Impact of Post-Traumatic Stress Disorder Duration on Volumetric and Microstructural Parameters of the Hippo-Campus, Amygdala, and Prefrontal Cortex: A Multiparametric Magnetic Resonance Imaging Study with Correlation Analysis
Abstract
1. Introduction
2. Results
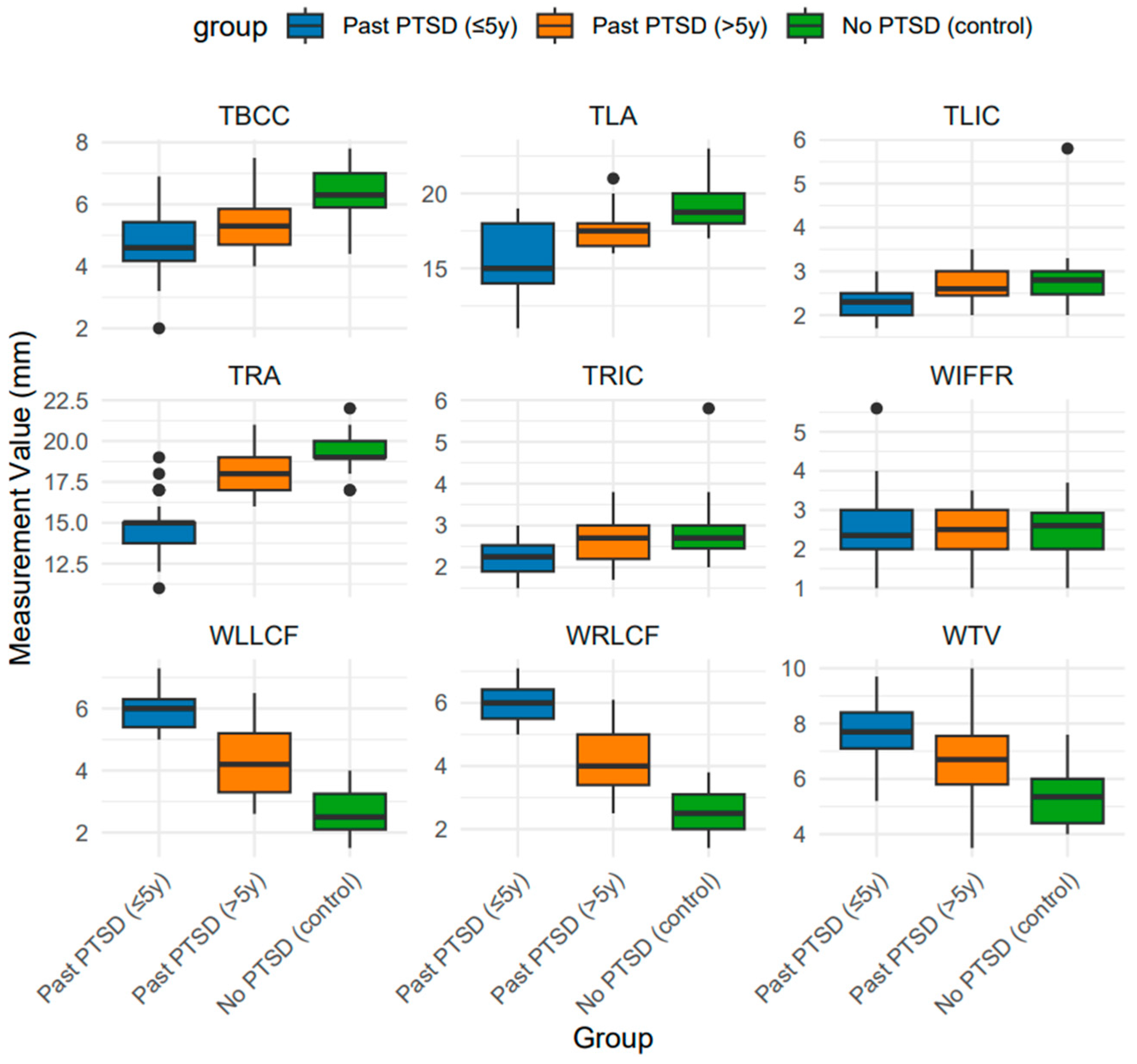
2.1. Brain Measurements and Magnetic Resonance Imaging Characteristics Across PTSD Groups
2.2. Correlation Analysis Between Neuroimaging Metrics and Brain Measurements Across PTSD Groups
2.2.1. Right Hippocampal Region (RHR) vs. Brain Measurements

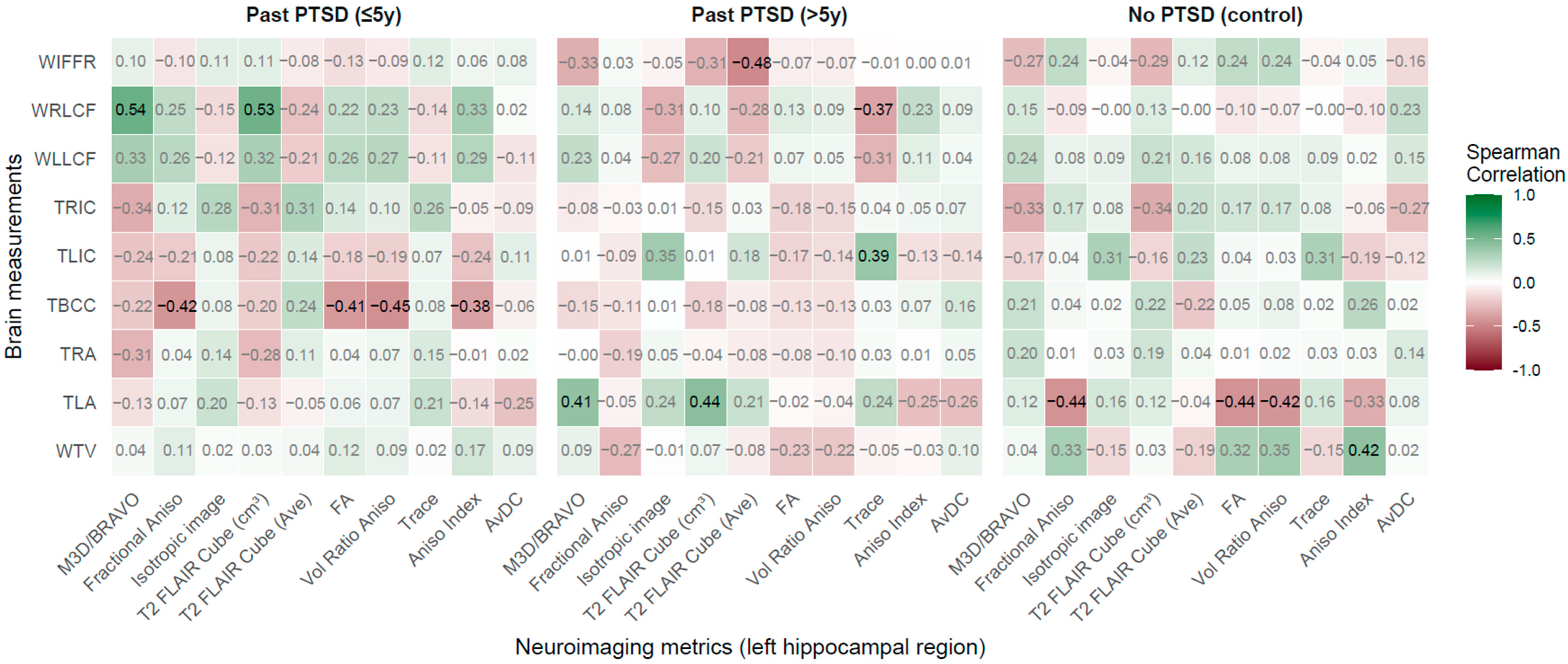

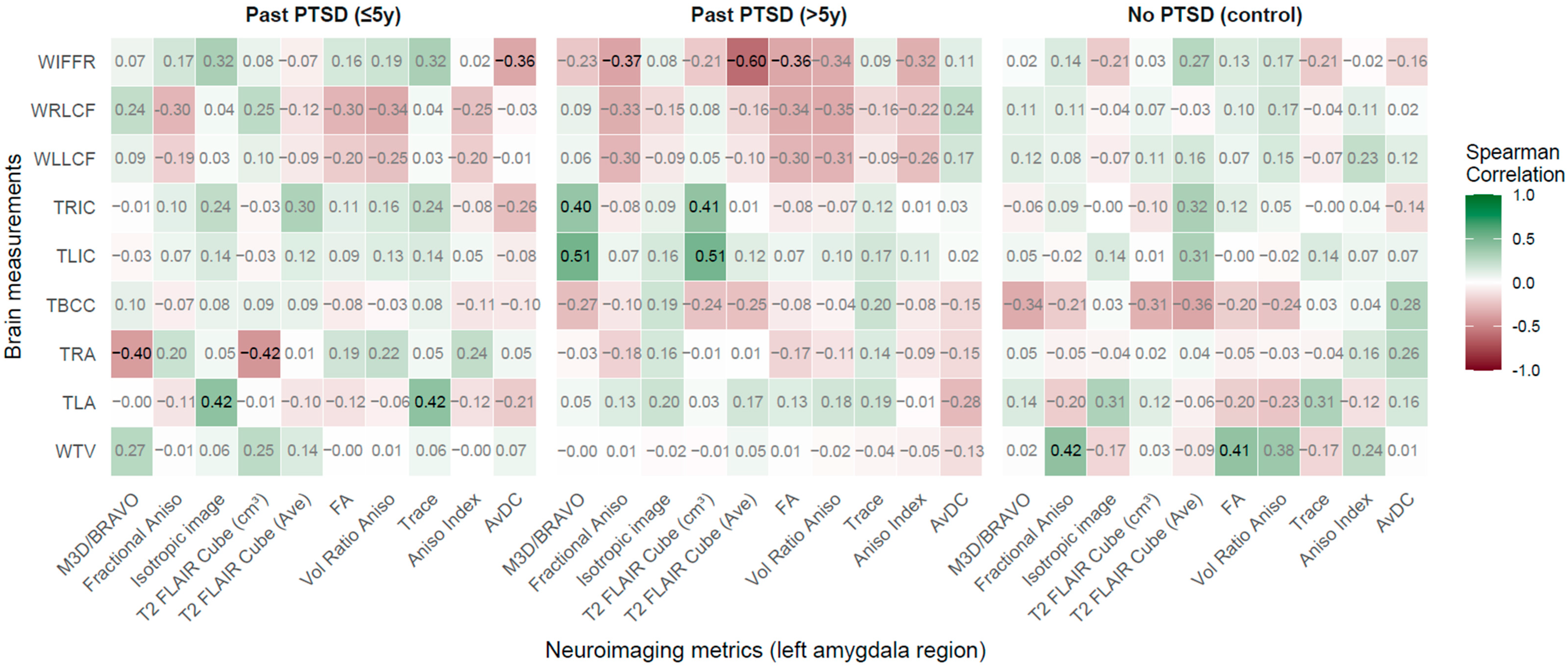
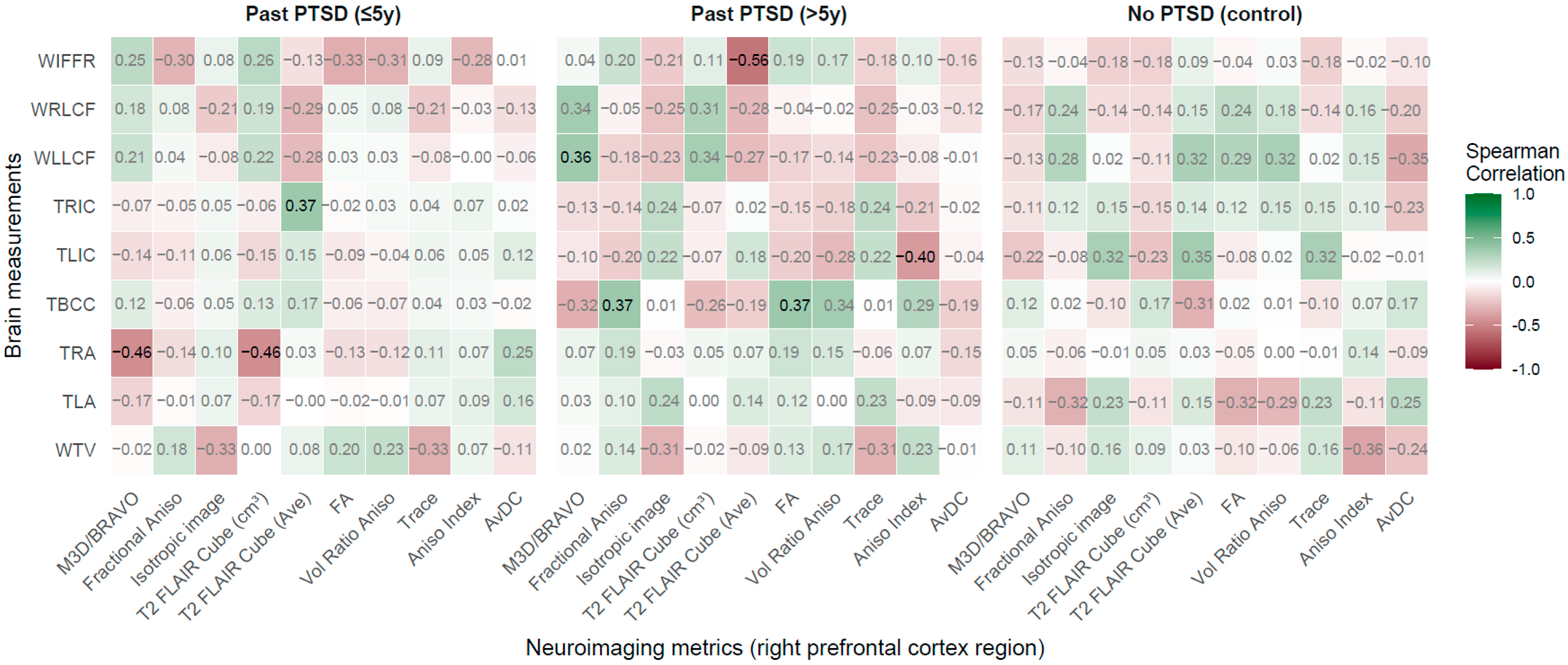
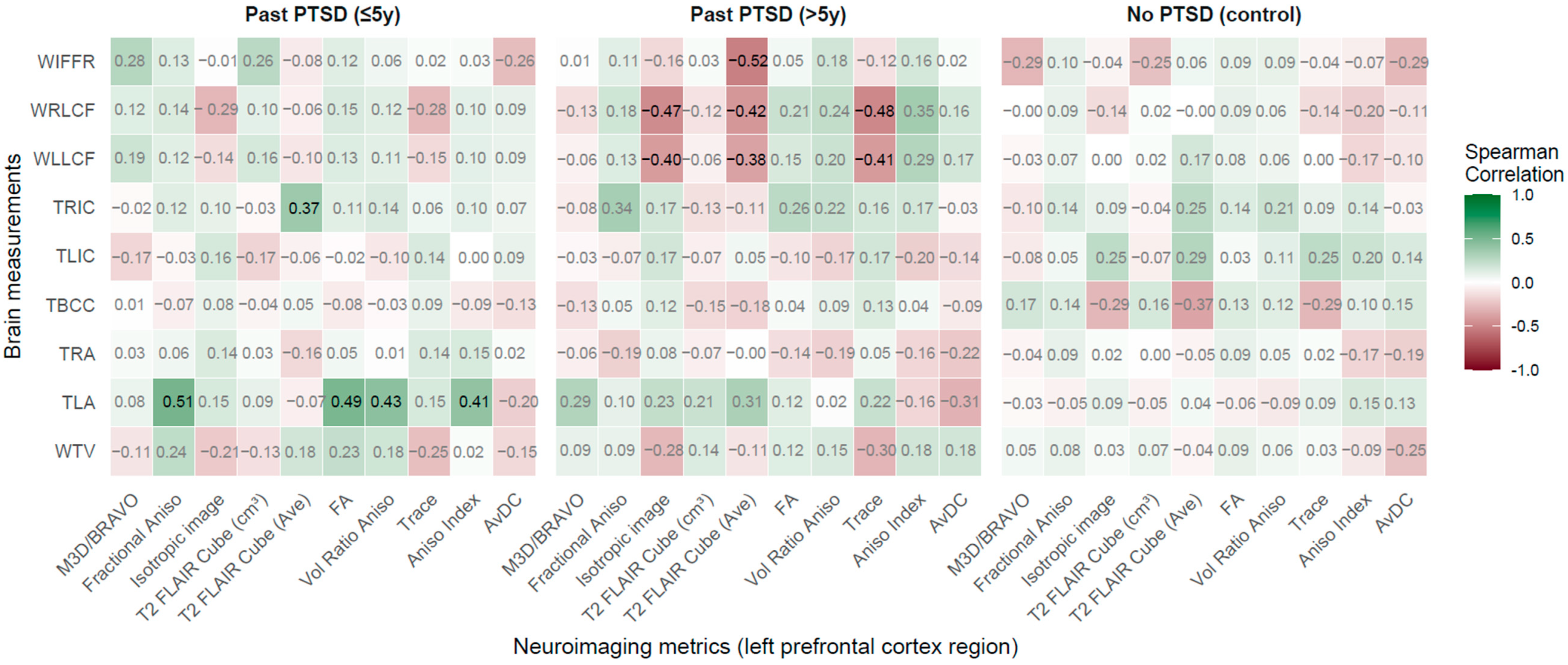
2.2.2. Left Hippocampal Region (LHR) vs. Brain Measurements
2.2.3. Right Amygdala Region vs. Brain Measurements
2.2.4. Left Amygdala Region vs. Brain Measurements
2.2.5. Right Prefrontal Cortex Region vs. Brain Measurements
2.2.6. Left Prefrontal Cortex Region (LPCR) vs. Brain Measurements
2.2.7. Integrated Findings from Group-Specific Correlations
3. Discussion
4. Materials and Methods
4.1. Characteristics of the Participants
4.2. MRI and Diffusion Tensor Imaging Methodology Using 32 Diffusion Directions
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTSD | Post-Traumatic Stress Disorder |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| HPA axis | Hypothalamic–Pituitary–Adrenal Axis |
| MRI | Magnetic Resonance Imaging |
| DTI | Diffusion Tensor Imaging |
| fMRI | Functional Magnetic Resonance Imaging |
| ROI | Region of Interest |
| 3D Ax BRAVO | Three-Dimensional Axial Brain Volume Imaging (BRAVO) |
| Cube T2 FLAIR | Cube T2-Weighted Fluid-Attenuated Inversion Recovery |
| FA | Fractional Anisotropy |
| MD | Mean Diffusivity |
| CLD | Compact Letter Display |
| WTV | Width of the Third Ventricle |
| TLA | Thickness of the Left Amygdala |
| TRA | Thickness of the Right Amygdala |
| TBCC | Thickness of the Body of the Corpus Callosum |
| TLIC | Thickness of the Left Insular Cortex |
| TRIC | Thickness of the Right Insular Cortex |
| WLLCF | Width of the Left Lateral Cerebral Fissure |
| WRLCF | Width of the Right Lateral Cerebral Fissure |
| WIFFR | Width of the Interhemispheric Fissure in the Frontal Region |
| RHR | Right Hippocampal Region |
| LHR | Left Hippocampal Region |
| RAR | Right Amygdala Region |
| LAR | Left Amygdala Region |
| RPCR | Right Prefrontal Cortex Region |
| LPCR | Left Prefrontal Cortex Region |
| M3D/BRAVO | Three-Dimensional Axial BRAVO MRI Sequence |
| M3D/CubeT2flair | Three-Dimensional Cube T2-Weighted Fluid-Attenuated Inversion Recovery Sequence |
| Vol Ratio Aniso | Volume Ratio Anisotropy |
| Aniso Index | Anisotropy Index |
| AvDC | Average Diffusivity Coefficient |
| DMN | Default Mode Network |
| pACC | Pre-Genual Anterior Cingulate Cortex |
| CAPS-5 | Clinician-Administered PTSD Scale for DSM-5 |
| MP-RAGE | Magnetization Prepared Rapid Gradient Echo |
| TR | Repetition Time |
| TE | Echo Time |
| TI | Inversion Time |
| FOV | Field of View |
| TIV | Total Intracranial Volume |
| EPI | Echo Planar Imaging |
| SNR | Signal-to-Noise Ratio |
| AD | Axial Diffusivity |
| RD | Radial Diffusivity |
| FSL | FMRIB Software Library |
| TBSS | Tract-Based Spatial Statistics |
| MNI152 | Montreal Neurological Institute 152 Standard Brain Template |
| MRS | Magnetic Resonance Spectroscopy |
| CBT | Cognitive Behavioral Therapy |
| TMS | Transcranial Magnetic Stimulation |
| EMDR | Eye Movement Desensitization and Reprocessing |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
References
- Ogłodek, E.A.; Just, M.J.; Szromek, A.R.; Araszkiewicz, A. Assessing the serum concentration levels of NT-4/5, GPX-1, TNF-α, and l-arginine as biomediators of depression severity in first depressive episode patients with and without posttraumatic stress disorder. Pharmacol. Rep. 2017, 69, 1049–1058. [Google Scholar] [CrossRef]
- Tiet, Q.Q.; Tiet, T.N. Diagnostic Accuracy of the Primary Care PTSD for DSM-5 Screen (PC-PTSD-5) in Demographic and Diagnostic Subgroups of Veterans. J. Gen. Intern. Med. 2024, 39, 2017–2022. [Google Scholar] [CrossRef]
- Ogłodek, E.A.; Szota, A.M.; Just, M.J.; Araszkiewicz, A.; Szromek, A.R. Sense of alexithymia in patients with anxiety disorders comorbid with recurrent urticaria. Neuropsychiatr. Dis. Treat. 2016, 12, 995–1004. [Google Scholar] [CrossRef]
