Abstract
Background/Objectives: The potential role of robotic surgery in segmental colectomy for the treatment of splenic flexure and mid-transverse colon cancers remains underexplored. These sites are technically demanding because of the occurrence of vascular variability, the need for dual lymphatic drainage, and the close anatomical relationship to surrounding organs. This systematic review evaluated surgical strategies, anastomotic techniques, perioperative outcomes, and the oncological adequacy of robotic segmental colectomies in this context. Methods: The review followed the PRISMA guidelines (PROSPERO ID: CRD420251119736). Studies were eligible if they included ≥3 patients who were undergoing a robotic segmental colectomy for malignant tumors of the splenic flexure or mid-transverse colon. Data on patient demographics, operative details, complications, and oncological outcomes were extracted. The risk of bias was assessed using the Newcastle–Ottawa Scale and ROBINS-I. Results: Five retrospective studies reporting on 74 patients were included. All the procedures involved a fully robotic approach. Vascular ligation was uniform for transverse tumors (middle colic vessels point of origin), but varied for splenic flexure lesions. Anastomotic reconstruction was extracorporeal stapled (55.4%), intracorporeal stapled (16.2%), or intracorporeal hand sewn (4.1%). Operative times were in the range of 157.5–268 min; conversion occurred in 4.1% of cases. The overall morbidity was 16.2%, with anastomotic leaks in 5.4% of cases. No 30-day mortality was observed, and one reoperation was required. All patients achieved R0 resection, with a mean lymph node yield of 16.9. Only one recurrence was documented during the follow-up period. Conclusions: Robotic segmental colectomy for splenic flexure and mid-transverse colon malignancies is feasible and safe, achieving consistent perioperative and oncological outcomes. Larger multicenter prospective studies are needed to validate the oncological adequacy, standardize anastomotic strategies, and assess the cost effectiveness of the approach.
1. Introduction
Oncologically, splenic flexure tumors, defined as those arising between the distal third of the transverse colon and within 10 cm of the descending colon, together with mid-transverse lesions, represent some of the most technically demanding sites for colorectal resection. Surgical complexity arises from the need to mobilize both colonic flexures, working across multiple abdominal quadrants, in close proximity to the spleen, pancreas, stomach, and left kidney. Adequate lymphadenectomy requires precise identification and skeletonization of the middle colic vessels, whose branching pattern and variability may complicate vascular control [1,2,3]. Moreover, patients are often diagnosed at an advanced stage of disease, because subocclusive symptoms are usually the first manifestation and indicate tumors that are already voluminous. Finally, there is no consensus in the literature regarding the optimal surgical strategy, with proponents advocating for extended right or left hemicolectomy versus more conservative segmental resections [4,5].
Robotic surgery may facilitate these complex resections by enabling precise dissection, secure vascular ligation, and improved intracorporeal reconstruction [6]. Nevertheless, current evidence on the perioperative outcomes and oncological safety of such procedures is fragmented, being limited to case reports and a small heterogeneous series of studies. In addition, the choice of anastomotic technique (manual vs. mechanical, intracorporeal vs. extracorporeal) could affect the complication rates and recovery, but data specific to this setting remain scarce [7].
The aim of this systematic review is to critically assess and synthesize the available evidence on robotic segmental colectomies for malignant tumors of the splenic flexure and mid-transverse colon, focusing on surgical strategies, anastomotic techniques, and perioperative and oncological outcomes.
2. Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8] (Figure 1). The study protocol was developed a priori and registered in the PROSPERO database (Registration ID: CRD420251119736).
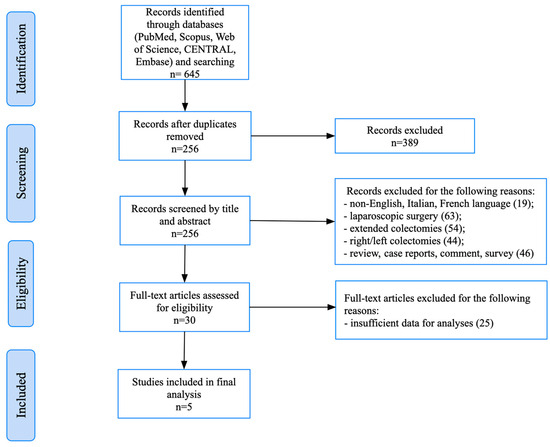
Figure 1.
PRISMA flowchart.
2.1. Search Strategy
A comprehensive literature search was performed using the electronic databases PubMed (MEDLINE), Embase, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL), from their inception to August 2025. The search strategy combined terms related to robotic surgery, splenic flexure, transverse colon, segmental colectomy, and colorectal malignancy, with database-specific syntax adjustments applied. The reference lists of relevant reviews were also screened to identify additional studies, although these sources were not included in the final analysis.
