Pulmonary Embolism in Hematologic Malignancies: Predictive Value of the D-Dimer/Albumin Ratio and Proposal of the Hema-PE Score
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Patient Characteristics
3.2. D-Dimer/Albumin Ratio
3.3. Development of the Hema-PE Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Porras, J.R.; Mateo, J.; Gonzalez-Calle, V.; Marco, P.; Garcia-Gutierrez, V.; Reverter, J.C.; Lecumberri, R. Prevention of venous thromboembolism in hematologic neoplasms: An expert consensus from SEHH-SETH. Clin. Transl. Oncol. 2022, 24, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razeq, H.; Ma’koseh, M.; Mansour, A.; Bater, R.; Amarin, R.; Abufara, A.; Halahleh, K.; Manassra, M.; Alrwashdeh, M.; Almomani, M.; et al. The Application of the ThroLy Risk Assessment Model to Predict Venous Thromboembolism in Patients with Diffuse Large B-Cell Lymphoma. Clin. Appl. Thromb. Hemost. 2021, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W. Risk prediction of cancer-associated thrombosis: Appraising the first decade and developing the future. Thromb. Res. 2018, 164, S70–S76. [Google Scholar] [CrossRef]
- Antic, D.; Milic, N.; Nikolovski, S.; Todorovic, M.; Bila, J.; Djurdjevic, P.; Andjelic, B.; Djurasinovic, V.; Sretenovic, A.; Vukovic, V.; et al. Development and validation of multivariable predictive model for thromboembolic events in lymphoma patients. Am. J. Hematol. 2016, 91, 1014–1019. [Google Scholar] [CrossRef]
- Li, W.; Liu, R.; Shen, Y.; Gao, G.; Yang, R.; Wang, Y.; Yang, R.; Lin, Z.; Dong, R.; Zhao, W.; et al. Incidence, Risk Factors, and Modified Risk Assessment Model of Venous Thromboembolism in Non-Hodgkin Lymphoma Patients. Cancer Med. 2024, 13, e70510. [Google Scholar] [CrossRef] [PubMed]
- Kekre, N.; Connors, J.M. Venous thromboembolism incidence in hematologic malignancies. Blood Rev. 2019, 33, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Frequency, risk factors, and trends for venous thrombo-embolism among hospitalized cancer patients. Cancer 2007, 110, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Dunkler, D.; Simanek, R.; Thaler, J.; Koder, S.; Marosi, C.; Zielinski, C.; Pabinger, I. Prediction of Venous Thromboembolism in Patients with Cancer by Measuring Thrombin Generation: Results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2011, 29, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, E.; Pannunzio, A.; Palumbo, I.M.; Bartimoccia, S.; Cammisotto, V.; Castellani, V.; Porfidia, A.; Pignatelli, P.; Violi, F. Risk of venous thromboembolism and arterial events in patients with hypoalbuminemia: A comprehensive meta-analysis of more than 2 million patients. J. Thromb. Haemost. 