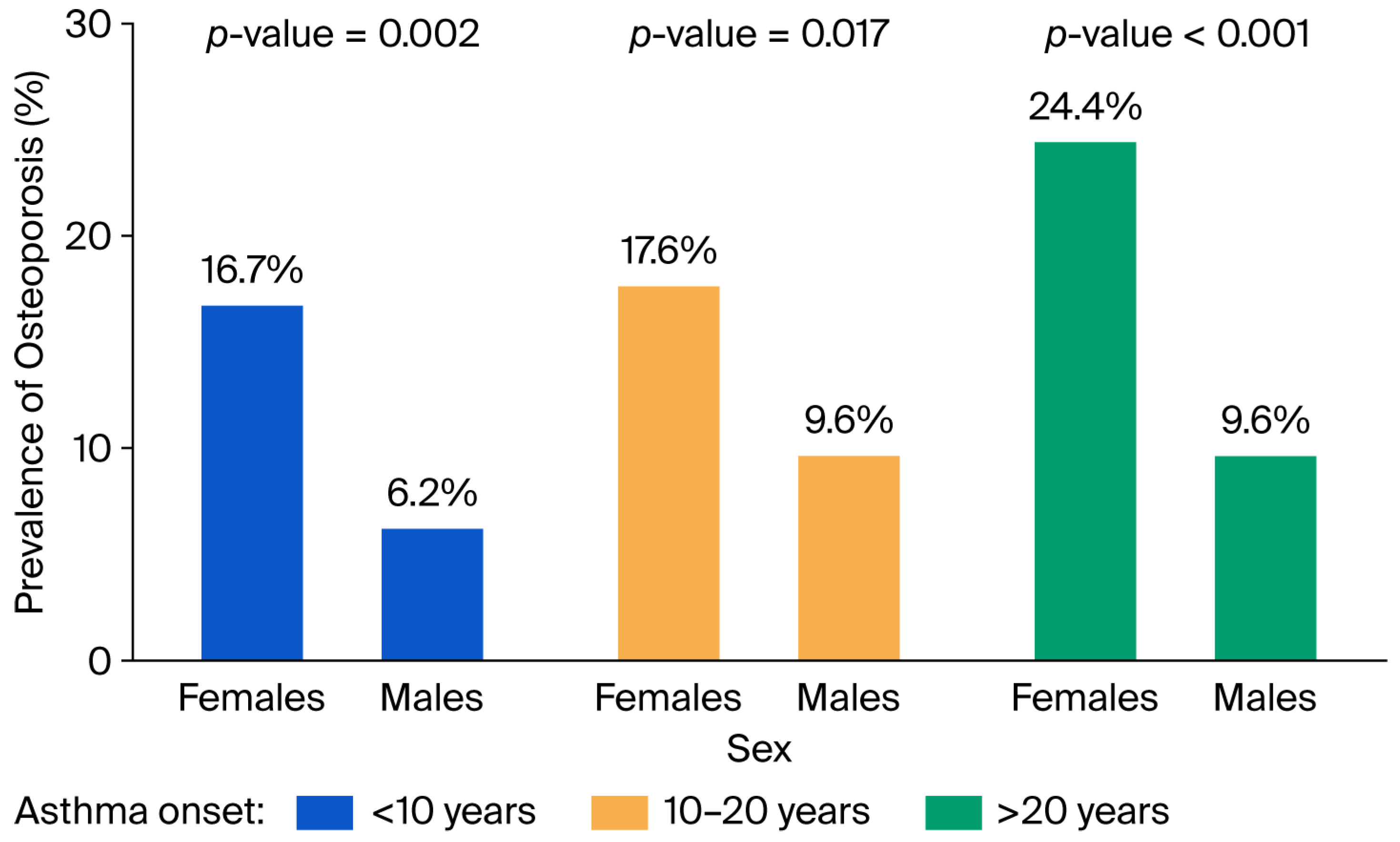
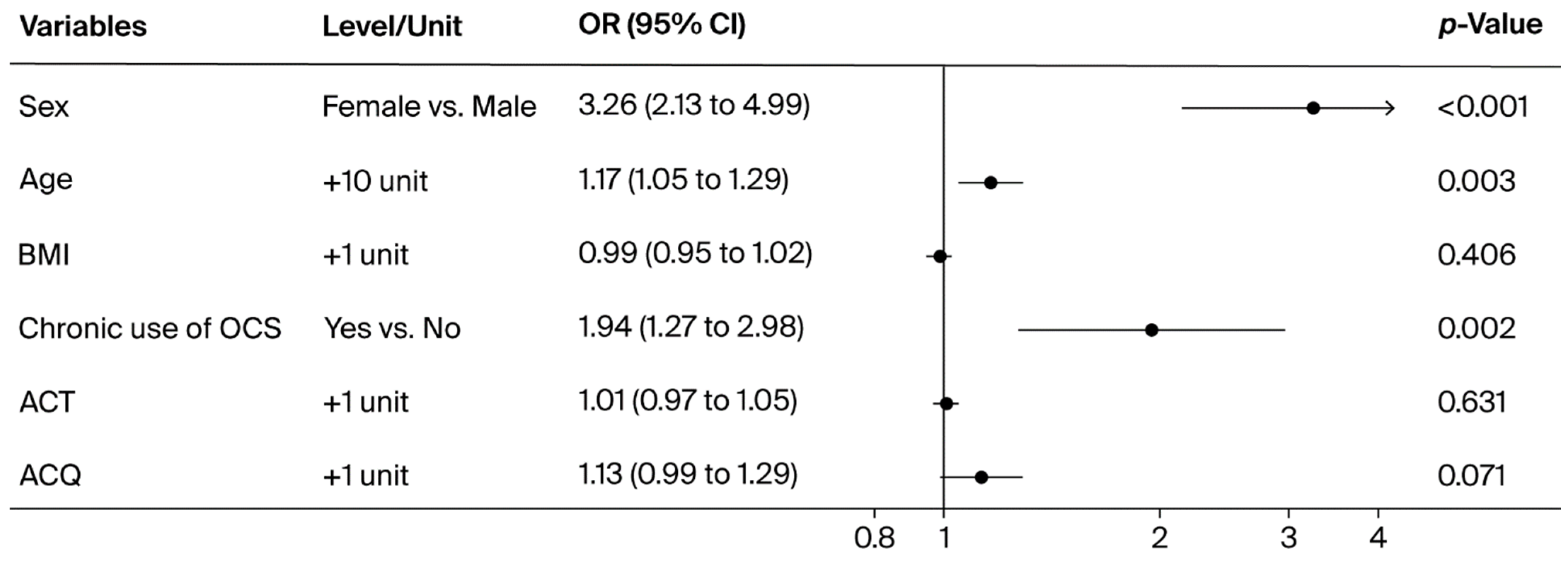
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACO | Asthma–COPD overlap |
| ACT | Asthma Control Test |
| ACQ | Asthma Control Questionnaire |
| ATS | American Thoracic Society |
| BMI | Body mass index |
| BMD | Bone mineral density |
| CPRD | Clinical Practice Research Datalink |
| COPD | Chronic obstructive pulmonary disease |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| DXA | Dual-energy X-ray absorptiometry |
| EGPA | Eosinophilic granulomatosis with polyangiitis |
| ERS | European Respiratory Society |
| FeNO | Fractional exhaled nitric oxide |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| GINA | Global Initiative for Asthma |
| HES | Hospital Episode Statistics |
| ICSs | Inhaled corticosteroids |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| IQR | Interquartile range |
| ISAR | International Severe Asthma Registry |
| ISTAT | Istituto Nazionale di Statistica |
| LABAs | Long-acting β2-agonists |
| LAMAs | Long-acting muscarinic antagonists |
| mAbs | Monoclonal antibodies |
| nOP | Non-osteoporosis |
| OCSs | Oral corticosteroids |
| OP | Osteoporosis |
| OSAS | Obstructive sleep apnea syndrome |
| SANI | Severe Asthma Network Italy |
| TNF-α | Tumor necrosis factor-alpha |
| TSLP | Thymic stromal lymphopoietin |
| WHO | World Health Organization |
Appendix A
- The SANI study group †Diego Bagnasco 16, Luisa Brussino 17, Cecilia Calabrese 18, Gianna Camiciottoli 19, Marco Caminati 12,20, Giovanna Elisiana Carpagnano 21,22, Cristiano Caruso 23, Angelo Guido Corsico 24, Maria Teresa Costantino 25, Claudia Crimi 26, Alice D’Adda 27, Simona D’Alo 28, Maria D’Amato 29, Leda D’Amico 30, Corrado D’Andria 31, Fabiano Di Marco 32, Nicola Cosimo Facciolongo 33, Alessandro Farsi 34, Manlio Milanese 35, Michele Mondoni 36, Eustachio Nettis 37, Vincenzo Patella 38, Girolamo Pelaia 39, Luisa Ricciardi 40, Fabio Luigi Massimo Ricciardolo 41, Luca Richeldi 42, Erminia Ridolo 43, Pierachille Santus 44, Nicola Scichilone 45, Giulia Scioscia 46, Giuseppe Spadaro 47, Antonio Spanevello 48, Paolo Tarsia 49, Massimo Triggiani 50, Mona-Rita Yacoub 5116 Clinica di Malattie Respiratorie e Allergologia, Dip. Medicina Interna, Univ. degli Studi di Genova, IRCCS-AOU San Martino, Genoa, Italy17 S.S.D.D.U. Immunologia Clinica e Allergologia, Ospedale