2.2. Eligibility Criteria
Studies were considered eligible if they specifically reported on robotic segmental colectomy for malignant tumors of the splenic flexure or mid-transverse colon, and if they provided sufficient surgical and perioperative outcomes. We excluded single case reports and 2 case technical notes to avoid studies involving sparse, non-standardized reporting and to ensure minimally interpretable event rates (e.g., morbidity, anastomotic leakage). A ≥3 case threshold also improved the comparability across cohorts within our descriptive, single-arm framework and in regard to the subsequent GRADE appraisal.
Publications in English, French, or Italian were accepted. Conversely, case reports involving fewer than three patients, studies limited to right or left colectomies, or case series describing non-robotic or hybrid procedures without any clearly distinguishable robotic data were excluded. Reviews, conference abstracts without full text, editorials, and letters were also not considered. Primary endpoints were the conversion rate, postoperative morbidity, anastomotic leakage, and mortality, while secondary endpoints included operative variables, recovery outcomes, and oncological adequacy, assessed according to the R0 resection, lymph node yield, and recurrence rate.
2.3. Study Selection and Data Extraction
The selection process was conducted independently by two reviewers (A.M. and S.G.), who first screened the titles and abstracts and, subsequently, assessed the full text of potentially relevant articles. Any disagreements were resolved through discussions, with recourse to a third reviewer when necessary (G.L.). For each study, the data were systematically extracted using a predefined template. The variables collected included the study characteristics, patient demographics, and tumor location, surgical details, such as the type of resection performed, operative time, blood loss, and conversion rate, as well as information on the anastomotic technique used (manual versus mechanical, intracorporeal versus extracorporeal). Postoperative outcomes were also recorded. Oncological parameters, such as the lymph node yield, R0 resection rate, and recurrence rate, when available, were noted.
The results were summarized in structured tables, and pooled descriptive statistics were calculated when possible, using SPSS software, version 12.0 (SPSS Inc., Chicago, IL, USA). Given the heterogeneity of the study design and reporting, no formal meta-analysis was performed. Instead, weighted and unweighted pooled values were presented for the most relevant perioperative and oncological endpoints. Forest plots and tests for interaction were not conducted because the included studies reported single-arm outcomes, without consistent comparator arms or extractable effect sizes. Accordingly, this review provides a descriptive, non-comparative synthesis of the results, rather than a meta-analysis.
2.4. Risk of Bias Assessment
Due to the anticipated predominance of non-randomized and retrospective studies, the methodological quality and risk of bias were assessed using the Newcastle–Ottawa Scale (NOS) and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) [9,10]. Each study was evaluated independently by two authors.
2.5. Certainty of Evidence (GRADE)
We rated the certainty for each key outcome (conversion, overall morbidity, anastomotic leakage, R0 resection, and ≥12 lymph nodes) using the GRADE framework. This method considers five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Because the evidence base was small, retrospective, and heterogeneously reported, we anticipated concerns regarding the risk of bias, inconsistency, and imprecision. To avoid an overly conservative, blanket downgrading approach in this single-arm surgical context, we applied a pragmatic adaptation: objective oncologic endpoints with consistent reporting (R0, ≥12 nodes) were rated as moderate, whereas perioperative outcomes (conversion, morbidity, leakage) were rated as low, due to sparse data and reporting variability.
3. Results
3.1. Study Selection
The initial database search yielded a total of 645 records. After the removal of duplicates and screening of the titles and abstracts, 30 full-text articles were assessed for eligibility. Of these, five retrospective studies met the predefined inclusion criteria and were included in the final analysis [11,12,13,14,15]. A PRISMA flow diagram illustrating the study selection process is provided in Figure 1.
3.2. Risk of Bias Assessment
Table 1 provides a comprehensive overview of the risk of bias evaluation across the included studies. The methodological quality of the included studies was generally high, despite their retrospective design. The NOS scores ranged from 7 to 8, corresponding to high quality in regard to the comparative cohorts and the single-surgeon series [11,12,13,14]. Jung’s case series could not be assessed with the NOS and was considered at serious risk of bias, according to the ROBINS-I tool, due to the very small sample size and the lack of a control group [15]. Two studies were rated as having a moderate risk of bias, while the remainder were judged to be at low risk. The main concerns were residual confounding, retrospective data collection, incomplete perioperative information, and the limited generalizability of single-surgeon series.

Table 1.
Risk of bias assessment.
Overall, the strength of the evidence can be considered moderate, constrained by the retrospective design, small patient cohorts, and potential selection bias. Consequently, the conclusions should be interpreted with caution.
