Integrating Emotional Stress and Lipid Lowering in Cardiovascular Disease Management: The Future of Precision Cardiovascular Prevention
Abstract
1. Introduction
2. The Traditional Lipid-Centric Paradigm
- (i)
- Refinement of lipid targets: Therapeutic strategies should incorporate apoB or remnant cholesterol measurement into routine clinical practice—moving beyond LDL-C alone—to identify individuals at heightened risk who might benefit from additional metabolic interventions (e.g., targeting triglyceride-rich lipoproteins, ANGPTL3/4 modulation).
- (ii)
- Integration with non-lipid risk factors: Lipid lowering, while necessary, is not sufficient. Residual risk stems from multifactorial contributors—including inflammation, emotional stress, endothelial dysfunction, and microvascular pathology—that lipid-centric interventions do not address.
3. Emotional Stress: The Underappreciated Culprit
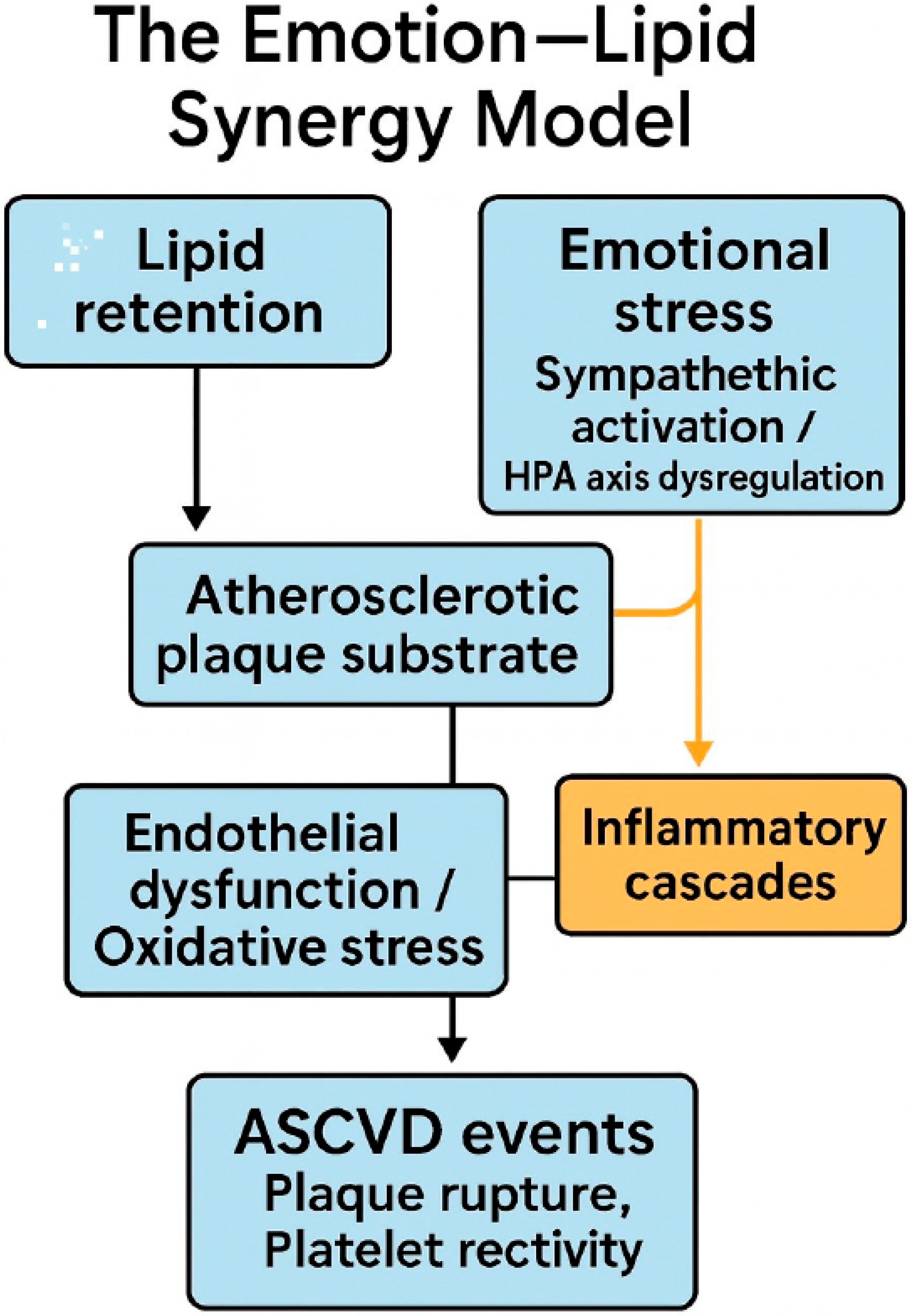
4. Mechanistic Insights: The Emotion–Lipid Synergy Model
5. Clinical and Therapeutic Implications
- (i)
- Emotion-targeted interventions: Combining pharmacologic options (e.g., low-dose SSRIs, centrally acting agents) with structured behavioral therapies such as CBT or mindfulness-based stress reduction (MBSR).