- Barone, D.A. Trauma-Associated Sleep Disorder. Sleep Med. Clin. 2024, 19, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Slavova, D.; Ortiz, V.; Blaise, M.; Bairachnaya, M.; Giros, B.; Isingrini, E. Role of the locus coeruleus-noradrenergic system in stress-related psychopathology and resilience: Clinical and pre-clinical evidences. Neurosci. Biobehav. Rev. 2024, 167, 105925. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, A.; Yasaka, K.; Akai, H.; Kunimatsu, N.; Abe, O. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imaging 2020, 52, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Jagger-Rickels, A.; Rothlein, D.; Stumps, A.; Evans, T.C.; Bernstein, J.; Milberg, W.; McGlinchey, R.; DeGutis, J.; Esterman, M. An executive function subtype of PTSD with unique neural markers and clinical trajectories. Transl. Psychiatry 2022, 12, 262. [Google Scholar] [CrossRef]
- Hunt, K.J.; Knight, L.K.; Depue, B.E. Related neural networks underlie suppression of emotion, memory, motor processes as identified by data-driven analysis. BMC Neurosci. 2023, 24, 44. [Google Scholar] [CrossRef]
- Wen, Z.; Seo, J.; Pace-Schott, E.F.; Milad, M.R. Abnormal dynamic functional connectivity during fear extinction learning in PTSD and anxiety disorders. Mol. Psychiatry 2022, 27, 2216–2224. [Google Scholar] [CrossRef]
- Mu, C.; Dang, X.; Luo, X.J. Mendelian randomization analyses reveal causal relationships between brain functional networks and risk of psychiatric disorders. Nat. Hum. Behav. 2024, 8, 1417–1428. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.; Zhang, X.; Zhang, Y.; Ge, Y.; Lu, Z.; Ma, M.; Song, Y.; Tan, H.Y.; Zhang, D.; et al. Deviations From Normative Functioning Underlying Emotional Episodic Memory Revealed Cross-Scale Neurodiverse Alterations Linked to Affective Symptoms in Distinct Psychiatric Disorders. Biol. Psychiatry Glob. Open Sci. 2025, 5, 100534. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Moeller, S.J.; Ali, F.; DeLorenzo, C.; Domschke, K.; Horga, G.; Jutla, A.; Kotov, R.; Paulus, M.P.; Rubio, J.M.; et al. Candidate biomarkers in psychiatric disorders: State of the field. World Psychiatry 2023, 22, 236–262. [Google Scholar] [CrossRef]
- Bonne, O.; Brandes, D.; Gilboa, A.; Gomori, J.M.; Shenton, M.E.; Pitman, R.K.; Shalev, A.Y. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am. J. Psychiatry 2001, 158, 1248–1251. [Google Scholar] [CrossRef]
- Ju, Y.; Ou, W.; Su, J.; Averill, C.L.; Liu, J.; Wang, M.; Wang, Z.; Zhang, Y.; Liu, B.; Li, L.; et al. White matter microstructural alterations in posttraumatic stress disorder: An ROI and whole-brain based meta-analysis. J. Affect. Disord. 2020, 266, 655–670. [Google Scholar] [CrossRef]
- Chen, H.J.; Guo, Y.; Ke, J.; Qiu, J.; Zhang, L.; Xu, Q.; Zhong, Y.; Lu, G.M.; Qin, H.; Qi, R.; et al. Characterizing typhoon-related posttraumatic stress disorder based on multimodal fusion of structural, diffusion, and functional magnetic resonance imaging. Neuroscience 2024, 537, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Woon, F.L.; Hedges, D.W. Gender does not moderate hippocampal volume deficits in adults with posttraumatic stress disorder: A meta-analysis. Hippocampus 2011, 21, 243–252. [Google Scholar] [CrossRef] [PubMed]
- de Nooij, L.; Wirz, L.; Heling, E.; Pais, M.; Hendriks, G.J.; Verkes, R.J.; Roozendaal, B.; Hermans, E.J. Exogenous glucocorticoids to improve extinction learning for post-traumatic stress disorder patients with hypothalamic-pituitary-adrenal-axis dysregulation: A study protocol description. Eur. J. Psychotraumatol. 2024, 15, 2364441. [Google Scholar] [CrossRef] [PubMed]
- Harnett, N.G.; Fleming, L.L.; Clancy, K.J.; Ressler, K.J.; Rosso, I.M. Affective Visual Circuit Dysfunction in Trauma and Stress-Related Disorders. Biol. Psychiatry 2025, 97, 405–416. [Google Scholar] [CrossRef]
- Dolcos, F.; Denkova, E.; Iordan, A.D.; Shafer, A.T.; Fernández, G.; Dolcos, S. Dissociating and linking divergent effects of emotion on cognition: Insights from current research and emerging directions. Front. Psychol. 2025, 16, 1483373. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Wang, X.; Liu, Y.; Liu, S.; Zhao, J.; Liu, L.; Yu, P.; Ren, Z. Exploring emotion regulation in PTSD with Insomnia: A task-based fMRI study. J. Psychiatr. Res. 2025, 189, 125–137. [Google Scholar] [CrossRef]
- Kangarlou, K.; Raminfard, S.; Zebardast, J.; Faghihzadeh, E.; Kondori, B.J. Age-dependent brain subcortical white and gray matter disruptions in patients with posttraumatic stress disorder. Anat. Cell Biol. 2025, 58, 220–228. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Bierer, L.M.; Bader, H.N.; Chu, K.W.; Tang, C.Y.; Murphy, K.M.; Hazlett, E.A.; Flory, J.D.; Yehuda, R. Cingulate and hippocampal subregion abnormalities in combat-exposed veterans with PTSD. J. Affect. Disord. 2022, 311, 432–439. [Google Scholar] [CrossRef]