Mauriziano, Torino, Italy18 Department of Translational Medical Sciences, University of Campania “Luigi Vanvitelli”, Naples, Italy19 UNIT ASMA GRAVE - Ambulatorio Asma Grave Pneumologia e Fisiopatologia Toraco-Polmonare, Azienda Ospedaliera Universitaria Careggi (FI), Florence, Italy20 Department of Medicine, University of Verona, Verona, Italy21 Department of Translational Biomedicine and Neuroscience “DiBraiN”, University of Bari Aldo Moro, Bari, Italy22 Section of Respiratory Diseases, Policlinico Hospital of Bari, Italy23 Allergologic Unit, Policlinico Agostino Gemelli, Rome, Italy24 Division of Respiratory Diseases, IRCCS Policlinico San Matteo, Foundation and Department of Internal Medicine and Therapeutics, University of Pavia, Italy25 Allergy and Clinical Immunology Unit, Department of Medicine, “Carlo Poma” Hospital Mantova, Italy26 Respiratory Medicine Unit, Policlinico “G. Rodolico-San Marco” University Hospital, Catania, Italy27 Broncopneumologia, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy28 UOC Allergologia - PO Civitanova Marche - AREA VASTA 3, Civitanova Marche, Italy29 Department of Respiratory Medicine, University “Federico II” of Naples, Italy30 UOC Pneumologia Arnas Garibaldi, Catania, Italy31 Internal and Respiratory Medicine, SS Annunziata Hospital, Taranto, Italy32 Pulmonary Medicine Unit, ASST Papa Giovanni XXIII Hospital, Bergamo, Italy33 UOC di Pneumologia, Arcispedale S. Maria Nuova Azienda USL di Reggio Emilia, Italy34 SOSD Allergology and Clinical Immunology, Ospedale S. Stefano, USL Toscana Centro, Prato, Italy35 Pulmonology Unit, Santa Corona Hospital, Pietra Ligure (Savona), Italy36 Respiratory Unit, ASST Santi Paolo e Carlo, San Paolo Hospital, Department of Health Sciences, University of Milan, Milan, Italy37 Department of Emergency and Organ Transplantation, School and Chair of Allergology and Clinical Immunology, University of Bari - Aldo Moro, Bari, Italy38 Division of Allergy and Clinical Immunology, Department of Medicine ASL Salerno, Santa Maria Della Speranza Hospital, Salerno, Italy39 U.O. Malattie dell’Apparato Respiratorio, A.O.U. Mater Domini, Catanzaro, Italy40 Allergologia e Immunologia Clinica, AOU Policlinico G. Martino - Università di Messina, Messina Italy41 Department of Clinical and Biological Sciences, University of Turin, San Luigi Gonzaga University Hospital, Orbassano, Turin, Italy42 