- (ii)
- Digital therapeutics: Deploying eHealth and mHealth platforms capable of delivering CBT, relaxation, or emotion-regulation modules, particularly suited for long-term adherence and monitoring.
- (iii)
- Exercise integration: Leveraging structured recovery programs—not only to improve physical conditioning post-MI or takotsubo—but also as psychological therapy, capitalizing on the mutually reinforcing benefits of physical activity and emotional regulation.
- (iv)
- Dietary patterns, microbiome, and emotion-aware prevention: Diet is a tractable mediator at the intersection of lipid metabolism, inflammation, and stress physiology. In primary prevention, Mediterranean-style eating reduces major cardiovascular events (PREDIMED; randomized re-analysis) [28]. At the immune–microbiome interface, a randomized diet trial in healthy adults showed that fermented foods (vs high-fiber alone) increased microbiome diversity and decreased inflammatory markers over 10 weeks [50]. Preclinical and translational data support the microbiota–gut–brain axis and short-chain fatty acids (SCFAs) as mediators of stress and neuroimmune signaling, nominating diet as a pathway to alter stress biology [51,92]. In nutritional psychiatry, the SMILES randomized trial demonstrated that a structured diet intervention improved depressive symptoms with an NNT ≈ 4 for remission [93]. Taken together, diet can be integrated with lipid-lowering and emotion-targeted strategies to reduce residual risk through apoB/LDL pathways, inflammatory tone, and stress reactivity.
6. Digital Health and Precision Prevention
- (i)
- From numbers to states: Continuous lipid metrics are static; emotional state is dynamic. In patients with established CAD (or post-MI), we advocate pairing apoB/LDL targets with state-space monitoring (HRV, EDA, breathing, SCG) to detect “high-risk emotional states” that can precipitate ischemia or arrhythmia, then responding in minutes—not months—with a micro-intervention (paced breathing, brief CBT, or even short-acting pharmacologic modulation) delivered as a JITAI [47].
- (ii)
- Precision triggers, layered treatments: Use patient-specific physiological fingerprints (e.g., a characteristic HRV drop + EDA surge) as digital triggers that titrate the intensity of the intervention: from a subtle haptic cue to breathe, up to a coached CBT module, and, for selected patients, protocolized β-blocker up-titration or anti-inflammatory strategies if repeated stress-peaks correlate with symptoms or biomarkers. (Trials should test these stepped algorithms explicitly).
- (iii)
- On-device, privacy-preserving AI: Deploy SSL-pretrained models on the phone/watch to keep raw data local, fine-tune to the individual, and stream only low-dimensional risk scores to the clinic (i.e., brief, clinically interpretable indices such as a stress-reactivity score derived from HRV/EDA)—minimizing privacy/latency issues while maximizing clinical actionability [96].
- (iv)
- Equity by design: Because stress burden is socially patterned, digital prevention must not widen disparities. Plan for device access (loaner wearables, subsidized programs), connectivity (offline/sms pathways where broadband is limited), and digital literacy (multilingual, simple UI). Co-design with communities at highest risk and evaluate in pragmatic trials that oversample under-resourced settings [103,104,105,106,107,108,109]. Test feasibility alongside outcomes to ensure inclusive benefit, not just technical success [101,110].
7. Equity & Social Determinants
From Perspective to Action
8. Future Directions and Research
8.1. Flagship Outcomes Trial: EMOTION-MI
8.2. Mechanistic Research Priorities
8.3. Precision Phenotyping and Biobanking
8.4. Implementation Science and Real-World Integration
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reijnders, E.; van der Laarse, A.; Jukema, J.W.; Cobbaert, C.M. High residual cardiovascular risk after lipid-lowering: Prime time for Predictive, Preventive, Personalized, Participatory, and Psycho-cognitive medicine. Front. Cardiovasc. Med. 2023, 10, 1264319. [Google Scholar] [CrossRef]