3.3. Study Characteristics
The five included studies were all retrospective in design and collectively reported on 74 patients undergoing robotic resection for malignancies located at the splenic flexure or mid-transverse colon. The studies were conducted in four countries: Italy (n = 2), France, China, and South Korea. Three were comparative case series (two with propensity score matching) and two were single-surgeon case series. All the patients underwent a robotic segmental colectomy, with three studies including comparative laparoscopic cohorts. The robotic platforms utilized included the da Vinci S, Si, and Xi System® (Intuitive, Sunnyvale, CA, USA).
A detailed overview of the study characteristics is provided in Table 2.

Table 2.
Study and patient characteristics.
3.4. Patient and Tumor Characteristics
The patient demographics were variably reported. Patients were generally in the sixth to seventh decade of life, with a slight male predominance across the cohorts (overall distribution approximating 2:1). The American Society of Anesthesiologists (ASA) status, when available, indicated that most patients were classified as I–II. The tumor location was the splenic flexure in 31 patients (41.9%) and the mid-transverse colon in 43 patients (58.1%).
3.5. Surgical Technique and Intraoperative Outcomes
The technical details and intraoperative results are summarized in Table 3. All the procedures were performed using a robotic approach, although the extent of the robotic use varied depending on the anastomotic strategy. In the resections of the mid-transverse colon, vascular control invariably involved ligation of the middle colic vessels at their origin [11,12,14]. For splenic flexure cancers, the vascular strategies were more heterogeneous: Zhang et al. described artery-guided dissection, with division of the inferior mesenteric vein and left colic artery, while Monsellato et al. reported on a lymphadenectomy of the inferior mesenteric vein, inferior mesenteric artery, left colic artery, and both the main trunk and left branch of the middle colic vessels, while preserving the IMV [13,15]. Anastomotic reconstruction also varied: two studies performed extracorporeal stapled side-to-side anastomoses, one reported an intracorporeal hand-sewn end-to-end technique, and one described an intracorporeal stapled side-to-side approach. In the multicenter study by Milone et al., both intra- and extracorporeal anastomoses were performed, but the specific distribution among the 18 robotic cases was not reported, as the analysis was pooled with the laparoscopic group [14]. The use of indocyanine green (ICG) fluorescence was inconsistently reported. The operative time ranged from 157.5 to 267 min. Blood loss was consistently low (~100 mL). Conversion to open surgery was reported exclusively by Monsellato et al. (3 of 12 patients, 25%) [15].

Table 3.
Reported pre- and intraoperative data.
3.6. Postoperative Outcomes and GRADE Certainty
The length of stay was typically 7–9 days. Across the five case series (n = 74), postoperative morbidity was 16.2% (12/74; study range 0–33.3%) and anastomotic leakage occurred in 5.4% cases (4/74; 0–11.1%). Thirty-day mortality was 0; reoperation occurred in 1/74 (1.4%) cases; one patient developed severe pulmonary failure. Comparator data were inconsistent or absent, so the relative effects could not be estimated. Using a pragmatic GRADE approach for single-arm surgical data, the certainty for these perioperative endpoints (conversion rate, morbidity) was rated low due to the small retrospective cohorts involved, reporting heterogeneity, and the occurrence of only a few events. The study-level outcomes are reported in Table 4, and the pooled absolute risks, along with the study ranges and GRADE certainty, are summarized in Table 5.

Table 4.
Reported postoperative data.

Table 5.
Summary of Findings for Robotic Segmental Resection with GRADE Certainty. Aggregated absolute risks across five retrospective robotic series (n = 74), shown with study ranges and GRADE certainty. These are single-arm descriptive estimates (not comparative).
3.7. Oncological Outcomes
R0 resection was achieved in all 74 cases (100%). The tumor size was generally 3–5 cm. The lymph node yield varied across the case series, ranging from 6 to 8 nodes in the small single-surgeon report by Jung et al., to a mean of approximately 16–20 nodes in larger comparative case series, exceeding the oncological minimum of 12 in most patients. The resection margins were consistently negative (R0). The follow-up duration was heterogeneous, ranging from early postoperative reporting to up to 72 months. When reported, long-term oncologic outcomes were favorable: no recurrence in the cohorts described by De Angelis, Zhang, or Jung, and one recurrence with synchronous liver and lung metastases in the study by Monsellato (per-study details in Table 4).