Fondazione Policlinico Universitario A. Gemelli, IRCCS Catholic University of Rome, Italy43 Department of Medicine and Surgery, University of Parma, Parma, Italy44 Department of Clinical and Biomedical Sciences, University of Milan, Respiratory Diseases, Sacco University Hospital, ASST Fatebenefratelli-Sacco, Milan, Italy45 U.O.C. Pneumologia, Azienda Ospedaliera Universitaria Policlinico P. Giaccone di Palermo, Italy46 Malattie Apparato Respiratorio, Dipartimenti delle funzioni Mediche e Sanitarie, Azienda Ospedaliero Universitaria - Ospedali Riuniti, Foggia, Italy47 Center for Basic and Clinical Immunology Research (CISI), University of Naples Federico II, Naples, Italy48 Pneumologia Riabilitativa, ICS Maugeri IRCCS Tradate, Tradate (VA), Italy49 Pneumology Unit, Grande Ospedale Metropolitano Niguarda, Milan, Italy50 Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy51 Unit of Immunology, Rheumatology, Allergy and Rare Diseases, IRCCS San Raffaele Hospital, Milan, Italy
References
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, ITC17–ITC32. [Google Scholar] [CrossRef]
- Lacativa, P.G.S.; Farias, M.L.F. Osteoporosis and inflammation. Arq. Bras. Endocrinol. Metabol. 2010, 54, 123–132. [Google Scholar] [CrossRef]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef] [PubMed]
- Kearney, D.M.; Lockey, R.F. Osteoporosis and asthma. Ann. Allergy Asthma Immunol. 2006, 96, 769–774; quiz 775–778, 857. [Google Scholar] [CrossRef] [PubMed]
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: A focused review of the impact data in the literature. Eur. Respir. J. 2018, 52, 1800703. [Google Scholar] [CrossRef]
- Watanabe, H.; Sugiyama, K.; Otsuji, N.; Nakano, K.; Arifuku, H.; Wakayama, T.; Tokita, S.; Koyama, K.; Hirata, H.; Arima, M.; et al. Effect of inhaled corticosteroids on bone mineral density in patients with asthma. Asian Pac. J. Allergy Immunol. 2023, 41, 45–52. [Google Scholar] [CrossRef]
- Matsunaga, K.; Adachi, M.; Nagase, H.; Okoba, T.; Hayashi, N.; Tohda, Y. Association of low-dosage systemic corticosteroid use with disease burden in asthma. npj Prim. Care Respir. Med. 2020, 30, 35. [Google Scholar] [CrossRef] [PubMed]
- Shackleford, A.; Heaney, L.G.; Redmond, C.; McDowell, P.J.; Busby, J. Clinical remission attainment, definitions, and correlates among patients with severe asthma treated with biologics: A systematic review and meta-analysis. Lancet Respir. Med. 2025, 13, 23–34. [Google Scholar] [CrossRef]