- Machanahalli Balakrishna, A.; Kaushik, S.; Tandalam Palanivelu, S.; Monther, N.; Ponamgi, S.P.; Alla, V.M.; Patil, S.M. Safety and Efficacy of Achieving Very Low LDL Cholesterol Concentrations with PCSK9 Inhibitors. J. Clin. Med. 2025, 14, 4562. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Ma, S.; Qian, L.; Jing, Y.; Chen, X.; Yang, J. Efficacy and safety of PCSK9 inhibitors, potent statins, and their combinations for reducing low-density lipoprotein cholesterol in hyperlipidemia patients: A systematic network meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1415668. [Google Scholar] [CrossRef]
- Gomez-Delgado, F.; Raya-Cruz, M.; Katsiki, N.; Delgado-Lista, J.; Perez-Martinez, P. Residual cardiovascular risk: When should we treat it? Eur. J. Intern. Med. 2024, 120, 17–24. [Google Scholar] [CrossRef]
- Banach, M.; Surma, S.; Toth, P.P. 2023: The year in cardiovascular disease—the year of new and prospective lipid lowering therapies. Can we render dyslipidemia a rare disease by 2024? Arch. Med. Sci. 2023, 19, 1602–1615. [Google Scholar] [CrossRef] [PubMed]
- Capuozzo, M.; Ottaiano, A.; Cinque, C.; Farace, S.; Ferrara, F. Cardiovascular risk management beyond statins: Review of new therapies available in Italy. Egypt. Heart J. 2025, 77, 68. [Google Scholar] [CrossRef]
- Safarova, M.; Bimal, T.; Soffer, D.E.; Hirsh, B.; Shapiro, M.D.; Mintz, G.; Cha, A.; Gianos, E. Advances in targeting LDL cholesterol: PCSK9 inhibitors and beyond. Am. J. Prev. Cardiol. 2024, 19, 100701. [Google Scholar] [CrossRef] [PubMed]
- Manolis, T.A.; Manolis, A.A.; Manolis, A.S. Emotional Stress in Cardiac and Vascular Diseases. Curr. Vasc. Pharmacol. 2025, 23, 172–195. [Google Scholar] [CrossRef]
- Garcia, M.; Moazzami, K.; Almuwaqqat, Z.; Young, A.; Okoh, A.; Shah Amit, J.; Sullivan, S.; Lewis Tené, T.; Elon, L.; Ko, Y.-A.; et al. Psychological Distress and the Risk of Adverse Cardiovascular Outcomes in Patients with Coronary Heart Disease. JACC Adv. 2024, 3, 100794. [Google Scholar] [CrossRef]
- Munir, L.; Toit, E. Impact of Chronic Psychological Stress on Cardiovascular Disease Risk: A Narrative Review. Heart Mind 2024, 8, 268–278. [Google Scholar] [CrossRef]
- Shimbo, D.; Cohen, M.T.; McGoldrick, M.; Ensari, I.; Diaz, K.M.; Fu, J.; Duran, A.T.; Zhao, S.; Suls, J.M.; Burg, M.M.; et al. Translational Research of the Acute Effects of Negative Emotions on Vascular Endothelial Health: Findings from a Randomized Controlled Study. J. Am. Heart Assoc. 2024, 13, e032698. [Google Scholar] [CrossRef]
- Vaccarino, V.; Bremner, J.D. Stress and cardiovascular disease: An update. Nat. Rev. Cardiol. 2024, 21, 603–616. [Google Scholar] [CrossRef]
- Ebong, I.A.; Quesada, O.; Fonkoue, I.T.; Mattina, D.; Sullivan, S.; Oliveira, G.M.M.d.; Spikes, T.; Sharma, J.; Commodore, Y.; Ogunniyi, M.O. The role of psychosocial stress on cardiovascular disease in women: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2024, 84, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, S.; Sullivan, S. Toxic stress is associated with cardiovascular disease among younger but not older women in the United States: Results from the research goes red registry. Prev. Med. Rep. 2025, 51, 102992. [Google Scholar] [CrossRef]
- Civieri, G.; Abohashem, S.; Grewal Simran, S.; Aldosoky, W.; Qamar, I.; Hanlon, E.; Choi Karmel, W.; Shin Lisa, M.; Rosovsky Rachel, P.; Bollepalli Sandeep, C.; et al. Anxiety and Depression Associated with Increased Cardiovascular Disease Risk Through Accelerated Development of Risk Factors. JACC Adv. 2024, 3, 101208. [Google Scholar] [CrossRef]
- El-Malahi, O.; Mohajeri, D.; Bäuerle, A.; Mincu, R.I.; Rammos, C.; Jansen, C.; Teufel, M.; Rassaf, T.; Lortz, J. The Influence of eHealth Stress Management Interventions on Psychological Health Parameters in Patients with Cardiovascular Disease: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2025, 27, e67118. [Google Scholar] [CrossRef]
- García-Sánchez, E.; Santamaría-Peláez, M.; González-Bernal, J.J.; González-Santos, J.; Sedano García, M.A.; De Juana Velasco, I.; Sánchez Hernández, J.; García Pardo, H.; Fernández-Solana, J. Impact of cardiac rehabilitation on anxiety, depression, and health-related quality of life in cardiovascular patients. Egypt. Heart J. 2025, 77, 64. [Google Scholar] [CrossRef]
- Su, J.J.; Lin, R.; Batalik, L.; Wong, A.K.C.; Grace, S.L. Psychological eHealth Interventions for Patients with Cardiovascular Diseases: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2025, 27, e57368. [Google Scholar] [CrossRef]
