Central Bone Mineral Density Is Not a Reliable Surrogate for Assessing Suitable Bone Strength for Cementless Total Knee Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Central BMD Assessment
2.3. Clinical Fixation Type Selection Protocol
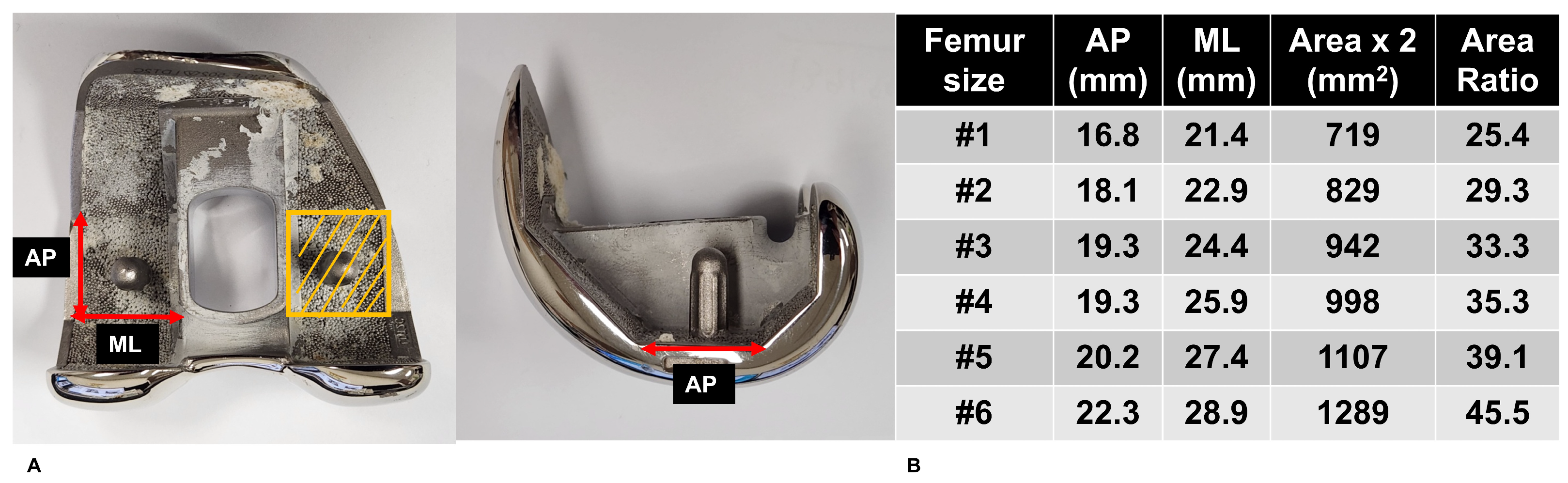
2.4. Bone Strength Determination
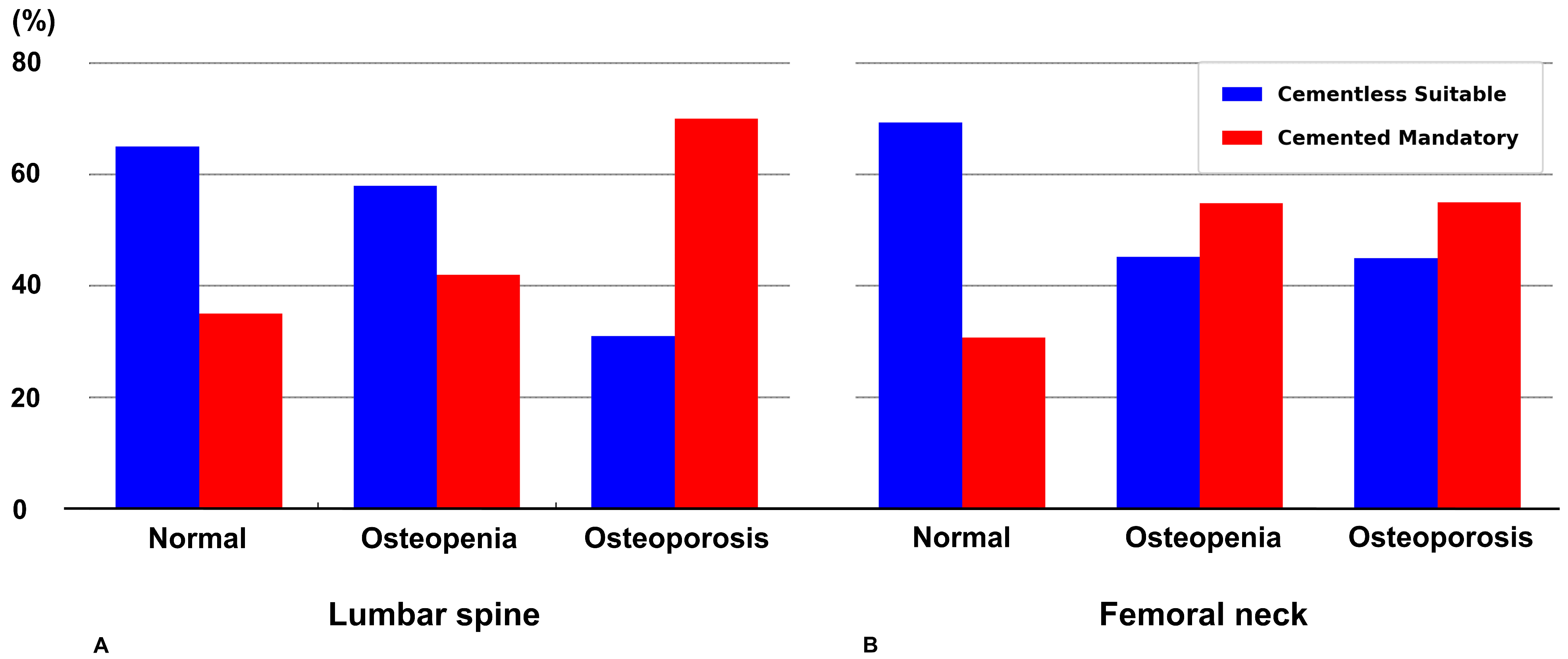
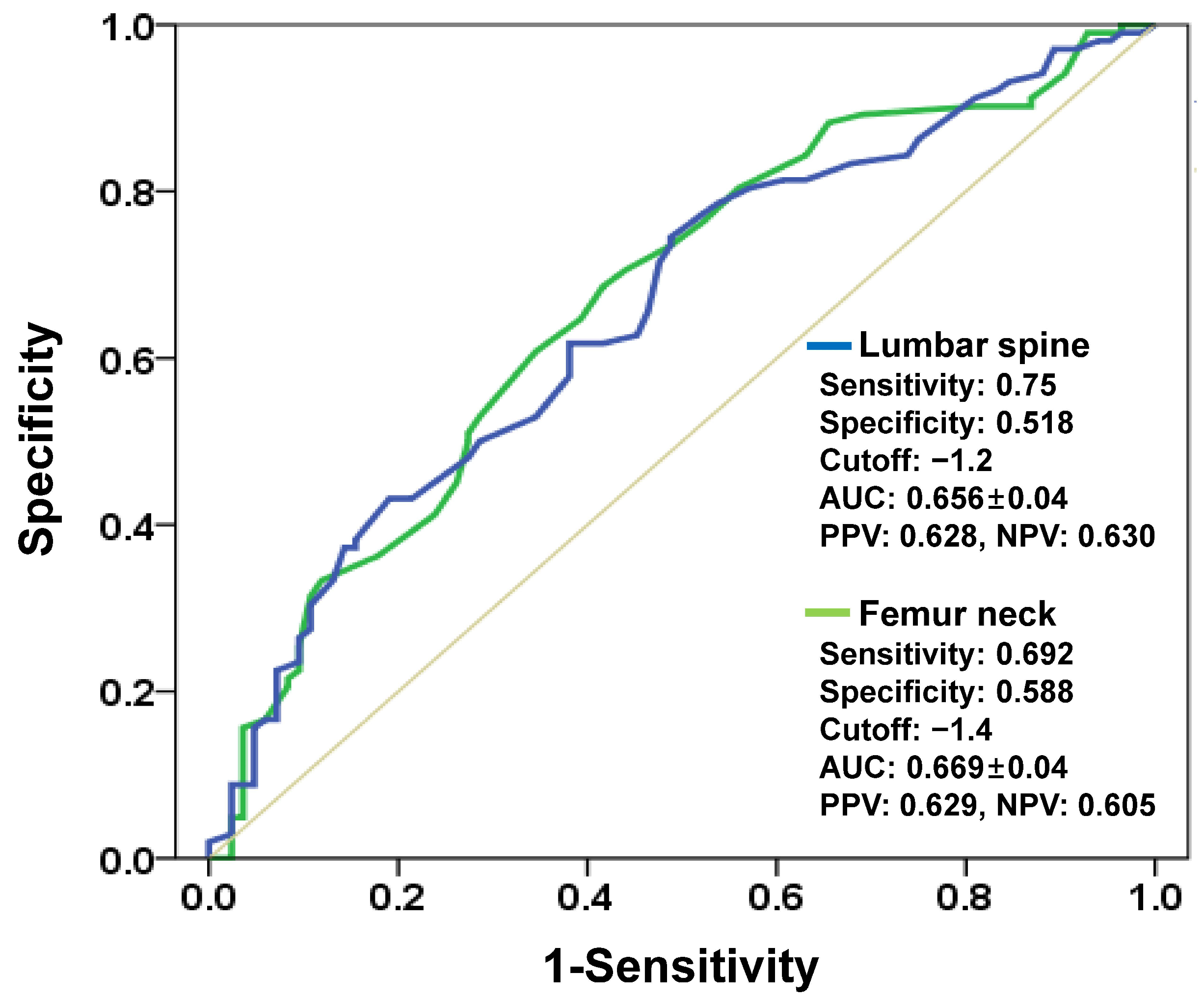
2.5. Defining Strength-Based Criteria for Cementless TKA
2.6. Statistical Analysis
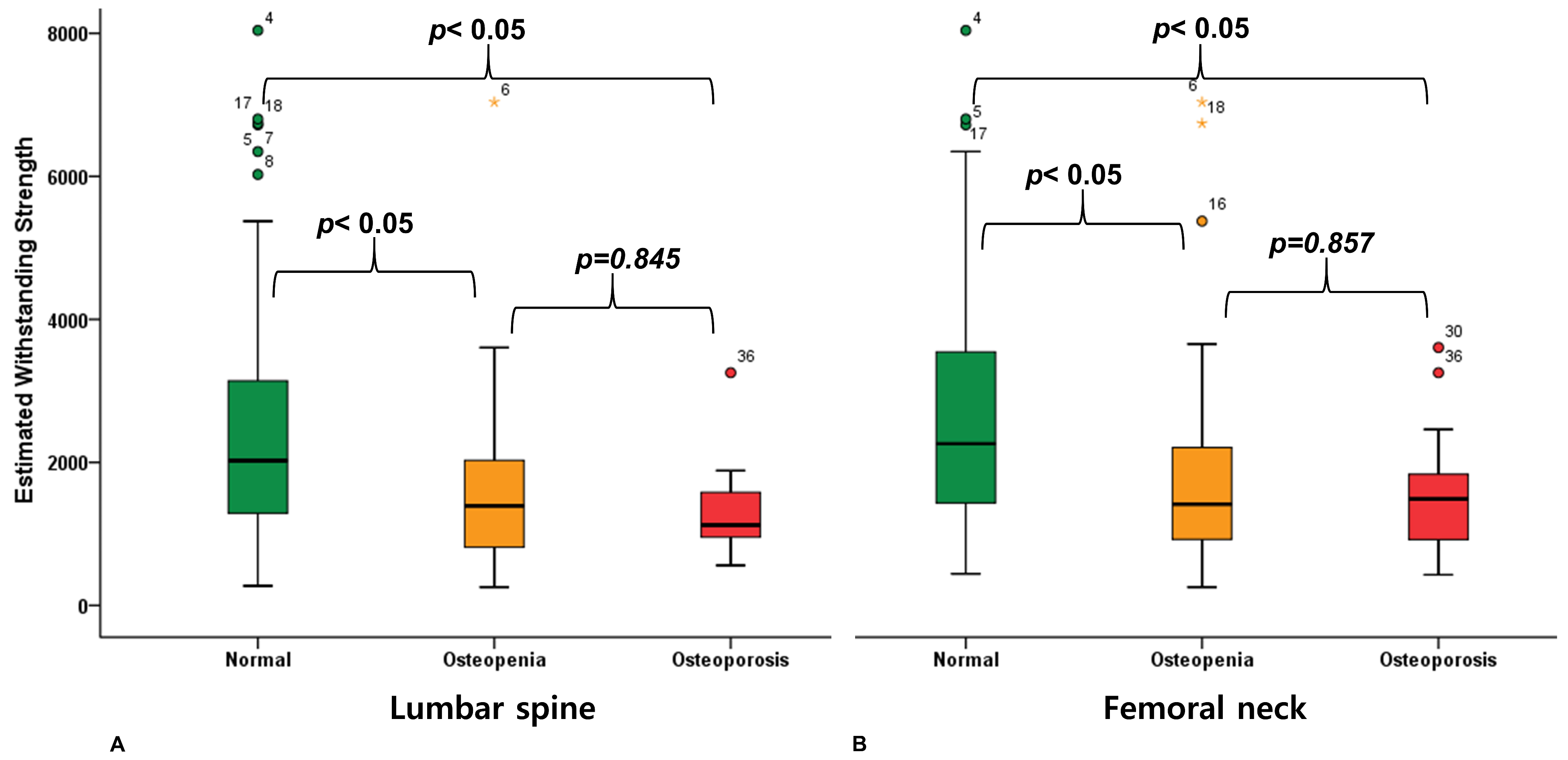
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TKA | Total Knee Arthroplasty |
| cBMD | Central Bone Mineral Density |
| DXA | Dual-energy X-ray Absorptiometry |
| BMI | Body Mass Index |
| HU | Hounsfield Unit |
| MRS | Minimum Required Strength |
| EWS | Estimated Withstanding Strength |
| AUC | Area Under the Curve |
| ANOVA | Analysis of Variance |
| HSD | Honestly Significant Difference |
References
- Ashkenazi, I.; Lawrence, K.W.; Kaplan, M.; Arshi, A.; Rozell, J.C.; Schwarzkopf, R.; Lajam, C.M. Demographic and Socioeconomic Trends of Patients Undergoing Total Knee Arthroplasty from 2013 to 2022-An Analysis from an Urban Orthopaedic Hospital. J. Arthroplast. 2024, 39, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Choi, B.S.; Ro, D.H.; Lee, M.C.; Han, H.S. Fixed-Bearing and Higher Postoperative Knee Flexion Angle as Predictors of Satisfaction in Asian Patients Undergoing Posterior-Stabilized Total Knee Arthroplasty. Clin. Orthop. Surg. 2024, 16, 733–740. [Google Scholar] [CrossRef]
- Salem, H.S.; Tarazi, J.M.; Ehiorobo, J.O.; Marchand, K.B.; Mathew, K.K.; Sodhi, N.; Mont, M.A. Cementless Fixation for Total Knee Arthroplasty in Various Patient Populations: A Literature Review. J. Knee Surg. 2020, 33, 848–855. [Google Scholar] [CrossRef]