- Bakaa, L.; Hoyte, F.; Perry, T.T.; Rivera-Spoljaric, K.; Sumino, K.; Chipps, B.; Oppenheimer, J.; Nyenhuis, S.M.; Israel, E.; McCabe, E.; et al. Triple therapy vs dual inhaler therapy for moderate-to-severe asthma: An updated systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2025, 135, 278–286.e11. [Google Scholar]
- Ding, W.; Huang, Y.; Li, G.; Dong, Y.; Li, X.; Wu, M.; Song, K.; Li, F. Higher risk of osteoporosis in adult-onset asthma than childhood-onset asthma: From genetic and prospective evidence. Osteoporos. Int. 2024, 35, 659–668. [Google Scholar] [CrossRef]
- Sweeney, J.; Patterson, C.C.; Menzies-Gow, A.; Niven, R.M.; Mansur, A.H.; Bucknall, C.; Chaudhuri, R.; Price, D.; Brightling, C.E.; Heaney, L.G.; et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: Cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016, 71, 339–346. [Google Scholar] [CrossRef]
- Scelo, G.; Torres-Duque, C.A.; Maspero, J.; Tran, T.N.; Murray, R.; Martin, N.; Menzies-Gow, A.N.; Hew, M.; Peters, M.J.; Gibson, P.G.; et al. Analysis of comorbidities and multimorbidity in adult patients in the International Severe Asthma Registry. Ann. Allergy Asthma Immunol. 2024, 132, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Hardtstock, F.; Krieger, J.; Wilke, T.; Lukas, M.; Ultsch, B.; Welte, R.; Quinzler, R.; Maywald, U.; Timmermann, H. Epidemiology, treatment and health care resource use of patients with severe asthma in Germany—A retrospective claims data analysis. J. Asthma 2023, 60, 1280–1289. [Google Scholar] [CrossRef]
- Perotin, J.M.; Gauquelin, L.; Just, N.; Devouassoux, G.; Chenivesse, C.; Bourdin, A.; Garcia, G.; Saint Raymond, C.; Boudjemaa, A.; Bonniaud, P.; et al. Severe asthma care trajectories: The French RAMSES cohort. ERJ Open Res. 2024, 10, 00837-2023. [Google Scholar] [CrossRef] [PubMed]
- von Bülow, A.; Hansen, S.; Sandin, P.; Ernstsson, O.; Janson, C.; Lehtimäki, L.; Kankaanranta, H.; Ulrik, C.; Aarli, B.B.; Geale, K.; et al. Severe asthma trajectories in adults: Findings from the NORDSTAR cohort. ERJ Open Res. 2023, 62, 2202474. [Google Scholar] [CrossRef] [PubMed]
- The International Osteoporosis Foundation (IOF) Website. Facts & Statistics. Available online: https://www.osteoporosis.foundation/facts-statistics (accessed on 18 April 2025).