- Chen, P.; Li, X. NLRP3 inflammasome in atherosclerosis: Mechanisms and targeted therapies. Front. Pharmacol. 2024, 15, 1430236. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, P.; Pitsillidi, A.; Pyrpyris, N.; Fragkoulis, C.; Leontsinis, I.; Koutsopoulos, G.; Mantzouranis, E.; Soulaidopoulos, S.; Kasiakogias, A.; Dimitriadis, K.; et al. Pregnancy-Associated Takotsubo Syndrome: A Narrative Review of the Literature. J. Clin. Med. 2025, 14, 2356. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.; Herrmann, M.J.; Lauer, M.; Förster, C.Y. Exploratory Review of the Takotsubo Syndrome and the Possible Role of the Psychosocial Stress Response and Inflammaging. Biomolecules 2024, 14, 167. [Google Scholar] [CrossRef]
- Mauriello, A.; Giudice, C.D.; Vecchio, G.E.D.; Correra, A.; Maratea, A.C.; Grieco, M.; Amata, A.; Quagliariello, V.; Maurea, N.; Proietti, R.; et al. Takotsubo Syndrome and Oxidative Stress: Physiopathological Linkage and Future Perspectives. Antioxidants 2025, 14, 522. [Google Scholar] [CrossRef]
- Fan, X.; Yang, G.; Kowitz, J.; Akin, I.; Zhou, X.; El-Battrawy, I. Takotsubo Syndrome: Translational Implications and Pathomechanisms. Int. J. Mol. Sci. 2022, 23, 1951. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Oikonomou, E.; Chasikidis, C.; Tsioufis, K.; Tousoulis, D. Inflammasomes in Atherosclerosis—From Pathophysiology to Treatment. Pharmaceuticals 2023, 16, 1211. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Katsiki, N.; Vrablik, M.; Banach, M.; Gouni-Berthold, I. Inclisiran, low-density lipoprotein cholesterol and lipoprotein (a). Pharmaceuticals 2023, 16, 577. [Google Scholar] [CrossRef]
- Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.J.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Lawrence, D.; Friedman, A. Inclisiran and cardiovascular events: A patient-level analysis of phase III trials. Eur. Heart J. 2023, 44, 129–138. [Google Scholar]
- Bashir, B.; Schofield, J.; Downie, P.; France, M.; Ashcroft, D.M.; Wright, A.K.; Romeo, S.; Gouni-Berthold, I.; Maan, A.; Durrington, P.N.; et al. Beyond LDL-C: Unravelling the residual atherosclerotic cardiovascular disease risk landscape—Focus on hypertriglyceridaemia. Front. Cardiovasc. Med. 2024, 11, 1389106. [Google Scholar] [CrossRef]
- Mushumba, P.; Uwineza, D.N.; Nsanzimana, V.; Mapira, H.T.; Gori, E.; Musarurwa, C. Stress, Cortisol, and Lipid Profiles Among Rwandan Undergraduate Students: A Cross-Sectional Study. Risk Manag. Healthc. Policy 2025, 18, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Arnaldi, G.; Scandali, V.M.; Trementino, L.; Cardinaletti, M.; Appolloni, G.; Boscaro, M. Pathophysiology of dyslipidemia in Cushing’s syndrome. Neuroendocrinology 2010, 92 (Suppl. 1), 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Dunbar, R.L.; Ketchum, S.B.; Tardif, J.C.; Martens, F.; Ballantyne, C.M.; et al. Cardiovascular Outcomes with Icosapent Ethyl by Baseline Low-Density Lipoprotein Cholesterol: A Secondary Analysis of the REDUCE-IT Randomized Trial. J. Am. Heart Assoc. 2025, 14, e038656. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Glynn, R.J.; Fruchart, J.-C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef]
- Hansen, M.K.; Mortensen, M.B.; Warnakula Olesen, K.K.; Thrane, P.G.; Maeng, M. Non-HDL cholesterol and residual risk of cardiovascular events in patients with ischemic heart disease and well-controlled LDL cholesterol: A cohort study. Lancet Reg. Health Eur. 2024, 36, 100774. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.F.; Wu, N.Q. Remnant Cholesterol and Residual Risk of Atherosclerotic Cardiovascular Disease. Rev. Cardiovasc. Med. 2025, 26, 25985. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tian, J.; Chang, X.; Liu, J.; Wang, G. Association between remnant cholesterol and the risk of cardiovascular-kidney-metabolic syndrome progression: Insights from the China health and retirement longitudinal study. Eur. J. Prev. Cardiol. 2025, 32, 1157–1165. [Google Scholar] [CrossRef]
- Mach, F.; Visseren, F.L.J.; Cater, N.B.; Salhi, N.; Soronen, J.; Ray, K.K.; Delgado, V.; Jukema, J.W.; Laufs, U.; Zamorano, J.-L.; et al. Addressing residual risk beyond statin therapy: New targets in the management of dyslipidaemias–A report from the European Society of Cardiology Cardiovascular Round Table. J. Clin. Lipidol. 2024, 18, e685–e700. [Google Scholar] [CrossRef]
