Geboes Histopathology Score Grade 3 Subclassification Predicts Treatment Response in Active Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
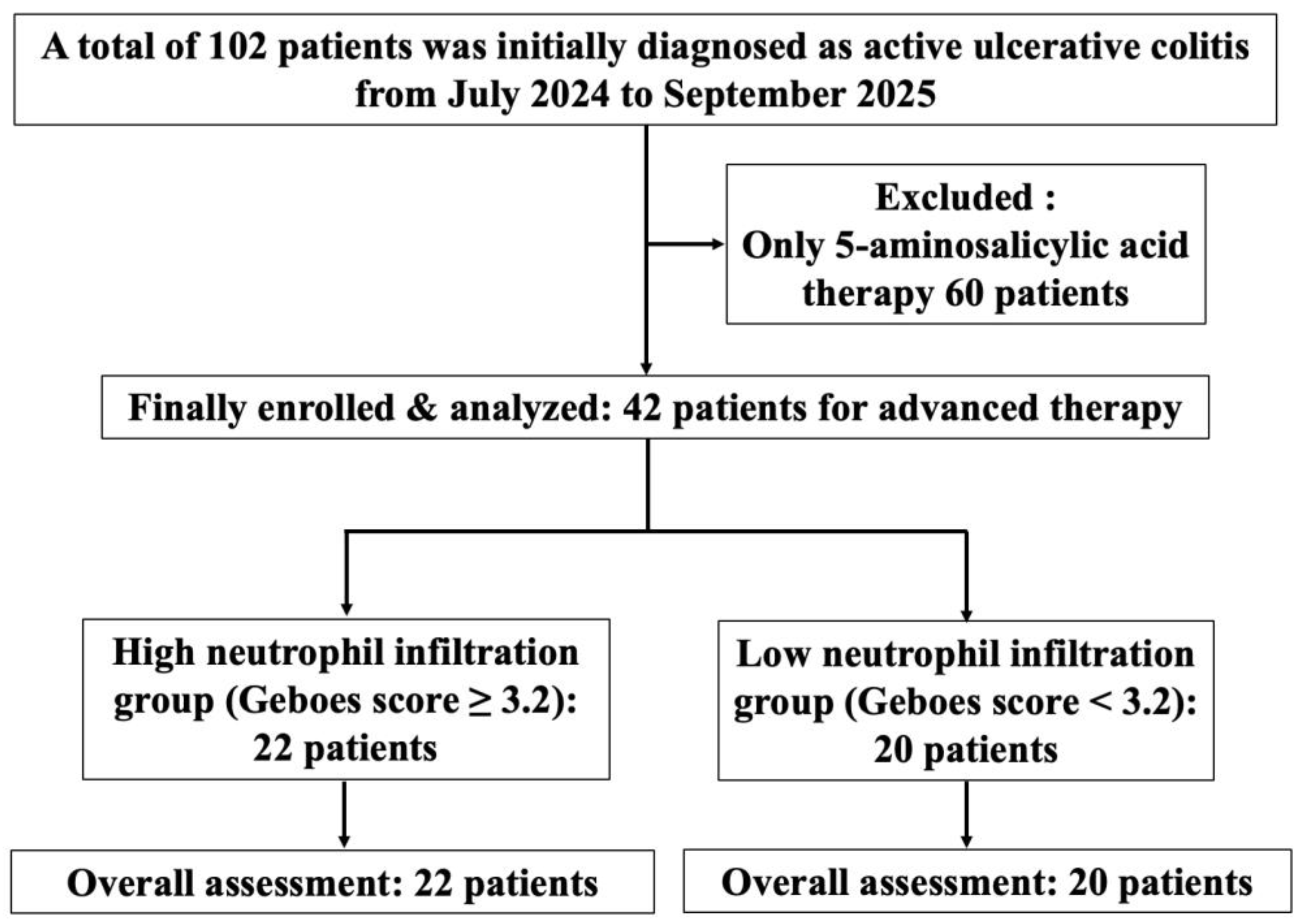
2.1. Study Design and Patients
2.2. Clinical, Endoscopic, and Histopathological Evaluations
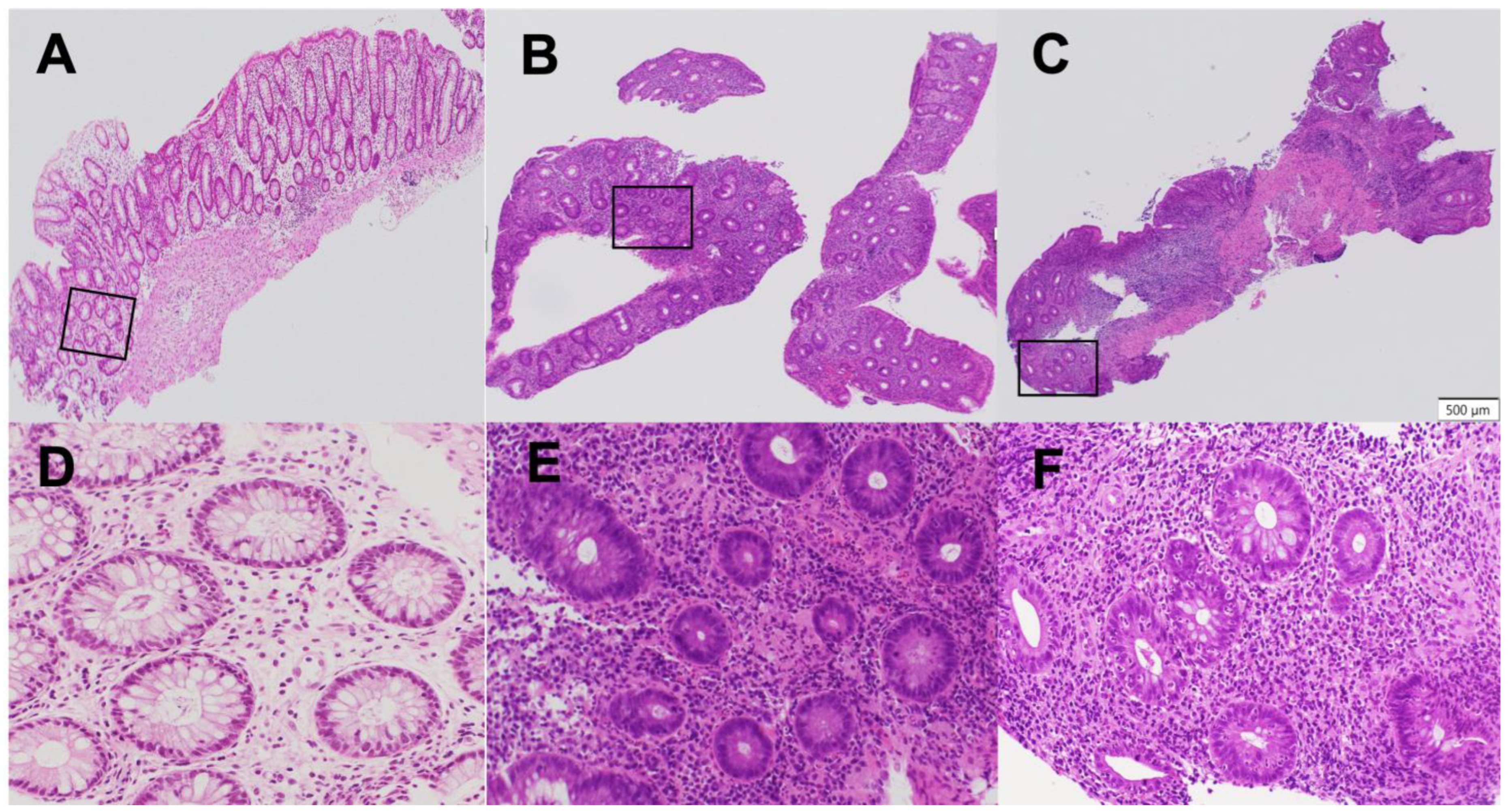
2.3. Komagane Evaluation Method
2.4. Raters and Reliability Testing
2.5. Treatment Stratification Based on the Komagane Subclassification of the Geboes Score Grade 3
2.6. Measurements of Serum C-Reactive Protein (CRP) and Leucine-Rich α2 Glycoprotein (LRG) Levels
2.7. Safety Monitoring
2.8. Outcome Measures
2.9. Statistical Analysis
3. Results
3.1. Patients’ Clinical Characteristics
3.2. Changes in Blood Biomarkers
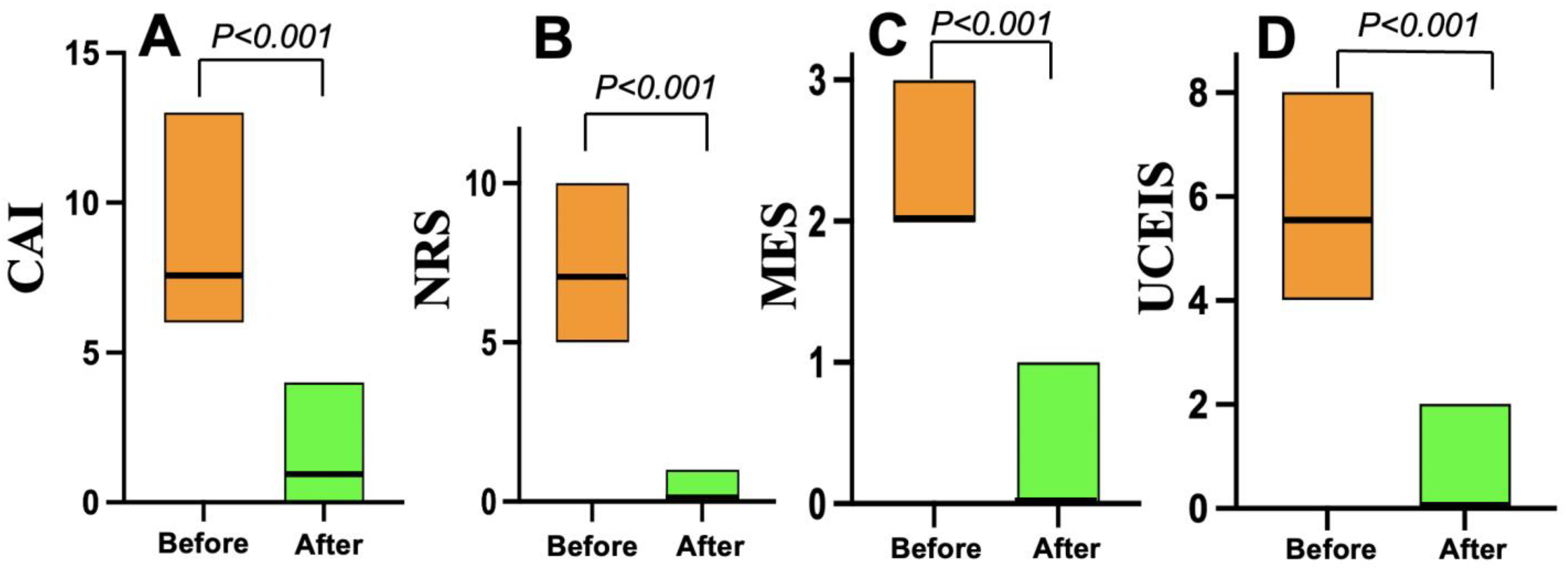
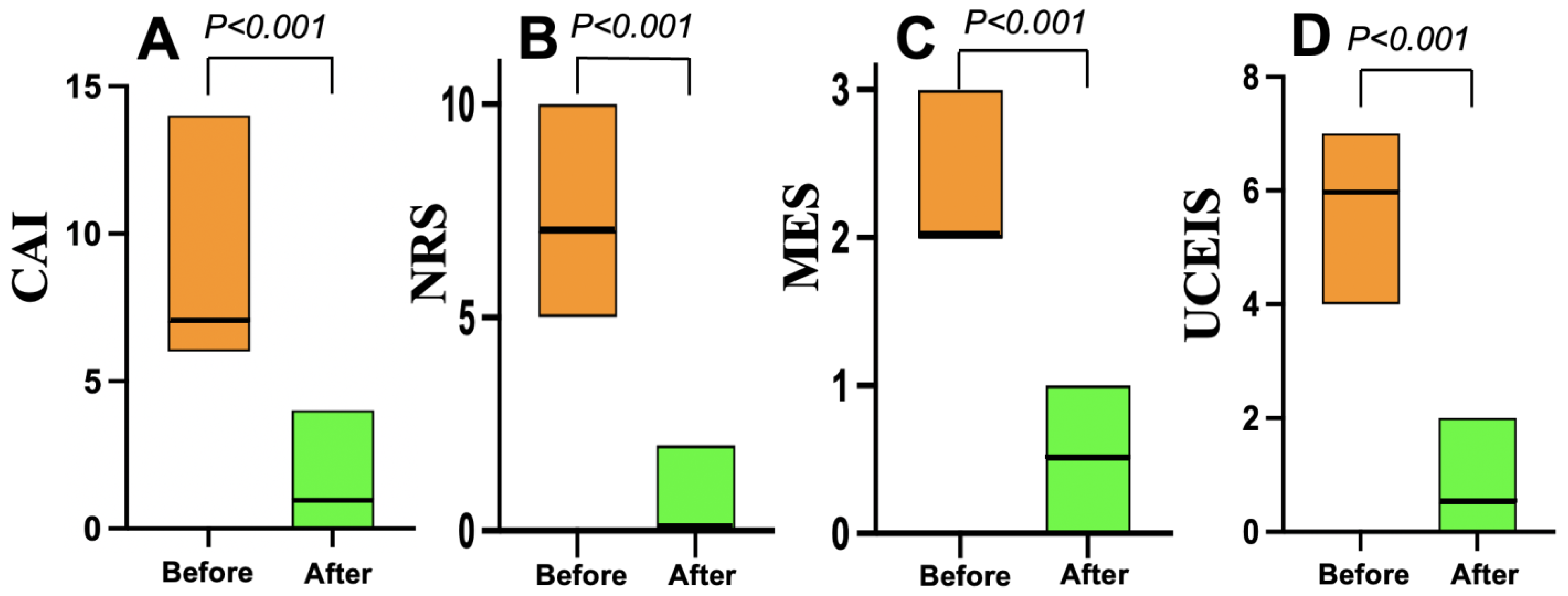
3.3. Treatment Efficacy
3.4. Safety and Adverse Events
4. Discussion
| Class/Agent | Targeted Pathway | Mucosal Healing Rate | Mechanistic Notes |
|---|---|---|---|
| Selective IL-23p19 inhibitors | IL-23 (p19 subunit) | High (30%+) [23,24] | Targets upstream of Th17 cells, highly specific. |
| Anti-TNF antibodies | TNF-α broader cytokines | Moderate (~20–25%) [23,28] | Blocks a key pro-inflammatory cytokine, less specific. Immunogenicity can limit long-term effectiveness. |
| Anti-Integrins (Vedolizumab) | Gut-selective cell trafficking | Moderate (~15–25%) [23,28] | Inhibits lymphocyte migration to gut. |
| JAK inhibitors (Tofacitinib) | JAK-STAT intracellular pathway | Moderate to Low [23,28] | Broad inhibition, can have higher infection risk. |