- Smith, B.M.; Thomasson, M.; Yang, Y.C.; Sibert, C.; Stocco, A. When fear shrinks the brain: A computational model of the effects of posttraumatic stress on hippocampal volume. Top. Cogn. Sci. 2021, 13, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.J.; Fillinger, C.; Waegaert, R.; Journée, S.H.; Hener, P.; Ayazgok, B.; Humo, M.; Karatas, M.; Thouaye, M.; Gaikwad, M.; et al. Amygdala hyperactivity in posttraumatic stress disorder: Disentangling predisposing from consequential factors using a prospective longitudinal design. Nat. Commun. 2023, 14, 2198. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Pan, M.; Li, C.X.; Zhang, Z.; Sun, M.; Liao, T.; Wang, Z.; Luo, J.; Shi, L.; et al. The medial prefrontal cortex-basolateral amygdala circuit mediates anxiety in Shank3 InsG3680 knock-in mice. Neurosci. Bull. 2025, 41, 77–92. [Google Scholar] [CrossRef]
- Bijanki, K.R.; van Rooij, S.J.H.; Ely, T.D.; Stevens, J.S.; Inman, C.S.; Fasano, R.E.; Carter, S.E.; Winters, S.J.; Baman, J.R.; Drane, D.L.; et al. Case series: Unilateral amygdala ablation ameliorates post-traumatic stress disorder symptoms and biomarkers. Neurosurgery 2020, 87, 796–802. [Google Scholar] [CrossRef]
- Morey, R.A.; Zheng, Y.; Bayly, H.; Sun, D.; Garrett, M.E.; Gasperi, M.; Maihofer, A.X.; Baird, C.L.; Grasby, K.L.; Huggins, A.A.; et al. Genomic structural equation modeling reveals latent phenotypes in the human cortex with distinct genetic architecture. Transl. Psychiatry 2024, 14, 451. [Google Scholar] [CrossRef]
- Ousdal, O.T.; Milde, A.M.; Hafstad, G.S.; Hodneland, E.; Dyb, G.; Craven, A.R.; Melinder, A.; Endestad, T.; Hugdahl, K. The association of PTSD symptom severity with amygdala nuclei volumes in traumatized youths. Transl. Psychiatry 2020, 10, 288. [Google Scholar] [CrossRef]
- Ahmed-Leitao, F.; Spies, G.; van den Heuvel, L.; Seedat, S. Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: A systematic review. Psychiatry Res. Neuroimaging 2016, 256, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Cobb, A.R.; Rubin, M.; Stote, D.L.; Baldwin, B.C.; Lee, H.J.; Hariri, A.R.; Telch, M.J. Hippocampal volume and volume asymmetry prospectively predict PTSD symptom emergence among Iraq-deployed soldiers. Psychol. Med. 2023, 53, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Bhadoria, R.; Watson, D.; Danson, P.; Ferrier, I.N.; McAllister, V.I.; Moore, P.B. Enlargement of the third ventricle in affective disorders. Indian J. Psychiatry 2003, 45, 147–150. [Google Scholar]
- Harricharan, S.; Nicholson, A.A.; Thome, J.; Densmore, M.; McKinnon, M.C.; Théberge, J.; Frewen, P.A.; Neufeld, R.W.J.; Lanius, R.A. PTSD and its dissociative subtype through the lens of the insula: Anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology 2020, 57, e13472. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, B.R.; Djurovic, B.; Marinkovic, S.; Stijak, L.; Aksic, M.; Nikolic, V.; Starcevic, A.; Radonjic, V. Volume changes of corpus striatum, thalamus, hippocampus and lateral ventricles in posttraumatic stress disorder (PTSD) patients suffering from headaches and without therapy. Cent. Eur. Neurosurg. 2011, 72, 133–137. [Google Scholar] [CrossRef]
- Verfaellie, M.; Patt, V.; Lafleche, G.; Hunsberger, R.; Vasterling, J.J. Imagining emotional future events in PTSD: Clinical and neurocognitive correlates. Cogn. Affect. Behav. Neurosci. 2023, 23, 1428–1444. [Google Scholar] [CrossRef]
- Cavicchioli, M.; Ogliari, A.; Maffei, C.; Mucci, C.; Northoff, G.; Scalabrini, A. Neural responses to emotional stimuli across the dissociative spectrum: Common and specific mechanisms. Psychiatry Clin. Neurosci. 2023, 77, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, K.H.; Offringa, R.; Pfaff, D.L.; Hughes, K.C.; Shin, L.M. The neural correlates of emotional memory in posttraumatic stress disorder. Biol. Psychiatry 2010, 68, 1023–1030. [Google Scholar] [CrossRef]
- Kondas, A.; McDermott, T.J.; Ahluwalia, V.; Haller, O.C.; Karkare, M.C.; Guelfo, A.; Daube, A.; Bradley, B.; Powers, A.; Stevens, J.S.; et al. White matter correlates of dissociation in a diverse sample of trauma-exposed women. Psychiatry Res. 2024, 342, 116231. [Google Scholar] [CrossRef]
- Graziano, R.; Bruce, S.; Paul, R. The corpus callosum and PTSD severity. J. Interpers. Violence 2021, 36, 7480–7494. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.A.; Lebois, L.A.M.; Ely, T.D.; van Rooij, S.J.H.; Bruce, S.E.; Murty, V.P.; Jovanovic, T.; House, S.L.; Beaudoin, F.L.; An, X.; et al. Internal capsule microstructure mediates the relationship between childhood maltreatment and PTSD following adulthood trauma exposure. Mol. Psychiatry 2023, 28, 5140–5149. [Google Scholar] [CrossRef]