- Chen, C.; Li, R. Cementless versus cemented total knee arthroplasty in young patients: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2019, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.J.; Cho, W.S.; Choi, N.Y.; Kim, T.K. Causes, risk factors, and trends in failures after TKA in Korea over the past 5 years: A multicenter study. Clin. Orthop. Relat. Res. 2014, 472, 316–326. [Google Scholar] [CrossRef]
- Lawrence, K.W.; Sobba, W.; Rajahraman, V.; Schwarzkopf, R.; Rozell, J.C. Does body mass index influence improvement in patient reported outcomes following total knee arthroplasty? A retrospective analysis of 3918 cases. Knee Surg. Relat. Res. 2023, 35, 21. [Google Scholar] [CrossRef]
- Kamath, A.F.; Siddiqi, A.; Malkani, A.L.; Krebs, V.E. Cementless Fixation in Primary Total Knee Arthroplasty: Historical Perspective to Contemporary Application. J. Am. Acad. Orthop. Surg. 2021, 29, e363–e379. [Google Scholar] [CrossRef]
- Abdel, M.P.; Carender, C.N.; Berry, D.J. Current Practice Trends in Primary Hip and Knee Arthroplasties Among Members of the American Association of Hip and Knee Surgeons. J. Arthroplast. 2023, 38, 1921–1927.e1923. [Google Scholar] [CrossRef]
- Yoon, C.; Chang, M.J.; Chang, C.B.; Chai, J.W.; Jeong, H.; Song, M.K.; Shin, J.H.; Kang, S.B. Bone Mineral Density Around the Knee Joint: Correlation with Central Bone Mineral Density and Associated Factors. J. Clin. Densitom. 2020, 23, 82–91. [Google Scholar] [CrossRef]
- Alavizadeh, S.A.; Mohajeri-Tehrani, M.R.; Rostamian, A.; Aghaei Meybodi, H.R.; Qorbani, M.; Keshtkar, A.A.; Panahi, S.S.; Rahdari, F.; Khashayar, P. Prevalence and associated factors of T-score discordance between different sites in Iranian patients with spinal cord injury. Spinal Cord 2014, 52, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Hongsdusit, N.; von Muhlen, D.; Barrett-Connor, E. A comparison between peripheral BMD and central BMD measurements in the prediction of spine fractures in men. Osteoporos. Int. 2006, 17, 872–877. [Google Scholar] [CrossRef]