| IL-12/23 inhibitors (Ustekinumab) | IL-12/IL-23 (p40 subunit) | Moderate to High [23,28] | Less specific, partly overlaps with IL-23p19 agents but may affect broader immune functions. |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X. Pathological mechanism and targeted drugs of ulcerative colitis: A review. Medicine 2023, 102, e35020. [Google Scholar] [CrossRef]
- Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Fumery, M.; Singh, S.; Dulai, P.S.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Sandborn, W.J. Natural history of adult ulcerative colitis in population-based cohorts: A systematic review. Clin. Gastroenterol. Hepatol. 2018, 16, 343–356.e3. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’aMico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Singh, S.; Loftus, E.D., Jr.; Limketkai, B.N.; Haydek, J.P.; Agrawal, M.; Scott, F.I.; Ananthakrishnan, A.N. AGA Clinical Guidelines Committee. Gastroenterology 2024, 167, 1307–1343. [Google Scholar] [CrossRef]
- Noviello, D.; Mager, R.; Roda, G.; Borroni, R.G.; Fiorino, F.; Vetrano, S. The IL23-IL17 immune axis in the treatment of ulcerative colitis: Successes, defeats, and ongoing challenges. Front. Immunol. 2021, 12, 611256. [Google Scholar] [CrossRef]
- Pastras, P.; Aggeletopoulou, I.; Papantoniou, K.; Triantos, C. Targeting the IL-23 receptor gene: A promising approach in inflammatory bowel disease treatment. Int. J. Mol. Sci. 2025, 26, 4775. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.; Nanda, K.S.; Zenlea, T.; Gifford, A.; Lawlor, G.O.; Falchuk, K.R.; Wolf, J.L.; Cheifetz, A.S.; Goldmith, J.D.; Moss, A.C. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission: Correlates of histological inflammation. Clin. Gastroenterol. Hepatol. 2013, 11, 991–996. [Google Scholar] [CrossRef]