- Shi, F.; Dou, J.; Zhang, X. Advancements in research to mitigate residual risk of atherosclerotic cardiovascular disease. Eur. J. Med. Res. 2025, 30, 735. [Google Scholar] [CrossRef]
- Raggi, P.; Quyyumi, A.A.; Henein, M.Y.; Vaccarino, V. Psychosocial stress and cardiovascular disease. Am. J. Prev. Cardiol. 2025, 22, 100968. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Osei, J.; Vaccarino, V.; Wang, M.; Shah, A.S.; Lampert, R.; Li, L.Y.; Ko, Y.-A.; Pearce, B.D.; Kutner, M.; Garcia, E.V.; et al. Stress-Induced Autonomic Dysfunction is Associated with Mental Stress–Induced Myocardial Ischemia in Patients with Coronary Artery Disease. Circ. Cardiovasc. Imaging 2024, 17, e016596. [Google Scholar] [CrossRef]
- Rahmani, M.H.; Symons, M.; Sobhani, O.; Berkvens, R.; Weyn, M. EmoWear: Wearable Physiological and Motion Dataset for Emotion Recognition and Context Awareness. Sci. Data 2024, 11, 648. [Google Scholar] [CrossRef]
- Pinge, A.; Gad, V.; Jaisighani, D.; Ghosh, S.; Sen, S. Detection and monitoring of stress using wearables: A systematic review. Front. Comput. Sci. 2024, 6, 1478851. [Google Scholar] [CrossRef]
- Schwerdtfeger, A.R.; Tatschl, J.M.; Rominger, C. Effectiveness of 2 Just-in-Time Adaptive Interventions for Reducing Stress and Stabilizing Cardiac Autonomic Function: Microrandomized Trials. J. Med. Internet Res. 2025, 27, e69582. [Google Scholar] [PubMed]
- Hébert, E.T.; Kendzor, D.E.; Vidrine, D.J.; Langford, J.S.; Kezbers, K.M.; Montgomery, A.; Chen, M.; Frank-Pearce, S.G.; Vesely, S.K.; Chen, S.; et al. Just-in-Time Adaptive Intervention for Smoking Cessation in Low-Income Adults: A Randomized Clinical Trial. JAMA Netw. Open 2025, 8, e2526691. [Google Scholar] [CrossRef]
- Bueno, H.; Deaton, C.; Farrero, M.; Forsyth, F.; Braunschweig, F.; Buccheri, S.; Dragan, S.; Gevaert, S.; Held, C.; Kurpas, D.; et al. 2025 ESC Clinical Consensus Statement on mental health and cardiovascular disease: Developed under the auspices of the ESC Clinical Practice Guidelines Committee: Developed by the task force on mental health and cardiovascular disease of the European Society of Cardiology (ESC) Endorsed by the European Federation of Psychologists’ Associations AISBL (EFPA), the European Psychiatric Association (EPA), and the International Society of Behavioral Medicine (ISBM). Eur. Heart J. 2025, ehaf191. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e00338-20. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Aldosoky, W.; Ong, M.B.H.; Mir, T.; Dar, T.; Abohashem, S. A Narrative Review on Mental Stress and Cardiovascular Disease: Evidence, Mechanisms, and Potential Interventions. Heart Mind 2023, 7, 62–69. [Google Scholar] [CrossRef]
- Jin, N.; Cheng, L.; Geng, Q. Multiomics on Mental Stress-Induced Myocardial Ischemia: A Narrative Review. Heart Mind 2024, 8, 15–20. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Bai, B.; Liu, Q.; Liu, Y.; Wang, H.; Wang, Y.; Liang, Y.Y.; Liu, A.; Yu, X.; et al. Myocardial blood flow mechanism of mental stress-induced myocardial ischemia in women with ANOCA. iScience 2024, 27, 111302. [Google Scholar] [CrossRef]
- Mehta, P.K.; Sharma, A.; Bremner, J.D.; Vaccarino, V. Mental Stress-Induced Myocardial Ischemia. Curr. Cardiol. Rep. 2022, 24, 2109–2120. [Google Scholar] [CrossRef]
- Hinterdobler, J.; Schott, S.; Jin, H.; Meesmann, A.; Steinsiek, A.-L.; Zimmermann, A.-S.; Wobst, J.; Müller, P.; Mauersberger, C.; Vilne, B.; et al. Acute mental stress drives vascular inflammation and promotes plaque destabilization in mouse atherosclerosis. Eur. Heart J. 2021, 42, 4077–4088. [Google Scholar] [CrossRef]
- Moazzami, K.; Garcia, M.; Sullivan, S.; Lewis, T.T.; Bremner, J.D.; Razavi, A.C.; Shallenberger, L.; Sun, Y.V.; Raggi, P.; Shah, A.J.; et al. Association Between Symptoms of Chronic Psychological Distress and Myocardial Ischemia Induced by Mental Stress in Patients with Coronary Artery Disease. J. Am. Heart Assoc. 2023, 12, e030305. [Google Scholar] [CrossRef]
- O’Hara, M.; Roy, R.; Altenburg, M.; Slivnick, J.; Patel, H. Examining the Disproportionate Burden of Microvascular Disease in Women. Curr. Atheroscler. Rep. 2025, 27, 65. [Google Scholar] [CrossRef]