- Chalitsios, C.V.; McKeever, T.M.; Shaw, D.E. Incidence of osteoporosis and fragility fractures in asthma: A UK population-based matched cohort study. Eur. Respir. J. 2021, 57, 2001251. [Google Scholar] [CrossRef]
- Chalitsios, C.V.; Shaw, D.E.; McKeever, T.M. Risk of osteoporosis and fragility fractures in asthma due to oral and inhaled corticosteroids: Two population-based nested case-control studies. Thorax 2021, 76, 21–28. [Google Scholar] [CrossRef]
- Canonica, G.W.; Malvezzi, L.; Blasi, F.; Paggiaro, P.; Mantero, M.; Senna, G.; Heffler, E.; Severe Asthma Network Italy (SANI). Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: Evidences from the Severe Asthma Network Italy (SANI) registry. Respir. Med. 2020, 166, 105947. [Google Scholar] [CrossRef]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef]
- Kelepouris, N.; Harper, K.D.; Gannon, F.; Kaplan, F.S.; Haddad, J.G. Severe osteoporosis in men. Ann. Intern. Med. 1995, 123, 452–460. [Google Scholar] [CrossRef]
- Ebeling, P.R. Osteoporosis in Men. N. Engl. J. Med. 2008, 358, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Kumarathas, I.; Harsløf, T.; Andersen, C.U.; Langdahl, B.; Hilberg, O.; Bjermer, L.; Løkke, A. The risk of osteoporosis in patients with asthma. Eur. Clin. Respir. J. 2020, 7, 1763612. [Google Scholar] [CrossRef]
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Pioggia, G.; Calapai, G.; Gangemi, S.; Mannucci, C. Alarmins in Osteoporosis, RAGE, IL-1, and IL-33 Pathways: A Literature Review. Medicina 2020, 56, 138. [Google Scholar] [CrossRef]
- Albano, G.D.; Gagliardo, R.P.; Montalbano, A.M.; Profita, M. Overview of the Mechanisms of Oxidative Stress: Impact in Inflammation of the Airway Diseases. Antioxidants 2022, 11, 2237. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Jeffery, P.K.; Busse, W.W.; Johnson, M.; Vignola, A.M. From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med. 2000, 161, 1720–1745. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Barbara, F.; Covelli, I.; Damiani, M.A.; Plantone, F.; Notarnicola, A.; Moretti, B.; Quaranta, N.; Ciprandi, G. Long-Term Therapy with Corticosteroids in Nasal Polyposis: A Bone Metabolism Assessment. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. S3), 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Magboul, N.A.; Alotaibi, M.; Aldokhayel, F.; Almazyad, L.M.; Alkwai, K.; Almutawa, N.; Alotaibi, M.; Alyousef, M.Y.; Alsaleh, S.; Alroqi, A. Association Between Serum Vitamin D Level and Uncontrolled Chronic Rhinosinusitis with Nasal Polyposis. Ear Nose Throat J. 2024, 1455613241302892. [Google Scholar] [CrossRef]
- van Staa, T.P.; Leufkens, H.G.; Cooper, C. The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporos. Int. 2002, 13, 777–787. [Google Scholar] [CrossRef]