- Sung, K.H.; Choi, Y.; Cho, G.H.; Chung, C.Y.; Park, M.S.; Lee, K.M. Peripheral DXA measurement around ankle joint to diagnose osteoporosis as assessed by central DXA measurement. Skelet. Radiol. 2018, 47, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.; Kwak, D.S.; Kim, Y.D.; Park, S.; Cho, N.; Koh, I.J. Central Bone Mineral Density Is Not a Useful Tool to Predict Bone Strength of the Distal Femur for Cementless Total Knee Arthroplasty. Clin. Orthop. Surg. 2024, 16, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, C.; Marwaha, R.K.; Tandon, N.; Kanwar, R.; Mani, K.; Narang, A.; Bhadra, K.; Singh, S. Correlation between bone mineral density measured by peripheral and central dual energy X-ray absorptiometry in healthy Indian children and adolescents aged 10–18 years. J. Pediatr. Endocrinol. Metab. 2013, 26, 695–702. [Google Scholar] [CrossRef]
- Lai, Y.M.; Qin, L.; Yeung, H.Y.; Lee, K.K.; Chan, K.M. Regional differences in trabecular BMD and micro-architecture of weight-bearing bone under habitual gait loading a pQCT and microCT study in human cadavers. Bone 2005, 37, 274–282. [Google Scholar] [CrossRef]
- Turunen, M.J.; Prantner, V.; Jurvelin, J.S.; Kroger, H.; Isaksson, H. Composition and microarchitecture of human trabecular bone change with age and differ between anatomical locations. Bone 2013, 54, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Lee, S.W.; In, Y.; Kim, M.S.; Kim, Y.D.; Lee, S.Y.; Lee, J.W.; Koh, I.J. Dual-Energy CT-Based Bone Mineral Density Has Practical Value for Osteoporosis Screening around the Knee. Medicina 2022, 58, 1085. [Google Scholar] [CrossRef]
- Dunham, C.E.; Takaki, S.E.; Johnson, J.A.; Dunning, C.E. Mechanical properties of cancellous bone of the distal humerus. Clin. Biomech. 2005, 20, 834–838. [Google Scholar] [CrossRef]