- Patel, V. Income Inequality and Psychiatric Admission in a Rich Country: Happiness Does Not Guarantee Mental Health Equity. JAMA Psychiatry 2020, 77, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.T.; Chang, J.; Manchanda, A.S.; Cook-Wiens, G.; Shufelt, C.L.; Anderson, R.D.; Petersen, J.W.; Naik, D.R.; Thomson, L.E.J.; Berman, D.S.; et al. Autoimmune rheumatic diseases in women with coronary microvascular dysfunction: A report from the Women’s Ischemia Syndrome Evaluation—Coronary Vascular Dysfunction (WISE-CVD) project. Front. Cardiovasc. Med. 2023, 10, 1155914. [Google Scholar] [CrossRef]
- Blaes, A.H.; Nair, C.; Everson-Rose, S.; Jewett, P.; Wolf, J.; Zordoky, B. Psychological measures of stress and biomarkers of inflammation, aging, and endothelial dysfunction in breast cancer survivors on aromatase inhibitors. Sci. Rep. 2023, 13, 1677. [Google Scholar] [CrossRef] [PubMed]
- Hage, Z.; Madeira, M.M.; Koliatsis, D.; Tsirka, S.E. Convergence of endothelial dysfunction, inflammation and glucocorticoid resistance in depression-related cardiovascular diseases. BMC Immunol. 2024, 25, 61. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Gamble, D.T.; Rudd, A.; Dospinescu, C.; Creaney, C.; Horgan, G.; Holme, A.; Wilson, H.M.; Newby, D.E.; Gray, S.R.; et al. Takotsubo syndrome: Cognitive behavioural therapy, physical training, and brain function recovery in the BREAKOUT trial. Eur. Heart J. 2025, ehaf441. [Google Scholar] [CrossRef]
- Smyth, A.; O’Donnell, M.; Lamelas, P.; Teo, K.; Rangarajan, S.; Yusuf, S. Physical Activity and Anger or Emotional Upset as Triggers of Acute Myocardial Infarction. Circulation 2016, 134, 1059–1067. [Google Scholar] [CrossRef]
- Brauer, M.; Roth, G.A.; Aravkin, A.Y.; Zheng, P.; Abate, K.H.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; et al. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef]
- Mostofsky, E.; Penner, E.A.; Mittleman, M.A. Outbursts of anger as a trigger of acute cardiovascular events: A systematic review and meta-analysis. Eur. Heart J. 2014, 35, 1404–1410. [Google Scholar] [CrossRef]
- Mostofsky, E.; Maclure, M.; Tofler, G.H.; Muller, J.E.; Mittleman, M.A. Relation of outbursts of anger and risk of acute myocardial infarction. Am. J. Cardiol. 2013, 112, 343–348. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality. Lancet 2003, 361, 1391–1392. [Google Scholar] [CrossRef]
- Canoy, D.; Nazarzadeh, M.; Copland, E.; Bidel, Z.; Rao, S.; Li, Y.; Rahimi, K. How Much Lowering of Blood Pressure Is Required to Prevent Cardiovascular Disease in Patients With and Without Previous Cardiovascular Disease? Curr. Cardiol. Rep. 2022, 24, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Bazmandegan, G.; Abbasifard, M.; Nadimi, A.E.; Alinejad, H.; Kamiab, Z. Cardiovascular risk factors in diabetic patients with and without metabolic syndrome: A study based on the Rafsanjan cohort study. Sci. Rep. 2023, 13, 559. [Google Scholar] [CrossRef]
- Nan, N.; Feng, L.; Dong, W.; Gao, B.; Zuo, H.; Mi, H.; Wang, G.; Song, X.; Zhang, H. The prognostic study of mental stress-induced myocardial ischemia in coronary revascularization patients with depression/anxiety: Rationale and design. BMC Cardiovasc. Disord. 2023, 23, 235. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, E.; Henein, M.Y. Mental stress and cardiovascular health—Part I. J. Clin. Med. 2022, 11, 3353. [Google Scholar] [CrossRef]
- Pepine Carl, J.; Petersen John, W.; Bairey Merz, C.N. A Microvascular-Myocardial Diastolic Dysfunctional State and Risk for Mental Stress Ischemia. JACC Cardiovasc. Imaging 2014, 7, 362–365. [Google Scholar] [CrossRef]
- Wei, J.; Cheng, S.; Bairey Merz, C.N. Coronary Microvascular Dysfunction Causing Cardiac Ischemia in Women. JAMA 2019, 322, 2334–2335. [Google Scholar] [CrossRef]
- Herrmann, H.C. A Novel Device for Coronary Microvascular Dysfunction and Refractory Angina. NEJM J. Watch. 2025, NA58314. [Google Scholar]
- Hampilos, K.; Merz, C.N.B.; Gulati, M.; Wei, J.; Cook-Wiens, G.; Dumitrascu, O. Relationships between retinal microvasculature and coronary microvascular function in women with ischemia and no obstructive coronary artery disease. Investig. Ophthalmol. Vis. Sci. 2024, 65, 307. [Google Scholar]
- Casagrande, M.; Forte, G.; Favieri, F.; Mingarelli, A.; Agostini, F.; Arcari, L.; Passaseo, I.; Semeraro, R.; Camastra, G.; Langher, V.; et al. Deciphering the Psychological Characteristics of Takotsubo Cardiomyopathy and Acute Myocardial Infarction. J. Pers. Med. 2025, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef]