- Canonica, G.W.; Colombo, G.L.; Bruno, G.M.; Di Matteo, S.; Martinotti, C.; Blasi, F.; Bucca, C.; Crimi, N.; Paggiaro, P.; Pelaia, G.; et al. Shadow cost of oral corticosteroids-related adverse events: A pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ. J. 2019, 12, 100007. [Google Scholar] [CrossRef]
- Latorre, M.; Rizzi, A.; Paggiaro, P.; Baiardini, I.; Bagnasco, D.; Del Giacco, S.; Lombardi, C.; Patella, V.; Nucera, E.; Parente, R.; et al. Asthma management, focused on the use of oral corticosteroids: The opinions of Italian asthmatic patients. J. Asthma 2024, 61, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Baastrup Søndergaard, M.; von Bülow, A.; Bjerrum, A.S.; Schmid, J.; Rasmussen, L.M.; Johnsen, C.R.; Ingebrigtsen, T.; Håkansson, K.E.J.; Johansson, S.L.; et al. Clinical Response and Remission in Patients With Severe Asthma Treated with Biologic Therapies. Chest 2024, 165, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.W.W.; Russell, R.J.; Boddy, C.E.; Balasundaram, K.; Hampson, E.; Bell, M.; Parnell, L.A.; Bonnington, M.A.; Mohammad, S.; Levy, M.; et al. Assessment of an oral corticosteroid withdrawal pathway for severe asthma patients receiving biologic therapies. J. Allergy Clin. Immunol. Pract. 2025, 3, 1807–1815.e4. [Google Scholar] [CrossRef]
- Pini, L.; Bagnasco, D.; Beghè, B.; Braido, F.; Cameli, P.; Caminati, M.; Caruso, C.; Crimi, C.; Guarnieri, G.; Latorre, M.; et al. Unlocking the Long-Term Effectiveness of Benralizumab in Severe Eosinophilic Asthma: A Three-Year Real-Life Study. J. Clin. Med. 2024, 13, 3013. [Google Scholar] [CrossRef]
- Domingo, C.; Rabe, K.F.; Price, D.; Brusselle, G.; Wechsler, M.E.; Xia, C.; Pandit-Abid, N.; Gall, R.; Rowe, P.J.; Deniz, Y.; et al. Long-term efficacy of dupilumab in severe asthma by baseline oral corticosteroid dose. ERJ Open Res. 2023, 9, 00056-2023. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bel, E.H.; Maspero, J.; Heaney, L.G.; Gurnell, M.; Wessman, P.; Martin, U.J.; Siddiqui, S.; Garcia Gil, E. Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE Trial. ERJ Open Res. 2019, 5, 00009-2019. [Google Scholar] [CrossRef]
- Bagnasco, D.; Povero, M.; Pradelli, L.; Brussino, L.; Rolla, G.; Caminati, M.; Menzella, F.; Heffler, E.; Canonica, G.W.; Paggiaro, P.; et al. Economic impact of mepolizumab in uncontrolled severe eosinophilic asthma, in real life. World Allergy Organ. J. 2021, 14, 100509. [Google Scholar] [CrossRef] [PubMed]
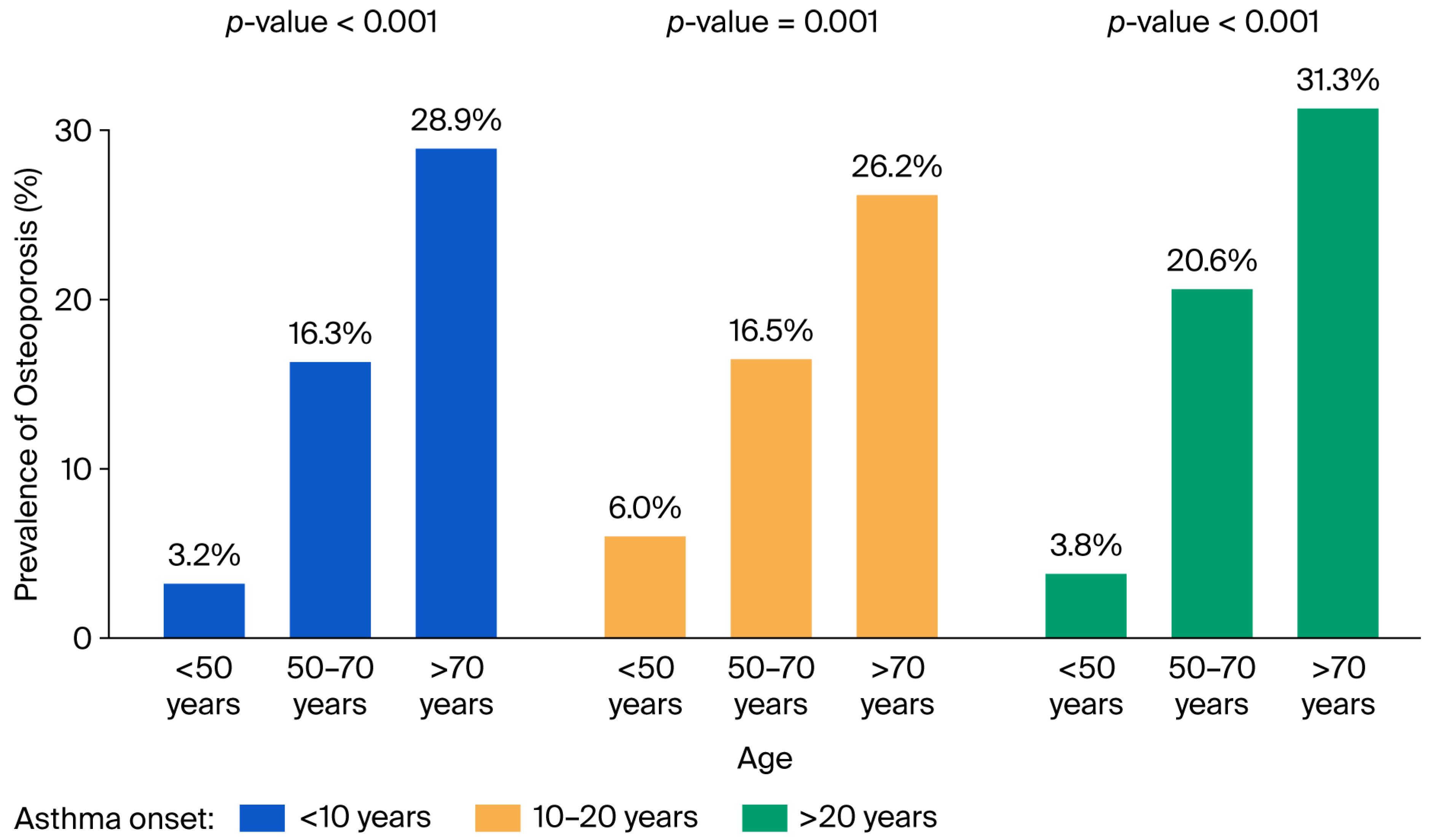
| Patients with Osteoporosis | Patients Without Osteoporosis | p-Value | |
|---|---|---|---|
| Inhaled therapy, n (%) | 282 (15.5) | 1531 (84.4) | 0.539 |