3.8. Descriptive Stratifications
The outcomes according to the tumor site, anastomotic technique, and center type are summarized in Table 6. Mid-transverse cases (n = 43) showed higher morbidity (20.9%) and leakage rates (7.0%) compared with splenic flexure cases (n = 31), which had lower morbidity (9.7%) and leakage rates (3.2%), but a higher conversion rate (9.7%, all from one single-surgeon case series). Extracorporeal stapled anastomoses (n = 41) had a morbidity of 12.2% and a leakage rate of 4.9% with no conversions, whereas intracorporeal anastomoses (n = 15) had lower morbidity (6.7%), no leaks, but higher conversions (20%, single-surgeon case series). The multicenter cohort (n = 18) showed higher morbidity (33.3%) and a higher leakage rate (11.1%) compared with pooled single-center cohorts (n = 56; morbidity 10.7%, leakage 3.6%, conversion 5.4%). Platform generation and ICG use were inconsistently reported and, thus, could not be analyzed. Subgroup effect sizes were not extractable, and no interaction tests were performed.

Table 6.
Descriptive stratifications for robotic segmental resection. Outcomes stratified by tumor site, anastomotic technique, and center type across five retrospective robotic series (n = 74). Results are presented as raw n/N (%) event rates. These are single-arm descriptive data, not comparative estimates. Platform generation and ICG use were inconsistently reported and therefore could not be analyzed. These data are descriptive only, with subgroup effect sizes not extractable. Therefore, no formal interaction testing was performed.
4. Discussion
4.1. Technical Challenges and Role of Robotic Assistance
The splenic flexure and mid-transverse colon represent some of the most technically demanding regions for colorectal surgery, due to their variable vascular anatomy, the need for dual lymphatic drainage, and the close proximity to the spleen, pancreas, and stomach [16,17]. The oncological adequacy of segmental resection in this region has long been debated, with many surgeons historically favoring extended colectomy to ensure comprehensive lymphadenectomy [18,19,20]. Nevertheless, recent evidence suggests that a more conservative approach may be equally effective. For transverse colon cancers, a systematic review and meta-analysis by Morarasu et al. demonstrated no significant differences in overall or disease-free survival between patients undergoing segmental and extended colectomies [21]. Segmental resections were further associated with a shorter operative time, reduced intraoperative blood loss, and preservation of the bowel length, while still achieving adequate lymph node harvest and R0 resection rates. Similarly, an analysis of the National Cancer Database (NCDB), involving more than 60,000 patients, confirmed that segmental colectomy offers survival outcomes comparable to extended resections [22]. For splenic flexure cancers, additional large-scale and multicenter study data have reported equivalent oncological results between segmental and extended colectomies, reinforcing the oncological adequacy of a limited resection in this region [23,24]. Collectively, these findings strengthen the rationale for using segmental resection as an oncologically sound strategy.
Prior studies have shown that laparoscopy can match open surgery, even in complex oncologic settings. Robotics is a natural evolution of this minimally invasive pathway [25,26]. The platform’s three-dimensional vision, wristed instruments, and stable ergonomics enable precise plane-based dissection and reliable control of the left colic and the left branch of the middle colic arteries [27]. Compared with laparoscopy, it reduces the risk of splenic or pancreatic injury and facilitates medial-to-lateral dissection, flexure takedown, and the identification of key pedicles, particularly in obese patients, with a thick abdominal wall and a short transverse mesocolon.
4.2. Anastomotic Strategies
Anastomotic reconstruction varied across the case series, underscoring the lack of a standardized approach in this region. Two studies reported extracorporeal stapled side-to-side anastomoses, one an intracorporeal hand-sewn end-to-end technique, and another, an intracorporeal stapled approach. In the multicenter case series by Milone et al., both intra- and extracorporeal methods were used, but the robotic subgroup was not stratified, because the analyses were pooled with laparoscopy [14]. This heterogeneity likely mirrors differences in surgical experience and institutional preference, rather than a proven superiority of one method over another.
The limited use of intracorporeal anastomosis is notable given that the robotic platform facilitates stable exposure, reduced mesenteric traction, and smaller extraction sites. Moreover, being performed entirely within the abdominal cavity, intracorporeal reconstruction spares the surgeon from having to perform the extensive mobilization otherwise required to exteriorize the colonic stumps when using extracorporeal techniques [28,29,30]. This advantage is exemplified by Jung et al., who reported on the feasibility of intracorporeal hand-sewn end-to-end anastomoses, without the need for undue colonic mobilization [12].
The analysis performed by Milone et al. represents an excellent example of the current evidence and illustrates well the points discussed above: in a propensity matched comparison of 33 intracorporeal versus 33 extracorporeal anastomoses, intracorporeal reconstruction was associated with a shorter operative time, faster recovery of the bowel function, and a markedly lower complication burden, including anastomotic leaks, compared with extracorporeal reconstruction, while the long-term oncologic outcomes remained equivalent [14].
These findings are consistent with evidence from right colectomies, such as the prospective ANCOR trial, where intracorporeal reconstruction similarly reduced complications and accelerated postoperative recovery, despite modestly longer operative times [31].