- Geboes, K.; Riddell, R.; Ost, A.; Jensfelt, B.; Persson, T.; Löfberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ukai, S.; Horiuchi, I.; Terashima, T.; Horiuchi, K.; Horiuchi, A. Assessment of ulcerative colitis patients with elevated neutrophilic infiltration in the colonic mucosal epithelium using the Komagane subclassification of the Geboes score Grade 3. J. Clin. Med. 2025, 14, 5180. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: A randomised trial. BMJ 1989, 298, 82–86. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Irving, P.M.; Panaccione, R.; Naegeli, A.N.; Potts-Bleakman, A.; Arora, V.; Shan, M.; Travis, S. Incorporating patient experience into drug development for ulcerative colitis: Development of the Urgency Numeric Rating Scale, a patient-reported outcome measure to assess bowel urgency in adults. J. Patient Rep. Outcomes 2022, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Travis, S.P.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.-F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef]
- D’Haens, G.; Dubinsky, M.; Kobayashi, T.; Irving, P.M.; Howaldt, S.; Pokrotnieks, J.; Krueger, K.; Laskowski, J.; Li, X.; Lissoos, T.; et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2023, 388, 2444–2455. [Google Scholar] [CrossRef]
- Shinzaki, S.; Matsuoka, K.; Iijima, H.; Mizuno, S.; Serada, S.; Fujimoto, M.; Arai, N.; Koyama, N.; Morii, E.; Watanabe, M.; et al. Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J. Crohn’s Colitis 2017, 11, 84–91. [Google Scholar] [CrossRef]
- Horiuchi, I.; Horiuchi, A.; Umemura, T. Serum leucine-rich α2 glycoprotein: A biomarker for predicting the presence of ulcerative colitis but not ulcerative proctitis. J. Clin. Med. 2022, 11, 6366. [Google Scholar] [CrossRef]