2024, 22, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, I.; Thaler, J.; Ay, C. Biomarkers for prediction of venous thromboembolism in cancer. Blood 2013, 122, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Ortel, T.L.; Francis, C.W. Impact of Venous Thromboembolism and Anticoagulation on Cancer and Cancer Survival. J. Clin. Oncol. 2009, 27, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Vormittag, R.; Dunkler, D.; Simanek, R.; Chiriac, A.-L.; Drach, J.; Quehenberger, P.; Wagner, O.; Zielinski, C.; Pabinger, I. D-Dimer and Prothrombin Fragment 1 + 2 Predict Venous Thromboembolism in Patients with Cancer: Results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2009, 27, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-F.; Luo, S.; Gage, B.F.; Schoen, M.W.; Afzal, A.; Carson, K.; Chang, S.-H.; Mahmoud, A.; Sanfilippo, K.M. Association between anticoagulant-related bleeding and mortality in patients with hematological malignancies and cancer-associated venous thromboembolism. Thromb. Res. 2025, 248, 109284. [Google Scholar] [CrossRef] [PubMed]
- Alsuhebany, N.; Alfehaid, L.; Alodhaibi, D.; Mahdali, M.; Almohareb, S.; Alshaya, A. Venous thromboembolism prophylaxis practices in hematology/oncology patients admitted to neurological intensive care units. Front. Cardiovasc. Med. 2025, 12, 1573080. [Google Scholar] [CrossRef] [PubMed]

| (A) Baseline demographics. | |||
| Variable | Control Group (n = 114) | Patient Group (n = 63) | p |
| Age [years, median (min–max)] | 62 | 66 | 0.530 |
| Sex | Female: 44 (38.6%) Male: 70 (61.4%) | Female: 27 (42.9%) Male: 36 (57.1%) | 0.580 |
| Smoking (n, %) | 44 (38.6%) | 22 (34.9%) | 0.628 |
| History of MI or stroke (n, %) | 9 (7.9%) | 2 (3.2%) | 0.332 |
| (B) Disease-related features. | |||
| Variable | Control Group (n = 114) | Patient Group (n = 63) | p |
| Disease type (n, %) | Leukemia: 29 (25.4%) Lymphoma: 28 (24.6%) MM: 57 (50.0%) | Leukemia: 11 (17.5%) Lymphoma: 23 (36.5%) MM: 29 (46.0%) | <0.001 * |
| Mediastinal LN involvement (n, %) | No: 8 (28.6%) Yes: 20 (71.4%) | No: 4 (20.0%) Yes: 16 (80.0%) | 0.737 |
| Extranodal involvement (n, %) | No: 8 (28.6%) Yes: 20 (71.4%) | No: 3 (35.0%) Yes: 17 (65.0%) | 0.755 |
| PE location (n, %) | - | Right: 17 (27.0%) Left: 10 (15.9%) Bilateral: 36 (57.1%) | - |