Overall, intracorporeal anastomosis appears to be a valid reconstructive option in robotic segmental colectomy, although its adoption remains inconsistent, likely reflecting variability in the relevant expertise and the absence of standardized protocols. Fluorescence angiography was not routinely reported. This support tool, already used in multiple laparoscopic contexts, is now incorporated into robotic systems (Firefly® mode), providing additional intraoperative guidance and potentially improving perfusion assessment [32,33,34].
4.3. Operative and Perioperative Outcomes
Across the pooled analysis of the five studies, the advantages of robotics were associated with consistent operative and perioperative results. The operative time was longer than in standard colectomies, reflecting the anatomical complexity rather than platform limitations. Conversion to open surgery was required in only 4.1% of patients, indicating stable procedural control, even in regard to anatomically demanding dissections. The morbidity (16.2%) and leakage rates (5.4%) were within expected colorectal benchmarks, indicating that robotic surgery does not carry an additional perioperative burden in this context. Importantly, mortality was zero and reoperation rare. R0 resection was universal, and the mean lymph node yield exceeded oncologic standards. Hospital stays ranged between 6 and 8.6 days, confirming that postoperative recovery is not negatively impacted, despite the demanding nature of the procedure.
This uniform adequacy, even across small and heterogeneous case series, highlights the ability of the robotic technique to ensure reproducible short-term outcomes and oncological reliability.
4.4. Operator Experience and Learning Curve
The concept of the learning curve has been widely investigated across different surgical fields and has been shown to vary substantially depending on the procedure and approach adopted. Different operative modalities (open, laparoscopic, robotic) are associated with distinct learning patterns, underscoring the multifactorial nature of surgical proficiency [35]. In colorectal surgery, robotics may shorten and standardize the learning curve through enhanced visualization, stable ergonomics, and wrist-based instrumentation [36,37,38]. Several of the reports included in this analysis are single-surgeon case series, which limits the generalizability of the findings and likely influences the technical outcomes. Conversions were clustered into one single-surgeon cohort (3/12; 25%), whereas the remaining case series reported 0% conversion; conversely, anastomotic leaks were absent in the single-surgeon cohorts (0/15), but occurred at a low frequency in the multi-operator comparative cohort. The operative time varied widely (≈157.5–268 min), consistent with differences in the case selection and stage along the learning curve. Although we could not formally model the learning curves (e.g., CUSUM) due to the small sample size and retrospective design of the studies included, future studies should report on the surgeon-level volume, sequence number, and proctoring to enable learning curve-adjusted benchmarking of the outcomes, such as conversion and leakage rates.
4.5. Cost-Effectiveness Considerations
The economic profile of robotic segmental colectomy likely varies by center and case mix. Capital and maintenance costs, instrument utilization, and case volume (learning curve and amortization effects) must be weighed against the potential gains, such as lower conversion, improved ergonomics, a comparable length of stay, and team-level efficiency [39,40]. Given the single-arm nature and small sample size in the included studies, comparative cost effectiveness remains uncertain. We advocate standardized cost reporting (fixed vs. variable costs, instrument counts, operative time, length of hospital stay, readmissions) and prospective, adequately powered comparative studies to determine whether the clinical benefits translate into net economic value across different healthcare settings.
4.6. Strengths
To our knowledge, this is the first focused synthesis of robotic segmental resections for splenic flexure and mid-transverse colon malignancies, which are two anatomically demanding locations. Beyond cataloguing technical variants (anastomotic strategy, vascular approach), this review shows consistent oncologic adequacy across the case series, with universal R0 reporting and a high proportion of patients meeting the ≥12 lymph node benchmark. These outcomes improve the interpretability of such data and may help to guide technical decisions in this context.
4.7. Limitations
The available evidence consists of retrospective, small cohorts, several of which are single-surgeon case series, with incomplete reporting of key modifiers (robot platform generation, ICG use), and substantial heterogeneity in regard to the case mix and technique used (tumor site, anastomosis type, platform). This precluded formal subgroup meta-analyses and tests for interactions. The follow-up period was short or variably reported, limiting long-term oncologic inferences. In regard to the NOS and ROBINS-I, the overall methodological quality was acceptable (comparative cohorts were generally higher; single-surgeon case series were at greater risk of bias). Using this pragmatic GRADE adaptation, the certainty ranged from low (perioperative endpoints) to moderate (objective oncologic endpoints). In conclusion, the results remain hypothesis generating and merit confirmation using larger, prospective multicenter studies.
4.8. Future Directions
Future research should aim to consolidate the role of the robotic approach in segmental colectomy for the treatment of splenic flexure and mid-transverse colon cancers through the use of prospective multicenter studies, with standardized protocols. Key areas for further investigation include the impact of the learning curve on surgical performance, the reproducibility of intracorporeal anastomosis as a reconstructive strategy, and the oncological validation of segmental colectomy as a safe alternative to extended resections.