- Gordon, K.D.; Duck, T.R.; King, G.J.; Johnson, J.A. Mechanical properties of subchondral cancellous bone of the radial head. J. Orthop. Trauma 2003, 17, 285–289. [Google Scholar] [CrossRef]
- D’Lima, D.D.; Patil, S.; Steklov, N.; Chien, S.; Colwell, C.W., Jr. In vivo knee moments and shear after total knee arthroplasty. J. Biomech. 2007, 40 (Suppl. S1), S11–S17. [Google Scholar] [CrossRef]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.; Beier, A.; Bergmann, G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef]
- Roessler, P.P.; Eich, J.; Wirtz, D.C.; Schildberg, F.A. Longitudinal Radiographic Bone Density Measurement in Revision Hip Arthroplasty and Its Correlation with Clinical Outcome. J. Clin. Med. 2023, 12, 2795. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.I.; Zheng, K.; Lin, C.; Mei, L.; Lu, L.; Li, W.; Chen, F.P.; Wang, Y.; Zhou, X.; Wang, F.; et al. Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nat. Commun. 2021, 12, 5472. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, C.; Busetto, R.; Bernardini, D.; Anselmi, C.; Zotti, A. Accuracy and precision of computer-assisted analysis of bone density via conventional and digital radiography in relation to dual-energy x-ray absorptiometry. Am. J. Vet. Res. 2012, 73, 381–384. [Google Scholar] [CrossRef]
- Johnson, C.C.; Gausden, E.B.; Weiland, A.J.; Lane, J.M.; Schreiber, J.J. Using Hounsfield Units to Assess Osteoporotic Status on Wrist Computed Tomography Scans: Comparison with Dual Energy X-Ray Absorptiometry. J. Hand Surg. Am. 2016, 41, 767–774. [Google Scholar] [CrossRef]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield units for assessing bone mineral density and strength: A tool for osteoporosis management. J. Bone Jt. Surg. Am. 2011, 93, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kwon, S.S.; Kim, H.S.; Yoo, J.H.; Kim, J.; Kim, J.Y.; Min, B.C.; Moon, S.J.; Sung, K.H. Reliability and validity of lower extremity computed tomography as a screening tool for osteoporosis. Osteoporos. Int. 2015, 26, 1387–1394. [Google Scholar] [CrossRef]