| ICSs + LAMAs | 8 (2.8) | 34 (2.2) | |
| ICSs + LABAs | 107 (37.9) | 629 (41.1) | |
| Triple therapy (ICSs + LABAs + LAMAs) | 167 (59.2) | 868 (56.7) | |
| Biologic therapy, n (%) | 227 (80.5) | 1189 (77.7) | 0.327 |
| Benralizumab | 43 (18.9) | 247 (20.8) | |
| Dupilumab | 15 (6.6) | 117 (9.8) | |
| Mepolizumab | 114 (50.2) | 406 (34.2) | |
| Omalizumab | 55 (24.2) | 416 (35.0) | |
| Tezepelumab | – | 2 (0.2) | |
| Mepolizumab + Omalizumab | – | 1 (0.1) |
| Patients with Osteoporosis N = 282 | Patients Without Osteoporosis N = 1531 | p-Value | |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 226 (80.1) | 885 (57.8) | <0.001 |
| Male | 56 (19.9) | 646 (42.2) | |
| Age (years), n (%) | |||
| <50 | 21 (7.5) | 471 (30.8) | <0.001 |
| 50–70 | 193 (68.4) | 884 (57.7) | |
| >70 | 68 (24.1) | 176 (11.5) | |
| mean (SD) | 63.2 (9.9) | 54.5 (13.4) | <0.001 |
| Age at asthma onset (years), mean (SD) | 35.6 (17.4) | 32.4 (17.0) | 0.007 |
| Age at diagnosis of asthma (years), mean (SD) | 38.8 (17.5) | 35.4 (17.1) | 0.003 |
| Years from asthma onset or diagnosis, n (%) | |||
| <10 | 52 (19.7) | 361 (26.2) | 0.008 |
| 10–20 | 67 (25.4) | 399 (29.0) | |
| >20 | 145 (54.9) | 618 (44.9) | |
| Not reported | 18 | 153 | |
| Median (IQR) | 21 (12–39) | 19 (9–30) | <0.001 |
| BMI (kg/m2), median (IQR) | 25.4 (22.7–28.4) | 25.5 (22.8–29.2) | 0.563 |
| Smoker, n (%) | |||
| No | 198 (70.5) | 1030 (68.2) | 0.187 |
| Yes | 7 (2.5) | 75 (5.0) | |
| Ex | 76 (27.0) | 406 (26.9) | |
| Pack years, median (IQR) | 10 (5–16) | 10 (4–20) | 0.981 |
| Not reported | 1 | 20 | |
| Blood eosinophils (cell/μL), median (IQR) | 305 (100–653) | 330 (120–620) | 0.758 |
| Blood eosinophils (%), median (IQR) | 4.0 (1.5–8.8) | 4.0 (1.5–8.2) | 0.792 |
| Blood neutrophils (cell/μL), median (IQR) | 4000 (3100–5330) | 3950 (3173–5198) | 0.771 |
| Blood neutrophils (%), median (IQR) | 55.7 (47.9–62.4) | 55.5 (49.1–62.7) | 0.676 |
| Max blood eosinophils (cell/μL), median (IQR) | 685 (413–1038) | 600 (350–1010) | 0.314 |
| FeNO (ppb), mean (SD) | 47.0 (41.4) | 43.9 (45.2) | 0.441 |
| Type 2 inflammation | |||
| No | 6 (2.6) | 16 (1.4) | 0.263 |
| Yes | 223 (97.4) | 1163 (98.6) | |
| Not reported | 53 | 352 | |
| Pre-bronchodilator FEV1 (L), mean (SD) | 1.7 (0.8) | 2.3 (2.1) | <0.001 |
| Pre-bronchodilator FEV1 (%), mean (SD) | 71.2 (25.1) | 75.6 (21.5) | 0.007 |
| Pre-bronchodilator FVC (L), mean (SD) | 2.6 (1.0) | 3.2 (1.0) | <0.001 |
| Pre-bronchodilator FVC (%), mean (SD) | 87.0 (24.9) | 90.9 (20.3) | 0.014 |
| Tiffeneau index pre-bronchodilator, mean (SD) | 66.0 (12.3) | 67.4 (12.2) | 0.132 |
| ACT, mean (SD) | 17.2 (5.3) | 19.9 (5.3) | 0.046 |
| ACQ, mean (SD) | 2.5 (1.6) | 2.0 (1.6) | 0.015 |