5. Conclusions
Robotic segmental colectomy for the treatment of splenic flexure and mid-transverse colon cancers appears feasible and safe, with acceptable short-term and oncologic outcomes, and it can be used to facilitate complex dissection and intracorporeal reconstruction. Yet, the current evidence is limited to small retrospective case series. Multicenter prospective studies are needed to confirm the oncologic safety, define the learning curves, and determine its cost effectiveness before broad adoption.
Author Contributions
Conceptualization, A.F. and G.C.; Methodology, A.I.; Software, A.M.; Validation, G.C., E.R. and A.I.; Formal Analysis, S.G. and A.M.; Investigation, G.L.; Data Curation, S.G. and G.L.; Writing—Original Draft Preparation, A.F. and G.C.; Writing—Review and Editing, E.R.; Visualization, A.I.; Supervision, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study have been extracted from the literature.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Nakagoe, T.; Sawai, T.; Tsuji, T.; Jibiki, M.; Ohbatake, M.; Nanashima, A.; Yamaguchi, H.; Yasutake, T.; Kurosaki, N.; Ayabe, H.; et al. Surgical treatment and subsequent outcome of patients with carcinoma of the splenic flexure. Surg. Today 2001, 31, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Nagai, S.; Manabe, T.; Koba, R.; Nagayoshi, K.; Nakamura, M.; Tanaka, M. Vascular anatomy of the transverse mesocolon and bidirectional laparoscopic D3 lymph node dissection for patients with advanced transverse colon cancer. Surg. Endosc. 2019, 33, 2257–2266. [Google Scholar] [CrossRef]
- Fukuoka, A.; Sasaki, T.; Tsukikawa, S.; Miyajima, N.; Ostubo, T. Evaluating distribution of the left branch of the middle colic artery and the left colic artery by CT angiography and colonography to classify blood supply to the splenic flexure. Asian J. Endosc. Surg. 2017, 10, 148–153. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, J.; Lee, S.Y.; Kim, C.H.; Kim, H.R. Distribution of lymph node metastasis and oncological outcomes of mid-transverse colon cancer: Extended versus transverse colectomy. Colorectal Dis. 2021, 23, 2007–2013. [Google Scholar] [CrossRef]
- Hajibandeh, S.; Hajibandeh, S.; Hussain, I.; Zubairu, A.; Akbar, F.; Maw, A. Comparison of extended right hemicolectomy, left hemicolectomy and segmental colectomy for splenic flexure colon cancer: A systematic review and meta-analysis. Colorectal Dis. 2020, 22, 1885–1907. [Google Scholar] [CrossRef]
- Morini, A.; Zizzo, M.; Zanelli, M.; Sanguedolce, F.; Palicelli, A.; Bonelli, C.; Mangone, L.; Fabozzi, M. Robotic versus laparoscopic colectomy for transverse colon cancer: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2025, 40, 79. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, S.; Wang, L.; Liu, Z.; Zhou, H.; Liu, Q.; Chen, Y.; Tang, J.; Wang, X. Short-term and long-term outcomes of intracorporeal anastomosis in laparoscopic segmental left colectomy for splenic flexure cancer—A multicenter retrospective cohort study of 342 cases. Int. J. Surg. 2024, 110, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 August 2025).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- De Angelis, N.; Alghamdi, S.; Renda, A.; Azoulay, D.; Brunetti, F. Initial experience of robotic versus laparoscopic colectomy for transverse colon cancer: A matched case-control study. World J. Surg. Oncol. 2015, 13, 295. [Google Scholar] [CrossRef]
- Jung, K.U.; Park, Y.; Lee, K.Y.; Sohn, S.K. Robotic transverse colectomy for mid-transverse colon cancer: Surgical techniques and oncologic outcomes. J. Robot. Surg. 2015, 9, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, Z.; Zhang, Y.; Ji, X.; Jing, X.; Shi, Y.; Cheng, X.; Zhao, R. Single-docking robotic-assisted artery-guided segmental splenic flexure colectomy for splenic flexure cancer—A propensity score-matching analysis. J. Gastrointest. Oncol. 2021, 12, 944–952. [Google Scholar] [CrossRef]