- Horiuchi, I.; Horiuchi, K.; Horiuchi, A.; Umemura, T. Serum leucine-rich α2 glycoprotein could be a useful biomarker to differentiate patients with normal colonic mucosa from those with inflammatory bowel disease or other forms of colitis. J. Clin. Med. 2024, 13, 2957. [Google Scholar] [CrossRef] [PubMed]
- Korta, A.; Kula, J.; Gomulka, K. The role of IL-23 in the pathogenesis and therapy of inflammatory bowel disease. Int. J. Mol. Sci. 2023, 24, 10172. [Google Scholar] [CrossRef]
- Parigi, T.L.; Lacucci, M.; Ghosh, S. Blockade of IL-23: What is in the pipeline? J. Crohns Colitis 2022, 16, ii64–ii72. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, Z.S.; Sands, B.E. Personalised medicine with IL-23 blockers: Myth or reality? J. Crohns Colitis 2022, 16, ii73–ii94. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Wang, Q.; Xiao, H. Efficacy and safety of IL-23 p19 inhibitors in the treatment for inflammatory bowel disease: A systematic review and meta-analysis. Front. Pharmacol. 2025, 16, 1490667. [Google Scholar] [CrossRef]
- Magro, F.; Pai, R.K.; Kobayashi, T.; Jairath, V.; Rieder, F.; Redondo, I.; Lissoos, T.; Morris, N.; Shan, M.; Park, M.; et al. Resolving histological inflammation in ulcerative colitis with mirikizumab in the LUCENT induction and maintenance trial programmes. J. Crohns Colitis 2023, 17, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Steere, B.; Beidler, C.; Martin, A.; Bright, S.; Kikly, K.; Benschop, R.J. Generation and characterization of mirikizumab, a humanized monoclonal antibody targeting the p19 subunit of IL23. J. Pharmacol. Exp. Ther. 2023, 387, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Salas, A.; Sands, B.E.; Abraham, C.; Leibovitzh, H.; Neurath, M.F.; Casteele, N.V. IL-12 and IL23 pathway inhibition in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 433–446. [Google Scholar] [CrossRef]