| Number of exacerbations in the previous 12 months, median (IQR) | 2 (0–3) | 1 (0–3) | 0.002 |
| Chronic use of OCSs, n (%) | |||
| No | 225 (79.8) | 1365 (89.2) | <0.001 |
| Yes | 57 (20.2) | 166 (10.8) |
| Patients with Osteoporosis N = 282 | Patients Without Osteoporosis N = 1531 | p-Value | |
|---|---|---|---|
| Rhinitis, n (%) | |||
| No | 112 (40.1) | 589 (38.6) | 0.888 |
| Yes, previously | 33 (11.8) | 188 (12.3) | |
| Yes, ongoing | 134 (48.0) | 748 (49.0) | |
| Not reported | 3 | 6 | |
| Chronic rhinosinusitis without polyps, n (%) | |||
| No | 202 (72.7) | 1120 (74.6) | 0.509 |
| Yes, previously | 35 (12.6) | 154 (10.3) | |
| Yes, ongoing | 41 (14.7) | 228 (15.2) | |
| Not reported | 4 | 29 | |
| Nasal polyposis, n (%) | |||
| No | 151 (53.7) | 831 (54.7) | 0.898 |
| Yes, confirmed by CT scan or nasal endoscopy | 121 (43.1) | 635 (41.8) | |
| Yes, suspected | 9 (3.2) | 54 (3.6) | |
| Age at diagnosis of nasal polyposis (years), median (IQR) | 46 (33–55) | 42 (33–50) | 0.083 |
| Atopic dermatitis, n (%) | |||
| No | 262 (93.6) | 1411 (92.8) | 0.545 |
| Yes | 12 (4.3) | 85 (5.6) | |
| Previously | 6 (2.1) | 24 (1.6) | |
| Not reported | 2 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latorre, M.; Costanzo, G.; Ledda, A.G.; Sambugaro, G.; Cardini, C.; Sala, I.; Oriecuia, C.; Bagnardi, V.; Blasi, F.; Paggiaro, P.; et al. Osteoporosis in Severe Asthmatic Patients: Data from the Severe Asthma Network in Italy (SANI) Registry. J. Clin. Med. 2025, 14, 7387. https://doi.org/10.3390/jcm14207387
Latorre M, Costanzo G, Ledda AG, Sambugaro G, Cardini C, Sala I, Oriecuia C, Bagnardi V, Blasi F, Paggiaro P, et al. Osteoporosis in Severe Asthmatic Patients: Data from the Severe Asthma Network in Italy (SANI) Registry. Journal of Clinical Medicine. 2025; 14(20):7387. https://doi.org/10.3390/jcm14207387
Chicago/Turabian StyleLatorre, Manuela, Giulia Costanzo, Andrea Giovanni Ledda, Giada Sambugaro, Cristina Cardini, Isabella Sala, Chiara Oriecuia, Vincenzo Bagnardi, Francesco Blasi, Pierluigi Paggiaro, and et al. 2025. "Osteoporosis in Severe Asthmatic Patients: Data from the Severe Asthma Network in Italy (SANI) Registry" Journal of Clinical Medicine 14, no. 20: 7387. https://doi.org/10.3390/jcm14207387
APA StyleLatorre, M., Costanzo, G., Ledda, A. G., Sambugaro, G., Cardini, C., Sala, I., Oriecuia, C., Bagnardi, V., Blasi, F., Paggiaro, P., Canonica, G. W., Heffler, E., Senna, G., Firinu, D., Puxeddu, I., Pini, L., Del Giacco, S., & on behalf of the SANI Study Group. (2025). Osteoporosis in Severe Asthmatic Patients: Data from the Severe Asthma Network in Italy (SANI) Registry. Journal of Clinical Medicine, 14(20), 7387. https://doi.org/10.3390/jcm14207387