- Milone, M.; Degiuli, M.; Velotti, N.; Manigrasso, M.; Vertaldi, S.; D’Ugo, D.; De Palma, G.D. Segmental transverse colectomy. Minimally invasive versus open approach: Results from a multicenter collaborative study. Updates Surg. 2022, 74, 127–135. [Google Scholar] [CrossRef]
- Monsellato, I.; Gatto, T.; Lodin, M.; Sangiuolo, F.; Palucci, M.; Del Basso, C.; Giannone, F.; Panaro, F. Robotic splenic flexure cancer resection: Technique and short-term outcomes. Minerva Surg. 2024, 79, 607–615. [Google Scholar] [CrossRef]
- Sakamoto, T.; Mukai, T.; Noguchi, T.; Matsui, S.; Yamaguchi, T.; Akiyoshi, T.; Kawachi, H.; Fukunaga, Y. Patterns of lymph node metastasis and long-term outcomes of splenic flexure colon cancer: A descriptive study from a Japanese high-volume center. Surg. Today 2025, 55, 1079–1087. [Google Scholar] [CrossRef]
- Murono, K.; Nozawa, H.; Kawai, K.; Sasaki, K.; Emoto, S.; Kishikawa, J.; Ishii, H.; Yokoyama, Y.; Abe, S.; Nagai, Y.; et al. Vascular anatomy of the splenic flexure: A review of the literature. Surg. Today 2022, 52, 727–735. [Google Scholar] [CrossRef]
- Milone, M.; Degiuli, M.; Allaix, M.E.; Ammirati, C.A.; Anania, G.; Barberis, A.; Belli, A.; Bianchi, P.P.; Bianco, F.; Bombardini, C.; et al. Mid-transverse colon cancer and extended versus transverse colectomy: Results of the Italian society of surgical oncology colorectal cancer network (SICO CCN) multicenter collaborative study. Eur. J. Surg. Oncol. 2020, 46, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.; Zanelli, M.; Sanguedolce, F.; Castro Ruiz, C.; Morini, A.; Ascani, S.; Manenti, A.; Giunta, A. Extended versus segmental colectomy for mid-transverse colon cancers: An unsolved question. Colorectal Dis. 2021, 23, 2772–2773. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; He, M.; Chen, K.; Liu, Z.; Khlusov, D.I.; Khorobrykh, T.V.; Cao, X.; Panova, P.D.; Efetov, S.K.; Kazaryan, A.M. Short- and long-term outcomes after surgical treatment of 5918 patients with splenic flexure colon cancer by extended right colectomy, segmental colectomy and left colectomy: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1244693. [Google Scholar] [CrossRef] [PubMed]
- Morarasu, S.; Clancy, C.; Cronin, C.T.; Matsuda, T.; Heneghan, H.M.; Winter, D.C. Segmental versus extended colectomy for tumours of the transverse colon: A systematic review and meta-analysis. Colorectal Dis. 2021, 23, 625–634. [Google Scholar] [CrossRef]
- Crippa, J.; Grass, F.; Achilli, P.; Behm, K.T.; Mathis, K.L.; Day, C.N.; Harmsen, W.S.; Mari, G.M.; Larson, D.W. Surgical approach to transverse colon cancer: Analysis of current practice and oncological outcomes using the National Cancer Database. Dis. Colon Rectum 2021, 64, 284–292. [Google Scholar] [CrossRef]
- Manceau, G.; Alves, A.; Meillat, H.; Benhaïm, L.; Ouaïssi, M.; Panis, Y.H.; Tuech, J.J.; Dousset, B.; Brigand, C.; Cotte, E.; et al. What is the optimal elective colectomy for splenic flexure cancer: End of the debate? A multicenter study from the GRECCAR Group with a propensity score analysis. Dis. Colon Rectum 2022, 65, 55–65. [Google Scholar] [CrossRef]
- Pang, A.J.; Marinescu, D.; Morin, N.; Vasilevsky, C.A.; Boutros, M. Segmental resection of splenic flexure colon cancers provides an adequate lymph node harvest and is a safe operative approach—An analysis of the ACS-NSQIP database. Surg. Endosc. 2022, 36, 5652–5659. [Google Scholar] [CrossRef]
- Di Carlo, S.; Siragusa, L.; Fassari, A.; Fiori, E.; La Rovere, F.; Izzo, P.; Usai, V.; Cavallaro, G.; Franceschilli, M.; Dhimolea, S.; et al. Laparoscopic versus open total gastrectomy for locally advanced gastric cancer: Short and long-term results. Curr. Oncol. 2022, 29, 8442–8455. [Google Scholar] [CrossRef]
- Koerner, C.; Rosen, S.A. How robotics is changing and will change the field of colorectal surgery. World J. Gastrointest. Surg. 2019, 11, 381–387. [Google Scholar] [CrossRef]
- Mithany, R.H.; Shaikh, A.; Murali, S.; Rafique, A.; Bebawy, P.S.; Nair, P.G.; Ramadan, W.; Abdelglil, M.; Gupta, A.; Sayed, M.A.; et al. A review of the current trends and future perspectives of robots in colorectal surgery: What have we got ourselves into? Cureus 2025, 17, e77690. [Google Scholar] [CrossRef]