| PE severity (n, %) | - | Massive: 5 (7.9%) Submassive: 1 (1.6%) Nonmassive: 57 (90.5%) | - |
| DVT (n, %) | - | Present: 9 (14.3%) | - |
| Diagnostic Test | ROC Curve | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Cut-off | Sensitivity | Specifity | PPV | NPV | AUC | (95% CI) | ||
| D-dimer/ Albumin | ≥205.33 | 92.1 | 57.0 | 54.2 | 92.9 | 0.882 (0.832–0.931) | <0.001 * | |
| Parameter | Cut-off | Points Assigned |
|---|---|---|
| D-dimer/Albumin ratio | >206.5 | 2 |
| Immobility | ≥3 days | 1 |
| Central venous catheter | Present | 1 |
| C-reactive protein (CRP) | >10 mg/L | 1 |
| Platelet count | >350 × 109/L | 1 |
| Hemoglobin | <10 g/Dl | 1 |
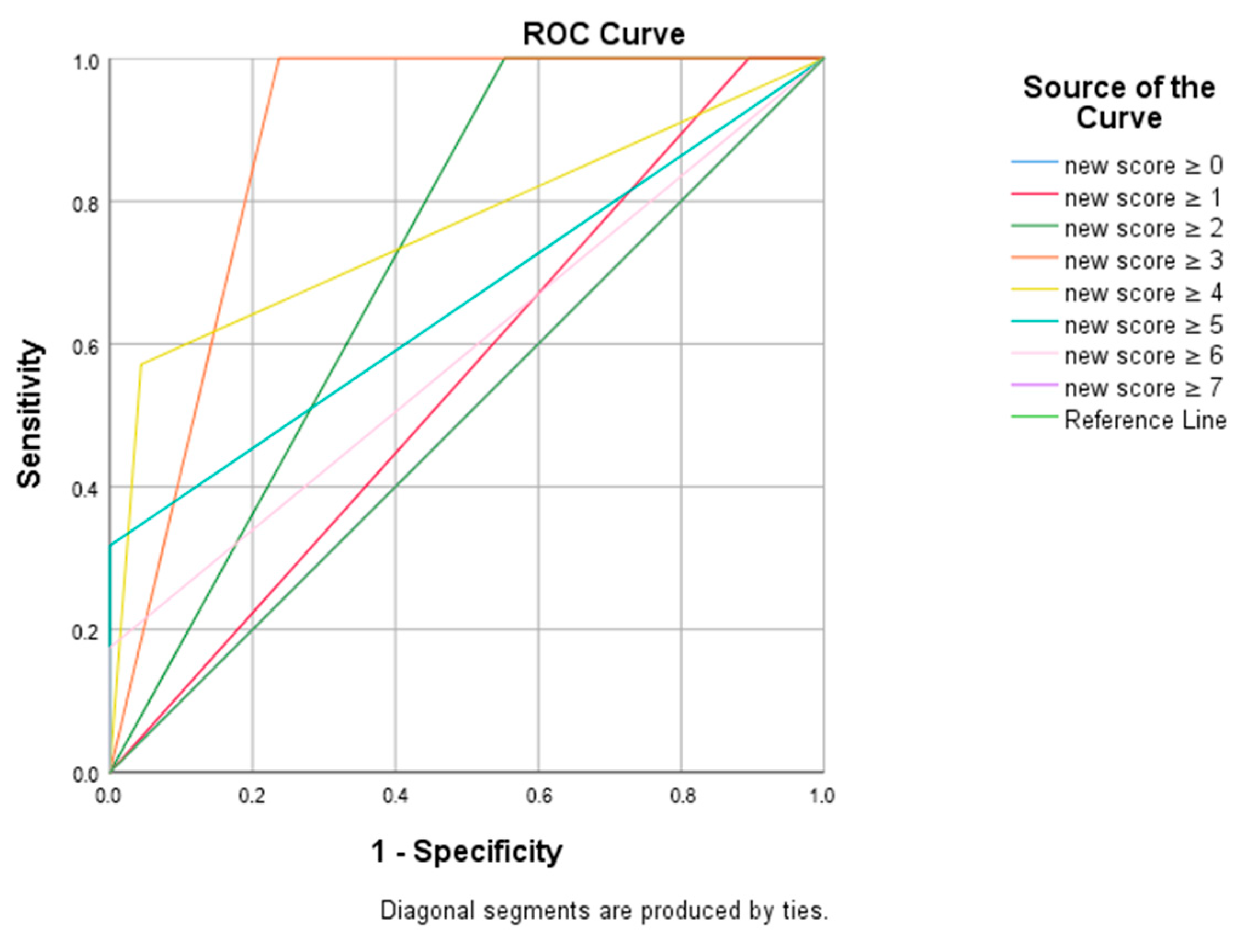
| Diagnostic Test | ROC Curve | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Cut-off | Sensitivity | Specifity | PPV | NPV | AUC | 95% CI | ||
| Score 2 | ≥2 | 100.0 | 44.7 | 50.0 | 100.0 | 0.724 | 0.651–0.796 | <0.001 * |
| Score 3 | ≥3 | 100.0 | 76.3 | 70.0 | 100.0 | 0.882 | 0.832–0.931 | <0.001 * |
| Score 4 | ≥4 | 57.1 | 95.6 | 87.8 | 80.1 | 0.764 | 0.682–0.845 | <0.001 * |
| Score 5 | ≥5 | 31.7 | 100.0 | 100.0 | 72.6 | 0.659 | 0.569–0.749 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkurt, E.S.; Duvenci Birben, O.; Akbulut, M.H.; Ozturk, B.N.; Ozad, A.; Dal, M.S.; Yenibertiz, D. Pulmonary Embolism in Hematologic Malignancies: Predictive Value of the D-Dimer/Albumin Ratio and Proposal of the Hema-PE Score. J. Clin. Med. 2025, 14, 7337. https://doi.org/10.3390/jcm14207337
Akkurt ES, Duvenci Birben O, Akbulut MH, Ozturk BN, Ozad A, Dal MS, Yenibertiz D. Pulmonary Embolism in Hematologic Malignancies: Predictive Value of the D-Dimer/Albumin Ratio and Proposal of the Hema-PE Score. Journal of Clinical Medicine. 2025; 14(20):7337. https://doi.org/10.3390/jcm14207337
Chicago/Turabian StyleAkkurt, Esma Sevil, Ozlem Duvenci Birben, Mehmet Hakan Akbulut, Beyza Nur Ozturk, Aleyna Ozad, Mehmet Sinan Dal, and Derya Yenibertiz. 2025. "Pulmonary Embolism in Hematologic Malignancies: Predictive Value of the D-Dimer/Albumin Ratio and Proposal of the Hema-PE Score" Journal of Clinical Medicine 14, no. 20: 7337. https://doi.org/10.3390/jcm14207337
APA StyleAkkurt, E. S., Duvenci Birben, O., Akbulut, M. H., Ozturk, B. N., Ozad, A., Dal, M. S., & Yenibertiz, D. (2025). Pulmonary Embolism in Hematologic Malignancies: Predictive Value of the D-Dimer/Albumin Ratio and Proposal of the Hema-PE Score. Journal of Clinical Medicine, 14(20), 7337. https://doi.org/10.3390/jcm14207337