- O’Doherty, D.C.M.; Ryder, W.; Paquola, C.; Tickell, A.; Chan, C.; Hermens, D.F.; Bennett, M.R.; Lagopoulos, J. White matter integrity alterations in post-traumatic stress disorder. Hum. Brain Mapp. 2018, 39, 1327–1338. [Google Scholar] [CrossRef]
- Klimova, A.; Korgaonkar, M.S.; Whitford, T.; Bryant, R.A. Diffusion tensor imaging analysis of mild traumatic brain injury and posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 81–90. [Google Scholar] [CrossRef]
- Riem, M.M.E.; van Hoof, M.J.; Garrett, A.S.; Rombouts, S.A.R.B.; van der Wee, N.J.A.; van IJzendoorn, M.H.; Vermeiren, R.R.J.M. General psychopathology factor and unresolved-disorganized attachment uniquely correlated to white matter integrity using diffusion tensor imaging. Behav. Brain Res. 2019, 359, 1–8. [Google Scholar] [CrossRef]
- Hinojosa, C.A.; George, G.C.; Ben-Zion, Z. Neuroimaging of posttraumatic stress disorder in adults and youth: Progress over the last decade on three leading questions of the field. Mol. Psychiatry 2024, 29, 3223–3244. [Google Scholar] [CrossRef]
- Chen, C.; Li, B.; Chai, L.; Liu, K.; Zhang, S. The amplitude of low-frequency fluctuations is correlated with birth trauma in patients with postpartum post-traumatic stress disorder. Transl. Psychiatry 2024, 14, 332. [Google Scholar] [CrossRef]
- O’Hare, M.A.; Rust, C.; Malan-Müller, S.; Pirovano, W.; Lowry, C.A.; Ramaboli, M.; van den Heuvel, L.L.; Seedat, S.; PGC—PTSD Microbiome Workgroup; Hemmings, S.M.J. Preliminary Insights Into the Relationship Between the Gut Microbiome and Host Genome in Posttraumatic Stress Disorder. Genes Brain. Behav. 2025, 24, e70025. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Tobia, A.; Stoner, M.; Wintering, N.; Matthews, M.; He, X.S.; Doucet, G.; Chervoneva, I.; Tracy, J.I.; Newberg, A.B.; et al. Neuro emotional technique effects on brain physiology in cancer patients with traumatic stress symptoms: Preliminary findings. J. Cancer Surviv. 2017, 11, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, Y.; Zhuang, K.; Lv, J.; Meng, J.; Sun, J.; Chen, Q.; Yang, W.; Qiu, J. Brain Structures Associated With Individual Differences in Somatic Symptoms and Emotional Distress in a Healthy Sample. Front. Hum. Neurosci. 2020, 14, 492990. [Google Scholar] [CrossRef]
- Suo, X.; Lei, D.; Li, W.; Chen, F.; Niu, R.; Kuang, W.; Huang, X.; Lui, S.; Li, L.; Sweeney, J.A.; et al. Large-scale white matter network reorganization in posttraumatic stress disorder. Hum. Brain Mapp. 2019, 40, 4801–4812. [Google Scholar] [CrossRef] [PubMed]
- Korgaonkar, M.S.; Felmingham, K.L.; Klimova, A.; Erlinger, M.; Williams, L.M.; Bryant, R.A. White matter anisotropy and response to cognitive behavior therapy for posttraumatic stress disorder. Transl. Psychiatry 2021, 11, 14. [Google Scholar] [CrossRef]
- Wen, J.; Fu, C.H.Y.; Tosun, D.; Veturi, Y.; Yang, Z.; Abdulkadir, A.; Mamourian, E.; Srinivasan, D.; Skampardoni, I.; Singh, A.; et al. Characterizing Heterogeneity in Neuroimaging, Cognition, Clinical Symptoms, and Genetics Among Patients With Late-Life Depression. JAMA Psychiatry 2022, 79, 464–474. [Google Scholar] [CrossRef]
- Dennis, E.L.; Disner, S.G.; Fani, N.; Salminen, L.E.; Logue, M.; Clarke, E.K.; Haswell, C.C.; Averill, C.L.; Baugh, L.A.; Bomyea, J.; et al. Altered White Matter Microstructural Organization in Post-Traumatic Stress Disorder across 3,047 Adults: Results from the PGC-ENIGMA PTSD Consortium. Mol. Psychiatry 2021, 26, 4315–4330. [Google Scholar] [CrossRef]
- Boldrini, M.; Hristov, A.; Austin, K.; Ardekani, B.; Cadet, J.L.; Carmody, T.J.; Cirrito, J.R.; Evans, S.E.; Jephson, C.; Kitamura, T.; et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol. Psychiatry 2012, 72, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Gado, M.H.; Kraemer, H.C. Untreated depression and hippocampal volume loss. Am. J. Psychiatry 2003, 160, 1516–1518. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.E., Jr.; Arellano Perez, A.D.; Vasudevan, K.; Ressler, R.L.; Garcia, G.M.; Parr, M.; Vierkant, V.M.; Bayer, H.; Maren, S. Hippocampal ensembles regulate circuit-induced relapse of extinguished fear. Mol. Psychiatry. 2025, 30, 4700–4709. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Gu, J.; Li, L.; Yan, Y.; Yan, C.; Zhang, T. Guilu Erxian Jiao remodels dendritic spine morphology through activation of the hippocampal TRPC6-CaMKIV-CREB signaling pathway and suppresses fear memory generalization in rats with post-traumatic stress disorder. J. Ethnopharmacol. 2025, 340, 119252. [Google Scholar] [CrossRef]