- Wong, M.; Papa, A.; Lang, T.; Hodis, H.N.; Labree, L.; Detrano, R. Validation of thoracic quantitative computed tomography as a method to measure bone mineral density. Calcif. Tissue Int. 2005, 76, 7–10. [Google Scholar] [CrossRef]
- Ramschutz, C.; Sollmann, N.; El Husseini, M.; Kupfer, K.; Paprottka, K.J.; Loffler, M.T.; Petzsche, M.R.H.; Schwarting, J.; Bodden, J.; Baum, T.; et al. Cervicothoracic volumetric bone mineral density assessed by opportunistic QCT may be a reliable marker for osteoporosis in adults. Osteoporos. Int. 2025, 36, 423–433. [Google Scholar] [CrossRef]
- Liu, X.S.; Cohen, A.; Shane, E.; Yin, P.T.; Stein, E.M.; Rogers, H.; Kokolus, S.L.; McMahon, D.J.; Lappe, J.M.; Recker, R.R.; et al. Bone density, geometry, microstructure, and stiffness: Relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J. Bone Min. Res. 2010, 25, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Arentsen, L.; Hansen, K.E.; Yagi, M.; Takahashi, Y.; Shanley, R.; McArthur, A.; Bolan, P.; Magome, T.; Yee, D.; Froelich, J.; et al. Use of dual-energy computed tomography to measure skeletal-wide marrow composition and cancellous bone mineral density. J. Bone Min. Metab. 2017, 35, 428–436. [Google Scholar] [CrossRef]
- Molwitz, I.; Leiderer, M.; Ozden, C.; Yamamura, J. Dual-Energy Computed Tomography for Fat Quantification in the Liver and Bone Marrow: A Literature Review. Rofo 2020, 192, 1137–1153. [Google Scholar] [CrossRef]
- Koh, I.J.; Kim, T.K.; Chang, C.B.; Cho, H.J.; In, Y. Trends in use of total knee arthroplasty in Korea from 2001 to 2010. Clin. Orthop. Relat. Res. 2013, 471, 1441–1450. [Google Scholar] [CrossRef]