- Elainein, M.A.A.; ElSherefy, S.S.; Yousef, N.M.; ElKady, S.M.; Hamam, N.G.; Elgarawany, A.; Aswa, D.W.; Hassan, A.N.E.; Allam, S. Efficacy and safety of Mirikizumab for ulcerative colitis: A systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol. 2025, 25, 307. [Google Scholar] [CrossRef]
- Dignass, A.; Ainsworth, C.; Hartz, S.; Dunnewind, N.; Redondo, I.; Sapin, C.; Kroep, S.; Halfpenny, N.; Arcà, E.; Hoque, S. Efficacy and safety of advanced therapies in moderately-to-severely active ulcerative colitis: A systematic review and network meta-analysis. Adv. Ther. 2024, 41, 4446–4462. [Google Scholar] [CrossRef]
| Geboes Score | Grade ≥ 3.2 | Grade < 3.2 | p |
|---|---|---|---|
| Number of patients | 22 | 20 | |
| Males/females, n (%) | 10 (45)/12 (55) | 9 (45)/11 (55) | 0.99 |
| Age, yrs; median (25th, 75th percentile) | 43 (32, 53) | 43 (31, 55) | 0.98 |
| Disease duration, years; median (25th, 75th percentile) | 2.0 (1.0, 3.0) | 2.0 (1.0, 7.8) | 0.37 |
| Disease extension, n (%): | 0.99 | ||
| Left-sided colitis | 10 (45) | 10 (50) | |
| Pancolitis | 12 (55) | 10 (50) | |
| CAI, median (25th, 75th percentile | 7.5 (7, 9) | 7 (6, 9) | 0.56 |
| NRS, median (25th, 75th percentile) | 7 (6, 7) | 7 (4, 8) | 0.25 |
| MES, median (25th, 75th percentile) | 2 (2, 2.25) | 2 (2, 2.5) | 0.99 |
| UCEIS, median (25th, 75th percentile) | 5.5 (5, 6) | 6 (5, 6) | 0.17 |
| Medication before induction therapy, n (%): | 0.15 | ||
| 5-ASA + steroids | 7 (32) | 10 (50) | |
| Infliximab | 7 (32) | 4 (20) | |
| Ustekinumab | 4 (18) | 0 | |
| Filgotinib | 4 (18) | 6 (30) |
| Parameter (Mean ± SD) | Before | After | p |