- Barksdale, B.R.; Enten, L.; DeMarco, A.; Kline, R.; Doss, M.K.; Nemeroff, C.B.; Fonzo, G.A. Low-intensity transcranial focused ultrasound amygdala neuromodulation: A double-blind sham-controlled target engagement study and unblinded single-arm clinical trial. Mol. Psychiatry 2025, 30, 4497–4511. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, C.; Pei, J.; Zhang, X.; Lu, H.; Ji, H.; Zhang, X.; Yuan, Y. Low-intensity transcranial ultrasound stimulation promotes the extinction of fear memory through the BDNF-TrkB signaling pathway. Neuroimage 2025, 319, 121441. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.J.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Lyoo, I.K.; Kim, J.E.; Yoon, S.J.; Hwang, J.; Bae, S.; Kim, D.J. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch. Gen. Psychiatry 2011, 68, 701–713. [Google Scholar] [CrossRef]
- Wolf, E.J.; Hawn, S.E.; Sullivan, D.R.; Miller, M.W.; Sanborn, V.; Brown, E.; Neale, Z.; Fein-Schaffer, D.; Zhao, X.; Logue, M.W.; et al. Neurobiological and genetic correlates of the dissociative subtype of posttraumatic stress disorder. J. Psychopathol. Clin. Sci. 2023, 132, 409–427. [Google Scholar] [CrossRef]
- Keefe, J.R.; Suarez-Jimenez, B.; Zhu, X.; Lazarov, A.; Durosky, A.; Such, S.; Marohasy, C.; Lissek, S.; Neria, Y. Elucidating behavioral and functional connectivity markers of aberrant threat discrimination in PTSD. Depress. Anxiety 2022, 39, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Crombie, K.M.; Adams, T.G.; Dunsmoor, J.E.; Greenwood, B.N.; Smits, J.A.; Nemeroff, C.B.; Cisler, J.M. Aerobic exercise in the treatment of PTSD: An examination of preclinical and clinical laboratory findings, potential mechanisms, clinical implications, and future directions. J. Anxiety Disord. 2023, 94, 102680. [Google Scholar] [CrossRef]
- Provencher, J.; Cernik, R.; Marin, M.F. Impact of Stress and Exercise on Fear Extinction. Curr. Top. Behav. Neurosci. 2023, 64, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Chen, Y.; Xu, X.; Chen, T.; Lui, S.; Huang, X.; Sweeney, J.A.; Li, K.; Gong, Q. The neurobiology of brain recovery from traumatic stress: A longitudinal DTI study. J. Affect. Disord. 2018, 225, 577–584. [Google Scholar] [CrossRef]
- Fonzo, G.A.; Goodkind, M.S.; Oathes, D.J.; Zaiko, Y.V.; Harvey, M.; Peng, K.K.; Weiss, M.E.; Thompson, A.L.; Zack, S.E.; Lindley, S.E.; et al. Amygdala and insula connectivity changes following psychotherapy for posttraumatic stress disorder: A randomized clinical trial. Biol. Psychiatry 2020, 89, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Klaming, R.; Spadoni, A.D.; Veltman, D.J.; Simmons, A.N. Expansion of hippocampal and amygdala shape in post-traumatic stress and early life stress. Neuroimage Clin. 2019, 24, 101982. [Google Scholar] [CrossRef]
- Saba, T.; Rehman, A.; Shahzad, M.N.; Latif, R.; Bahaj, S.A.; Alyami, J. Machine learning for post-traumatic stress disorder identification utilizing resting-state functional magnetic resonance imaging. Microsc. Res. Tech. 2022, 85, 2083–2094. [Google Scholar] [CrossRef]
- Paraniak-Gieszczyk, B. Applications of magnetic resonance imaging in the diagnosis of posttraumatic stress disorder. Pol. Merkur. Lekarski. 2025, 53, 273–276. [Google Scholar] [CrossRef]
- Sierra-Mercado, D.; Padilla-Coreano, N.; Quirk, G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 2011, 36, 529–538. [Google Scholar] [CrossRef]
- Zimmerman, J.M.; Maren, S. Prefrontal cortex: Fear extinction and persistent anxiety. Trends Neurosci. 2011, 34, 268–276. [Google Scholar] [CrossRef]
- Likhtik, E.; Paz, R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci. 2015, 38, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, D.; Yang, L.; Wang, P.; Xiao, J.; Zou, Z.; Min, W.; He, Y.; Yuan, C.; Zhu, H.; et al. Similarities and differences between post-traumatic stress disorder and major depressive disorder: Evidence from task-evoked functional magnetic resonance imaging meta-analysis. J. Affect. Disord. 2024, 361, 712–719. [Google Scholar] [CrossRef]
- Ireton, R.; Hughes, A.; Klabunde, M. A functional magnetic resonance imaging meta-analysis of childhood trauma. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Kamkar, N.; Varvani Farahani, M.; Nikolic, M.; Stewart, K.; Goldsmith, S.; Soltaninejad, M.; Rajabli, R.; Lowe, C.; Nicholson, A.A.; Morton, J.B.; et al. Adverse life experiences and brain function: A meta-analysis of functional magnetic resonance imaging findings. JAMA Netw. Open 2023, 6, e2340018. [Google Scholar] [CrossRef]
- McCall, A.; Forouhandehpour, R.; Celebi, S.; Richard-Malenfant, C.; Hamati, R.; Guimond, S.; Tuominen, L.; Weinshenker, D.; Jaworska, N.; McQuaid, R.J.; et al. Evidence for locus coeruleus-norepinephrine system abnormality in military posttraumatic stress disorder revealed by neuromelanin-sensitive magnetic resonance imaging. Biol. Psychiatry 2024, 96, 268–277. [Google Scholar] [CrossRef]