- Peacock, M.; Buckwalter, K.A.; Persohn, S.; Hangartner, T.N.; Econs, M.J.; Hui, S. Race and sex differences in bone mineral density and geometry at the femur. Bone 2009, 45, 218–225. [Google Scholar] [CrossRef]
- Misra, M.; Ackerman, K.E.; Bredella, M.A.; Stanford, F.C.; Faje, A.T.; Nordberg, A.; Derrico, N.P.; Bouxsein, M.L. Racial Differences in Bone Microarchitecture and Estimated Strength at the Distal Radius and Distal Tibia in Older Adolescent Girls: A Cross-Sectional Study. J. Racial Ethn. Health Disparities 2017, 4, 587–598. [Google Scholar] [CrossRef]
- Park, J.; Chaar, O.; Narayanakurup, J.; Abdelhamead, A.S.A.; Ro, D.H.; Kim, S.E. Do knee alignment patterns differ between Middle Eastern and East Asian populations? A propensity-matched analysis using artificial intelligence. Knee Surg. Relat. Res. 2025, 37, 11. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Wevers, H.W.; Cooke, T.D.; Griffin, M. Bone strength and histomorphometry of the distal femur. J. Arthroplast. 1994, 9, 307–315. [Google Scholar] [CrossRef]
- Christensen, P.; Kjaer, J.; Melsen, F.; Nielsen, H.E.; Sneppen, O.; Vang, P.S. The subchondral bone of the proximal tibial epiphysis in osteoarthritis of the knee. Acta Orthop. Scand. 1982, 53, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Wevers, H.W.; Cooke, T.D. Distribution of bone strength in the proximal tibia. J. Arthroplast. 1988, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F.; Moldovan, L. A Modeling Study for Hip Fracture Rates in Romania. J. Clin. Med. 2025, 14, 3162. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Values (n = 188) |
|---|---|
| First failure load (N) | 59.8 ± 38.1 (7.2~202.5) |
| Displacement at first failure load (mm) | 0.9 ± 0.3 (0.3~1.8) |
| Maximum failure load (N) | 81.5 ± 42.0 (14.6~244.0) |