|---|---|---|---|
| Geboes ≥ 3.2 Group (n = 22) | |||
| Hemoglobin (g/dL) | 13.1 ± 1.3 | 13.7 ± 1.4 | 0.36 |
| Albumin (g/dL) | 3.9 ± 0.52 | 4.1 ± 0.51 | 0.40 |
| CRP (mg/dL) | 1.5 ± 5.8 | 0.034 ± 0.03 | 0.12 |
| LRG (µg/mL) | 18.3 ± 3.6 | 11.0 ± 1.9 | <0.001 |
| Geboes < 3.2 Group (n = 20) | |||
| Hemoglobin (g/dL) | 13.6 ± 1.6 | 14.2 ± 1.4 | 0.44 |
| Albumin (g/dL) | 3.8 ± 0.32 | 4.1 ± 0.44 | 0.56 |
| CRP (mg/dL) | 2.1 ± 4.5 | 0.043 ± 0.04 | 0.33 |
| LRG (µg/mL) | 20.3 ± 4.1 | 15.0 ± 3.1 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiuchi, I.; Horiuchi, A.; Horiuchi, K.; Watanabe, S.; Terashima, T.; Sugimoto, K. Geboes Histopathology Score Grade 3 Subclassification Predicts Treatment Response in Active Ulcerative Colitis. J. Clin. Med. 2025, 14, 7214. https://doi.org/10.3390/jcm14207214
Horiuchi I, Horiuchi A, Horiuchi K, Watanabe S, Terashima T, Sugimoto K. Geboes Histopathology Score Grade 3 Subclassification Predicts Treatment Response in Active Ulcerative Colitis. Journal of Clinical Medicine. 2025; 14(20):7214. https://doi.org/10.3390/jcm14207214
Chicago/Turabian StyleHoriuchi, Ichitaro, Akira Horiuchi, Kaori Horiuchi, Shun Watanabe, Tsuyoshi Terashima, and Ken Sugimoto. 2025. "Geboes Histopathology Score Grade 3 Subclassification Predicts Treatment Response in Active Ulcerative Colitis" Journal of Clinical Medicine 14, no. 20: 7214. https://doi.org/10.3390/jcm14207214
APA StyleHoriuchi, I., Horiuchi, A., Horiuchi, K., Watanabe, S., Terashima, T., & Sugimoto, K. (2025). Geboes Histopathology Score Grade 3 Subclassification Predicts Treatment Response in Active Ulcerative Colitis. Journal of Clinical Medicine, 14(20), 7214. https://doi.org/10.3390/jcm14207214