- Very, E.; Leroy, A.; Richaud, L.; Vaiva, G.; Jardri, R.; Roullet, P.; Taib, S.; Bourcier, A.; Loubinoux, I.; Birmes, P. Hippocampal connectivity changes after traumatic memory reactivation with propranolol for posttraumatic stress disorder: A randomized fMRI study. Eur. J. Psychotraumatol. 2025, 16, 2466886. [Google Scholar] [CrossRef]
- McInnes, L.A.; Berman, R.M.; Worley, M.; Shih, E. A retrospective analysis of ketamine intravenous therapy for post-traumatic stress disorder in real-world care settings. Psychiatry Res. 2025, 352, 116689. [Google Scholar] [CrossRef]
- Moumgiakmas, S.S.; Vrochidou, E.; Papakostas, G.A. Mapping the brain: AI-driven radiomic approaches to mental disorders. Artif. Intell. Med. 2025, 168, 103219. [Google Scholar] [CrossRef]
- Gong, Q.; Lyu, H.; Kang, L.; Ma, S.; Zhang, N.; Xie, X.H.; Zhou, E.; Deng, Z.; Zhang, L.; Liu, J.; et al. The genetic relationships between post-traumatic stress disorder and its corresponding neural circuit structures. J. Affect. Disord. 2025, 393, 120323. [Google Scholar] [CrossRef]
- Klumpp, H.; Fitzgerald, D.A.; Angstadt, M.; Phan, K.L. Neural biomarkers of fear and anxiety: A meta-analysis of functional neuroimaging studies. Depress. Anxiety 2017, 34, 68–78. [Google Scholar] [CrossRef]
- Duval, E.; Dondaine, T.; Lebois, L.; Luminet, O.; Leconte, P. Neurobiological and psychological correlates of PTSD symptom clusters: A meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 2023, 147, 105120. [Google Scholar] [CrossRef]
- Liberzon, I.; Abelson, J.L. Context processing and the neurobiology of post-traumatic stress disorder. Neuron 2016, 92, 14–30. [Google Scholar] [CrossRef]
- Meijer, L.; Thomaes, K.; Blankers, M.; Deković, M.; Franz, M.R.; Kleber, R.; van de Putte, E.M.; van Ee, E.; Camisasca, E.; Fredman, S.J.; et al. Post-traumatic stress disorder, trauma and parenting stress: An individual participant data meta-analysis. Eur. J. Psychotraumatol. 2025, 16, 2538907. [Google Scholar] [CrossRef]
- Hedvall, H.; Andersson, E.; Ivanov, V.Z.; Bragesjö, M. Feasibility of written exposure therapy for PTSD in a psychiatric outpatient clinic: In-person and remote delivery. Eur. J. Psychotraumatol. 2025, 16, 2545048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.G.; Shang, Z.L.; Zhang, F.; Wu, L.L.; Sun, L.N.; Jia, Y.P.; Yu, H.B.; Liu, W.Z. PTSD: Past, present and future implications for China. Chin. J. Traumatol. 2021, 24, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.; Lubman, D.I.; Lam, T.; Grist, E.; Barnett, A.; Arunogiri, S.; Nielsen, S. Complex trauma and perceived barriers to treatment among people accessing a supervised injecting facility. Eur. J. Psychotraumatol. 2025, 16, 2558241. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D. Traumatic stress: Effects on the brain. Dialogues Clin. Neurosci. 2006, 8, 445–461. [Google Scholar] [CrossRef]
- Gilbar, O. Personality Disorder Severity-ICD-11 Scale and CPTSD ICD-11 Scale: Examining possible psychometric properties and diagnostic concept overlap. Eur. J. Psychotraumatol. 2025, 16, 2541472. [Google Scholar] [CrossRef]
- Diao, Z.; Zuo, Y.; Zhang, J.; Chen, K.; Liu, Y.; Wu, Y.; Miao, F.; Qiao, H. Transcutaneous auricular vagus nerve stimulation alleviates anxiety-like behaviors in mice with post-traumatic stress disorder by regulating glutamatergic neurons in the anterior cingulate cortex. Transl. Psychiatry. 2025, 15, 313. [Google Scholar] [CrossRef]
- Bremner, J.D.; Elzinga, B.M.; Schmahl, C.G.; Vermetten, E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog. Brain Res. 2008, 167, 171–186. [Google Scholar] [CrossRef]
- Koenigs, M.; Grafman, J. Posttraumatic stress disorder: The role of medial prefrontal cortex and amygdala. Neuroscientist 2009, 15, 540–548. [Google Scholar] [CrossRef]
- Young, G.; Thielen, H.; Samuelson, K.; Jin, J. Neurobiology of Chronic Pain, Posttraumatic Stress Disorder, and Mild Traumatic Brain Injury. Biology 2025, 14, 662. [Google Scholar] [CrossRef]
- Bennett, M.M.; Davis, K.E.; Fitzgerald, J.M. Neural Correlates of Reward Processing in the Onset, Maintenance, and Treatment of Posttraumatic Stress Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Zuo, C.; Lan, H.; Li, W.; Li, L.; Kemp, G.J.; Wang, S.; Gong, Q. Multilayer network analysis of dynamic network reconfiguration in adults with posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 15 July 2025).
- Makowski, D.; Lüdecke, D.; Patil, I.; Thériault, R.; Ben-Shachar, M.; Wiernik, B. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption. CRAN. 2023. Available online: https://easystats.github.io/report/ (accessed on 15 July 2025).