- Huscher, C.G.S.; Lazzarin, G.; Marchegiani, F.; Marks, J. Robotic right colectomy with robotic-sewn anastomosis: A pilot case series. J. Robot. Surg. 2023, 17, 427–434. [Google Scholar] [CrossRef]
- Scotton, G.; Contardo, T.; Zerbinati, A.; Tosato, S.M.; Orsini, C.; Morpurgo, E. From laparoscopic right colectomy with extracorporeal anastomosis to robot-assisted intracorporeal anastomosis to totally robotic right colectomy for cancer: The evolution of robotic multiquadrant abdominal surgery. J. Laparoendosc. Adv. Surg. Tech. A 2018, 28, 1216–1222. [Google Scholar] [CrossRef]
- Cuk, P.; Büyükuslu, M.; Möller, S.; Verwaal, V.J.; Al-Najami, I.; Ellebæk, M.B. Intracorporeal versus extracorporeal anastomosis in segmental resections for colon cancer: A retrospective cohort study of 328 patients. Langenbecks Arch. Surg. 2023, 408, 219. [Google Scholar] [CrossRef]
- Cleary, R.K.; Silviera, M.; Reidy, T.J.; McCormick, J.; Johnson, C.S.; Sylla, P.; Cannon, J.; Lujan, H.; Kassir, A.; Landmann, R.; et al. Intracorporeal and extracorporeal anastomosis for robotic-assisted and laparoscopic right colectomy: Short-term outcomes of a multi-center prospective trial. Surg. Endosc. 2022, 36, 4349–4358. [Google Scholar] [CrossRef]
- Fassari, A.; Bianucci, A.; Lucchese, S.; Santoro, E.; Lirici, M.M. Fluorescence cholangiography for laparoscopic cholecystectomy: How, when, and why? A single-center preliminary study. Minim. Invasive Ther. Allied Technol. 2023, 32, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Morimoto, M.; Denda, Y.; Nonoyama, K.; Murase, H.; Kato, T.; Hayashi, Y.; Imafuji, H.; Ogawa, R.; Takahashi, H.; et al. Accurate intraoperative real-time blood flow assessment of the remnant stomach during robot-assisted distal pancreatectomy with celiac axis resection using indocyanine green fluorescence imaging and da Vinci Firefly technology. Asian J. Endosc. Surg. 2023, 16, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ontario Health (Quality). Indocyanine green fluorescence imaging for colorectal surgery: A health technology assessment. Ont. Health Technol. Assess. Ser. 2025, 25, 1–124. [Google Scholar]
- Fassari, A.; Gurrado, A.; Iossa, A.; Micalizzi, A.; Polistena, A.; Sibio, S.; Crocetti, D.; Bononi, M.; Testini, M.; Avenia, N.; et al. Definition of learning curve for thyroidectomy: Systematic review on the different approaches. Gland Surg. 2023, 12, 989–1006. [Google Scholar] [CrossRef]
- Blumberg, D. Robotic colectomy with intracorporeal anastomosis is feasible with no operative conversions during the learning curve for an experienced laparoscopic surgeon developing a robotics program. J. Robot. Surg. 2019, 13, 545–555. [Google Scholar] [CrossRef]
- Flynn, J.; Larach, J.T.; Kong, J.C.H.; Waters, P.S.; Warrier, S.K.; Heriot, A. The learning curve in robotic colorectal surgery compared with laparoscopic colorectal surgery: A systematic review. Colorectal Dis. 2021, 23, 2806–2820. [Google Scholar] [CrossRef]
- Coco, D.; Leanza, S. Learning curve of robotic colectomy: A systematic review and meta-analysis of surgical proficiency, outcomes, and training protocols. J. Robot. Surg. 2025, 19, 509. [Google Scholar] [CrossRef]
- Ielpo, B.; Podda, M.; Burdio, F.; Sanchez-Velazquez, P.; Guerrero, M.A.; Núñez, J.; Toledano, M.; Morales-Conde, S.; Mayol, J.; Lopez-Cano, M.; et al. Cost-effectiveness of robotic vs. laparoscopic surgery for different surgical procedures: Protocol for a prospective, multicentric study (ROBOCOSTES). Front. Surg. 2022, 9, 866041. [Google Scholar] [CrossRef]
- Chok, A.Y.; Zhao, Y.; Tan, I.E.; Au, M.K.H.; Tan, E.J.K.W. Cost-effectiveness comparison of minimally invasive, robotic and open approaches in colorectal surgery: A systematic review and Bayesian network meta-analysis of randomized clinical trials. Int. J. Colorectal Dis. 2023, 38, 86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).