- Dhindsa, D.S.; Sandesara, P.B.; Shapiro, M.D.; Wong, N.D. The Evolving Understanding and Approach to Residual Cardiovascular Risk Management. Front. Cardiovasc. Med. 2020, 7, 88. [Google Scholar] [CrossRef]
- Calderone, A.; Marafioti, G.; Latella, D.; Corallo, F.; D’Aleo, P.; Quartarone, A.; Calabrò, R.S. Effectiveness of relaxation techniques for stress management and quality of life improvement in cardiovascular disease and hypertensive patients: A systematic review. Psychol. Health Med. 2025, 30, 1281–1352. [Google Scholar] [CrossRef] [PubMed]
- Lortz, J.; Rassaf, T.; Jansen, C.; Knuschke, R.; Schweda, A.; Schnaubert, L.; Rammos, C.; Köberlein-Neu, J.; Skoda, E.-M.; Teufel, M.; et al. A mHealth intervention to reduce perceived stress in patients with ischemic heart disease: Study protocol of the randomized, controlled confirmatory intervention “mStress-IHD” trial. Trials 2023, 24, 592. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Xie, Q.; Xu, Z.; Yang, X.; Luo, Y.; Wan, L.; Yang, Y.; Wang, Y.; Ding, H. Psychological stress and influence factors in elderly patients with mild coronary heart disease: A longitudinal follow-up study in Shanghai, China. Front. Psychol. 2024, 15, 1399061. [Google Scholar] [CrossRef]
- Särnholm, J.; Gaffey, A.E.; Turchio, M.R.; Biviano, A.; Burg, M.M. Psychological Interventions for Reducing Distress in Patients with Cardiac Arrhythmias. Curr. Cardiol. Rep. 2025, 27, 102. [Google Scholar] [CrossRef] [PubMed]
- Moazzami, K.; Sullivan, S.; Wang, M.; Okoh, A.K.; Almuwaqqat, Z.; Pearce, B.; Shah, A.J.; Sun, Y.V.; Ko, Y.A.; Raggi, P.; et al. Cardiovascular Reactivity to Mental Stress and Adverse Cardiovascular Outcomes in Patients with Coronary Artery Disease. J. Am. Heart Assoc. 2025, 14, e034683. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.C.; Vaccarino, V.; Blumenthal, R.S. Psychological Distress in Cardiovascular Disease. JACC Adv. 2025, 4, 101537. [Google Scholar] [CrossRef]
- Makaroff, K.E.; Van, C.; Grospe, V.; Edmunds, L.; Calfon-Press, M.A.; Watson, K.E.; Horwich, T. Novel Virtual Reality Intervention for Stress Reduction Among Patients with or at Risk for Cardiovascular Disease: Mixed Methods Pilot Study. JMIR Cardio 2025, 9, e66557. [Google Scholar] [CrossRef]
- Brown, T.M.; Pack, Q.R.; Aberegg, E.; Brewer, L.C.; Ford, Y.R.; Forman, D.E.; Gathright, E.C.; Khadanga, S.; Ozemek, C.; Thomas, R.J. Core components of cardiac rehabilitation programs: 2024 update: A scientific statement from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2024, 150, e328–e347. [Google Scholar] [CrossRef]
- Kachur, S.; Menezes, A.R.; De Schutter, A.; Milani, R.V.; Lavie, C.J. Significance of comorbid psychological stress and depression on outcomes after cardiac rehabilitation. Am. J. Med. 2016, 129, 1316–1321. [Google Scholar] [CrossRef]
- Gaffey, A.E.; Gathright, E.C.; Fletcher, L.M.; Goldstein, C.M. Screening for psychological distress and risk of cardiovascular disease and related mortality: A systematized review, meta-analysis, and case for prevention. J. Cardiopulm. Rehabil. Prev. 2022, 42, 404–415. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Anbuselvam, B.; Gunasekaran, B.M.; Srinivasan, S.; Ezhilan, M.; Rajagopal, V.; Nesakumar, N. Wearable biosensors in cardiovascular disease. Clin. Chim. Acta 2024, 561, 119766. [Google Scholar] [CrossRef]
- Jena, N.; Singh, P.; Chandramohan, D.; Garapati, H.N.; Gummadi, J.; Mylavarapu, M.; Shaik, B.F.; Nanjundappa, A.; Apala, D.R.; Toquica, C.; et al. Wearable Technology in Cardiology: Advancements, Applications, and Future Prospects. Rev. Cardiovasc. Med. 2025, 26, 39025. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, Y. Self supervised learning based emotion recognition using physiological signals. Front. Hum. Neurosci. 2024, 18, 1334721. [Google Scholar] [CrossRef]
- Ometov, A.; Mezina, A.; Lin, H.C.; Arponen, O.; Burget, R.; Nurmi, J. Stress and Emotion Open Access Data: A Review on Datasets, Modalities, Methods, Challenges, and Future Research Perspectives. J. Heal. Inf. Res. 2025, 9, 247–279. [Google Scholar] [CrossRef]
- Suh, J.; Howe, E.; Lewis, R.; Hernandez, J.; Saha, K.; Althoff, T.; Czerwinski, M. Toward tailoring just-in-time adaptive intervention systems for workplace stress reduction: Exploratory analysis of intervention implementation. JMIR Ment. Health 2024, 11, e48974. [Google Scholar] [CrossRef]