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Toomet, O.; Crowley, J.; Hofmann, H.; et al. GGally: Extension to ‘ggplot2’. R package, version 2.2.1. 2024. Available online: https://CRAN.R-project.org/package=GGally (accessed on 15 July 2025).
- Sjoberg, D.; Whiting, K.; Curry, M.; Lavery, J.; Larmarange, J. Reproducible Summary Tables with the gtsummary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R package ‘corrplot’: Visualization of a Correlation Matrix. GitHub, version 0.94. 2024. Available online: https://github.com/taiyun/corrplot (accessed on 15 July 2025).
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 15 July 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R package, version 1.1.4. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 15 July 2025).
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data. R package, version 1.3.1. 2024. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 15 July 2025).
| Characteristic | N | Overall (N = 92) | Past PTSD (≤5 year) (N = 33) | Past PTSD (>5 year) (N = 31) | No PTSD (Control) (N = 28) | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 92 | 34.0 (28.8, 41.0) | 34.0 (31.0, 41.0) | 36.0 (29.5, 41.0) | 33.5 (24.2, 41.5) | 0.524 |
| Brain Measurements (mm) | ||||||
| Width of the Third Ventricle (WTV) | 92 | 6.6 (5.5, 7.7) | 7.7 A (7.1, 8.4) | 6.7 B (5.8, 7.6) | 5.4 C (4.4, 6.0) | <0.001 |
| Width of the Left Amygdala (TLA) | 92 | 17.5 (16.0, 18.1) | 15.0 A (14.0, 18.0) | 17.5 B (16.5, 18.0) | 18.8 C (18.0, 20.0) | <0.001 |
| Width of the Right Amygdala (TRA) | 91 | 18.0 (15.0, 19.0) | 15.0 A (13.8, 15.0) | 18.0 B (17.0, 19.0) | 19.0 C (19.0, 20.0) | <0.001 |
| Thickness of the Body of the Corpus Callosum (TBCC) | 91 | 5.4 (4.5, 6.2) | 4.6 A (4.2, 5.4) | 5.3 B (4.7, 5.8) | 6.3 C (5.9, 7.0) | <0.001 |
| Thickness of the Left Insular Cortex (TLIC) | 91 | 2.5 (2.2, 2.9) | 2.3 A (2.0, 2.5) | 2.6 B (2.4, 3.0) | 2.8 B (2.5, 3.0) | <0.001 |
| Thickness of the Right Insular Cortex (TRIC) | 91 | 2.5 (2.1, 2.9) | 2.2 A (1.9, 2.5) | 2.7 B (2.2, 3.0) | 2.7 B (2.4, 3.0) | <0.001 |
| Width of the Left Lateral Cerebral Fissure (WLLCF) | 91 | 4.7 (3.1, 5.8) | 6.0 A (5.4, 6.3) | 4.2 B (3.3, 5.2) | 2.5 C (2.1, 3.2) | <0.001 |
| Width of the Right Lateral Cerebral Fissure (WRLCF) | 91 | 4.9 (3.0, 5.8) | 6.0 A (5.5, 6.4) | 4.0 B (3.4, 5.0) | 2.5 C (2.0, 3.1) | <0.001 |
| Width of the Interhemispheric Fissure in the Frontal Region (WIFFR) | 91 | 2.4 (2.0, 3.0) | 2.4 (2.0, 3.0) | 2.5 (2.0, 3.0) | 2.6 (2.0, 2.9) | 0.955 |
| Characteristic | Past PTSD (≤5 year) vs. Past PTSD (>5 year) | Past PTSD (≤5 year) vs. Control | Past PTSD (>5 year) vs. Control |
|---|---|---|---|
| WTV | 0.46 | 0.85 | 0.39 |
| TLA | 0.34 | 0.76 | 0.43 |
| TRA | 0.35 | 0.98 | 0.64 |
| TBCC | 0.45 | 0.73 | 0.27 |
| TLIC | 0.11 | 0.53 | 0.43 |
| TRIC | 0.10 | 0.50 | 0.41 |
| WLLCF | 0.48 | 1.03 | 0.55 |
| WRLCF | 0.48 | 1.05 | 0.57 |
| WIFFR | 0.03 | 0.03 | 0.00 |
| Characteristic | N | Overall (N = 92) | Past PTSD (≤5 year) (N = 33) | Past PTSD (>5 year) (N = 31) | No PTSD (Control) (N = 28) | p |
|---|---|---|---|---|---|---|
| Right Hippocampal Region (RHR) | ||||||
| M3D/BRAVO: 3D Ax BRAVO (cm3) | 90 | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) AB | 0.9 (0.8, 1.0) B | 0.7 (0.6, 0.9) A | 0.006 |
| Fractional Aniso | 90 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.516 |
| Isotropic image | 90 | 552.1 (521.7, 589.9) | 548.8 (518.2, 572.0) | 554.2 (527.7, 600.3) | 556.5 (517.7, 587.6) | 0.583 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS (cm3) | 90 | 0.8 (0.7, 0.9) | 0.8 (0.7, 1.0) AB | 0.9 (0.8, 1.0) B | 0.7 (0.6, 0.9) A | 0.006 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS | 90 | 1368.3 (1307.3, 1450.0) | 1355.3 (1312.8, 1448.3) | 1370.0 (1305.5, 1462.9) | 1368.5 (1322.4, 1449.1) | 0.959 |
| FA | 90 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.486 |
| Vol Ratio Aniso (×102) | 90 | 5.0 (4.0, 6.0) | 5.0 (4.0, 5.0) | 4.0 (4.0, 6.0) | 5.0 (4.0, 6.0) | 0.449 |
| Trace | 90 | 552.8 (522.4, 591.4) | 549.4 (518.8, 572.7) | 554.7 (529.0, 606.7) | 557.1 (518.2, 588.3) | 0.590 |
| Aniso Index (×102) | 90 | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.954 |
| AvDC (10−6 mm2/s) | 90 | 835.0 (814.2, 878.7) | 844.9 (822.7, 897.6) | 835.6 (811.6, 894.9) | 827.4 (813.5, 850.4) | 0.106 |
| Left Hippocampal Region (LHR) | ||||||
| M3D/BRAVO: 3D Ax BRAVO (cm3) | 90 | 0.8 (0.7, 0.9) | 0.9 (0.7, 1.0) | 0.8 (0.8, 0.9) | 0.7 (0.6, 0.9) | 0.231 |
| Fractional Aniso | 90 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.401 |
| Isotropic image | 90 | 546.5 (515.5, 584.0) | 540.9 (514.1, 561.4) | 546.4 (524.0, 574.1) | 580.8 (515.9, 590.0) | 0.589 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS (cm3) | 90 | 0.8 (0.7, 0.9) | 0.8 (0.6, 1.0) | 0.8 (0.7, 0.9) | 0.7 (0.6, 0.9) | 0.211 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS | 90 | 1363.0 (1267.8, 1451.8) | 1364.2 (1263.2, 1439.9) | 1368.1 (1295.8, 1503.2) | 1359.9 (1280.1, 1438.7) | 0.839 |
| FA | 90 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.565 |
| Vol Ratio Aniso (×102) | 90 | 5.0 (4.0, 5.0) | 4.0 (4.0, 5.0) | 5.0 (4.0, 5.0) | 5.0 (4.0, 6.0) | 0.576 |
| Trace | 90 | 546.9 (515.8, 584.3) | 541.3 (514.4, 561.8) | 546.8 (529.3, 574.5) | 581.6 (516.1, 590.4) | 0.550 |
| Aniso Index (×102) | 90 | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.888 |
| AvDC (10−6 mm2/s) | 90 | 870.5 (827.6, 929.4) | 886.8 (833.9, 928.5) | 869.6 (820.1, 970.9) | 857.9 (829.9, 887.6) | 0.470 |
| Right Amygdala Region (RAR) | ||||||
| M3D/BRAVO: 3D Ax BRAVO (cm3) | 90 | 1.5 (1.4, 1.7) | 1.5 (1.4, 1.7) | 1.5 (1.3, 1.7) | 1.5 (1.4, 1.8) | 0.742 |
| Fractional Aniso | 90 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.934 |