| Displacement at maximum failure load (mm) | 1.8 ± 0.3 (0.4~2.0) |
| Stiffness (N/mm) | 111.6 ± 80.5 (12.0~533.8) |
| Parameters | Values (n = 188) | |
|---|---|---|
| Demographic information * | ||
| Age (year) | 68.1 ± 5.4 (53~86) | |
| Gender (women) † | 154 (82) | |
| Height (cm) | 155.5 ± 7.0 (143.1~177.2) | |
| Weight (kg) | 65.7 ± 9.6 (45.4~94.3) | |
| BMI (kg/m2) | 27.2 ± 3.4 (19.6~36.5) | |
| Osteoporosis information | ||
| Lumbar spine | Femoral neck | |
| DXA (T-score) * | −0.5 ± 1.5 (−3.6~4.9) | −1.2 ± 1.1 (−3.5~2.8) |
| Prevalence † | ||
| Normal (T score > −1.0) | 113 (60) | 75 (40) |
| Osteopenia (−1.0 ≤ T score ≤ −2.5) | 62 (33) | 93 (50) |
| Osteoporosis (T score < −2.5) | 13 (7) | 20 (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.H.; Kwak, D.-S.; Kim, Y.D.; Cho, N.; Koh, I.J. Central Bone Mineral Density Is Not a Reliable Surrogate for Assessing Suitable Bone Strength for Cementless Total Knee Arthroplasty. J. Clin. Med. 2025, 14, 7384. https://doi.org/10.3390/jcm14207384
Lee DH, Kwak D-S, Kim YD, Cho N, Koh IJ. Central Bone Mineral Density Is Not a Reliable Surrogate for Assessing Suitable Bone Strength for Cementless Total Knee Arthroplasty. Journal of Clinical Medicine. 2025; 14(20):7384. https://doi.org/10.3390/jcm14207384
Chicago/Turabian StyleLee, Dong Hwan, Dai-Soon Kwak, Yong Deok Kim, Nicole Cho, and In Jun Koh. 2025. "Central Bone Mineral Density Is Not a Reliable Surrogate for Assessing Suitable Bone Strength for Cementless Total Knee Arthroplasty" Journal of Clinical Medicine 14, no. 20: 7384. https://doi.org/10.3390/jcm14207384
APA StyleLee, D. H., Kwak, D.-S., Kim, Y. D., Cho, N., & Koh, I. J. (2025). Central Bone Mineral Density Is Not a Reliable Surrogate for Assessing Suitable Bone Strength for Cementless Total Knee Arthroplasty. Journal of Clinical Medicine, 14(20), 7384. https://doi.org/10.3390/jcm14207384