| Isotropic image | 90 | 538.0 (512.4, 572.2) | 530.8 (491.2, 560.9) | 554.4 (521.3, 568.5) | 532.3 (512.9, 595.4) | 0.227 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS (cm3) | 90 | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) | 1.5 (1.4, 1.8) | 0.727 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS | 90 | 1255.6 (1197.9, 1347.0) | 1254.6 (1198.4, 1341.7) | 1270.3 (1208.3, 1387.8) | 1241.1 (1193.2, 1333.9) | 0.932 |
| FA | 90 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.908 |
| Vol Ratio Aniso (×102) | 90 | 4.0 (3.0, 4.0) | 4.0 (3.0, 5.0) | 4.0 (3.0, 4.0) | 4.0 (3.0, 4.0) | 0.902 |
| Trace | 90 | 538.3 (512.8, 572.7) | 531.3 (491.6, 561.5) | 555.0 (521.8, 569.0) | 532.8 (513.3, 595.9) | 0.215 |
| Aniso Index (×102) | 90 | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.510 |
| AvDC (10−6 mm2/s) | 90 | 973.8 (915.7, 1032.3) | 993.8 (927.0, 1041.8) | 958.5 (913.1, 1037.0) | 947.7 (900.5, 1003.6) | 0.200 |
| Left Amygdala Region (LAR) | ||||||
| M3D/BRAVO: 3D Ax BRAVO (cm3) | 90 | 1.6 (1.3, 1.8) | 1.5 (1.2, 1.7) | 1.6 (1.4, 1.8) | 1.6 (1.4, 2.0) | 0.274 |
| Fractional Aniso | 89 | 0.2 (0.17, 0.20) | 0.18 (0.18, 0.22) A | 0.18 (0.2, 0.2) B | 0.18 (0.17, 0.) AB | 0.022 |
| Isotropic image | 89 | 542.5 (504.3, 560.9) | 540.3 (503.4, 556.8) | 542.6 (508.9, 570.4) | 542.6 (505.5, 561.9) | 0.855 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS (cm3) | 89 | 1.6 (1.3, 1.8) | 1.5 (1.2, 1.7) | 1.6 (1.4, 1.8) | 1.6 (1.4, 1.9) | 0.231 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS | 89 | 1278.9 (1194.2, 1364.8) | 1275.8 (1196.6, 1351.8) | 1291.2 (1190.2, 1411.5) | 1282.1 (1194.5, 1364.4) | 0.856 |
| FA | 89 | 0.18 (0.18, 0.21) | 0.18 (0.17, 0.20) A | 0.19 (0.18, 0.22) B | 0.18 (0.17, 0.19) AB | 0.021 |
| Vol Ratio Aniso (×102) | 89 | 4.0 (4.0, 5.0) | 4.0 (4.0, 4.0) | 4.0 (4.0, 6.0) | 4.0 (3.0, 5.0) | 0.026 |
| Trace | 89 | 543.0 (505.9, 561.4) | 540.8 (503.8, 557.3) | 543.5 (516.6, 570.9) | 543.0 (506.0, 562.4) | 0.815 |
| Aniso Index (×102) | 89 | 3.0 (2.0, 4.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 3.0) | 0.682 |
| AvDC (10−6 mm2/s) | 89 | 985.9 (935.3, 1047.0) | 1006.0 (965.2, 1055.5) | 940.1 (912.6, 1031.0) | 1008.6 (956.9, 1039.5) | 0.079 |
| Right Prefrontal Cortex Region (RPCR) | ||||||
| M3D/BRAVO: 3D Ax BRAVO (cm3) | 90 | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.4) | 0.852 |
| Fractional Aniso | 88 | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.491 |
| Isotropic image | 88 | 526.6 (477.4, 559.2) | 519.0 (453.9, 582.6) | 519.0 (501.6, 553.6) | 529.7 (491.5, 552.3) | 0.796 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS (cm3) | 88 | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.3) | 0.889 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS | 88 | 1238.8 (1179.2, 1323.9) | 1237.2 (1176.8, 1310.9) | 1240.7 (1192.4, 1335.3) | 1214.3 (1175.9, 1326.1) | 0.774 |
| FA | 88 | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.606 |
| Vol Ratio Aniso (×102) | 88 | 7.0 (5.0, 9.0) | 6.0 (4.0, 9.0) | 7.0 (5.0, 9.0) | 7.0 (6.0, 8.0) | 0.522 |
| Trace | 88 | 527.2 (478.0, 570.4) | 519.5 (454.5, 583.4) | 519.7 (502.2, 569.0) | 530.2 (492.1, 553.0) | 0.770 |
| Aniso Index (×102) | 88 | 4.0 (3.0, 5.0) | 3.0 (3.0, 6.0) | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) | 0.640 |
| AvDC (10−6 mm2/s) | 88 | 928.1 (848.4, 1001.3) | 920.9 (850.4, 990.0) | 937.0 (855.1, 967.8) | 921.4 (844.3, 1026.0) | 0.936 |
| Left Prefrontal Cortex Region (LPCR) | ||||||
| M3D/BRAVO: 3D Ax BRAVO (cm3) | 90 | 0.3 (0.3, 0.4) | 0.3 (0.2, 0.3) | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.4) | 0.350 |
| Fractional Aniso | 90 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.421 |
| Isotropic image | 90 | 527.0 (482.4, 576.3) | 517.4 (483.2, 572.3) | 534.3 (483.0, 584.3) | 506.5 (482.5, 553.3) | 0.723 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS (cm3) | 90 | 0.3 (0.3, 0.3) | 0.3 (0.2, 0.3) | 0.3 (0.3, 0.4) | 0.3 (0.2, 0.4) | 0.257 |
| M3D/CubeT2flair: Sag T2 CUBE FLAIR FS | 90 | 1186.0 (1111.4, 1271.0) | 1173.3 (1116.4, 1227.8) | 1201.1 (1157.8, 1281.1) | 1175.4 (1063.8, 1276.4) | 0.461 |
| FA | 90 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) | 0.373 |
| Vol Ratio Aniso (×102) | 90 | 6.0 (4.0, 7.0) | 6.0 (5.0, 8.0) | 6.0 (4.0, 6.0) | 5.0 (4.0, 7.0) | 0.369 |
| Trace | 90 | 527.5 (483.0, 573.2) | 518.1 (483.8, 566.6) | 534.8 (483.7, 584.8) | 507.1 (483.1, 553.9) | 0.653 |
| Aniso Index (×102) | 90 | 3.0 (3.0, 5.0) | 3.0 (3.0, 4.0) | 3.0 (2.0, 4.0) | 4.0 (3.0, 5.0) | 0.287 |
| AvDC (10−6 mm2/s) | 90 | 966.6 (907.3, 1060.3) | 937.1 (876.5, 1013.5) | 968.0 (944.2, 1049.5) | 1022.0 (928.7, 1110.5) | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraniak-Gieszczyk, B.; Ogłodek, E.A. Impact of Post-Traumatic Stress Disorder Duration on Volumetric and Microstructural Parameters of the Hippo-Campus, Amygdala, and Prefrontal Cortex: A Multiparametric Magnetic Resonance Imaging Study with Correlation Analysis. J. Clin. Med. 2025, 14, 7242. https://doi.org/10.3390/jcm14207242
Paraniak-Gieszczyk B, Ogłodek EA. Impact of Post-Traumatic Stress Disorder Duration on Volumetric and Microstructural Parameters of the Hippo-Campus, Amygdala, and Prefrontal Cortex: A Multiparametric Magnetic Resonance Imaging Study with Correlation Analysis. Journal of Clinical Medicine. 2025; 14(20):7242. https://doi.org/10.3390/jcm14207242
Chicago/Turabian StyleParaniak-Gieszczyk, Barbara, and Ewa Alicja Ogłodek. 2025. "Impact of Post-Traumatic Stress Disorder Duration on Volumetric and Microstructural Parameters of the Hippo-Campus, Amygdala, and Prefrontal Cortex: A Multiparametric Magnetic Resonance Imaging Study with Correlation Analysis" Journal of Clinical Medicine 14, no. 20: 7242. https://doi.org/10.3390/jcm14207242
APA StyleParaniak-Gieszczyk, B., & Ogłodek, E. A. (2025). Impact of Post-Traumatic Stress Disorder Duration on Volumetric and Microstructural Parameters of the Hippo-Campus, Amygdala, and Prefrontal Cortex: A Multiparametric Magnetic Resonance Imaging Study with Correlation Analysis. Journal of Clinical Medicine, 14(20), 7242. https://doi.org/10.3390/jcm14207242