- Nishio, R.; Dohi, T.; Yokoyama, M.; Nakade, T.; Takahashi, N.; Chikata, Y.; Endo, H.; Nishiyama, H.; Okai, I.; Iwata, H. Wearable Devices in Remote Cardiac Rehabilitation With and Without Weekly Online Coaching for Patients with Coronary Artery Disease: Randomized Controlled Trial. JMIR mHealth uHealth 2025, 13, e63797. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.M.; Blay-Tofey, M.; Haeffner, C.E.; Raymond, C.N.; Tandilashvili, E.; Terry, N.; Kiderman, M.; Metcalf, O.; Brotman, M.A.; Lopez-Guzman, S. Just-in-Time Adaptive Interventions to Promote Behavioral Health: Protocol for a Systematic Review. JMIR Res. Protoc. 2025, 14, e58917. [Google Scholar] [CrossRef] [PubMed]
- van Genugten, C.R.; Thong, M.S.Y.; van Ballegooijen, W.; Kleiboer, A.M.; Spruijt-Metz, D.; Smit, A.C.; Sprangers, M.A.G.; Terhorst, Y.; Riper, H. Beyond the current state of just-in-time adaptive interventions in mental health: A qualitative systematic review. Front. Digit. Health 2025, 7, 1460167. [Google Scholar] [CrossRef]
- Zainal, N.H.; Liu, X.; Leong, U.; Yan, X.; Chakraborty, B. Bridging Innovation and Equity: Advancing Public Health Through Just-in-Time Adaptive Interventions. Annu. Rev. Public Health 2025, 46, 43–68. [Google Scholar] [CrossRef]
- Abdullah; Anwar, M.; Mufti, A.; Jamal, M.; Khan, S.; Mazhar, T.; Bangash, S. IMPACT OF SOCIOECONOMIC STATUS ON PSYCHOLOGICAL HEALTH AND CARDIOVASCULAR DISEASE RISK: A NARRATIVE REVIEW. Insights-J. Life Soc. Sci. 2024, 2, 104–111. [Google Scholar] [CrossRef]
- Bazoukis, G.; Loscalzo, J.; Hall, J.L.; Bollepalli, S.C.; Singh, J.P.; Armoundas, A.A. Impact of Social Determinants of Health on Cardiovascular Disease. J. Am. Heart Assoc. 2025, 14, e039031. [Google Scholar] [CrossRef]
- Bowles, N.P.; He, Y.; Huang, Y.-h.; Stecker, E.C.; Seixas, A.; Thosar, S.S. Cardiovascular disease risk: It is complicated, but race and ethnicity are key, a Bayesian network analysis. Front. Public Health 2024, 12, 1364730. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, H.A.; Beydoun, M.A.; Kinney, R.L.; Liu, S.; Yu, R.; Allison, M.; Wallace, R.B.; Xiao, Q.; Liu, L.; Gradidge, P.; et al. Pathways from Socioeconomic Factors to Major Cardiovascular Events Among Postmenopausal Veteran and Nonveteran Women: Findings from the Women’s Health Initiative. J. Am. Heart Assoc. 2024, 13, e037253. [Google Scholar] [CrossRef]
- Volpert-Esmond, H.I.; Bray, J.R.; Pages, S.M.; Danyluck, C. Cardiovascular reactivity during conversations about discrimination is buffered by social support among U.S. Latines. Sci. Rep. 2024, 14, 26964. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Nacer, H.; Lawson, C.A.; Khunti, K. Racial and Ethnic Disparities in Primary Prevention of Cardiovascular Disease. Can. J. Cardiol. 2024, 40, 1016–1030. [Google Scholar] [CrossRef]
- Scott, J.; Money, V.; Ellis, C.; Hughes-Halbert, C.; Birkett, M.A.; Magwood, G. Characterizing the influence of racism-related stress and pandemic-related changes in social connections on cardiovascular health: Study protocol and theoretical framework. PLoS ONE 2025, 20, e0324839. [Google Scholar] [CrossRef]
- Takeuchi, H.; Ishizawa, T.; Kishi, A.; Nakamura, T.; Yoshiuchi, K.; Yamamoto, Y. Just-in-Time Adaptive Intervention for Stabilizing Sleep Hours of Japanese Workers: Microrandomized Trial. J. Med. Internet Res. 2024, 26, e49669. [Google Scholar] [CrossRef] [PubMed]
- Hines, A.L.; Albert, M.A.; Blair, J.P.; Crews, D.C.; Cooper, L.A.; Long, D.L.; Carson, A.P. Neighborhood Factors, Individual Stressors, and Cardiovascular Health Among Black and White Adults in the US: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Netw. Open 2023, 6, e2336207. [Google Scholar] [CrossRef]
- Borkowski, P.; Borkowska, N.; Mangeshkar, S.; Adal, B.H.; Singh, N. Racial and Socioeconomic Determinants of Cardiovascular Health: A Comprehensive Review. Cureus 2024, 16, e59497. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, M.; Lal, L.S.; Abughosh, S.M.; Sansgiry, S.; Essien, E.J.; Sansgiry, S.S. Racial and ethnic disparities in perceived health status among patients with cardiovascular disease. Prev. Chronic Dis. 2024, 21, E89. [Google Scholar] [CrossRef]
- Moody, D.L.B.; Pantesco, E.J.; Novruz, A.; Tchangalova, N.; Sadler, R.C.; White Whilby, K.; Ashe, J.; Gee, G.C.; Hill, L.K.; Waldstein, S.R. Multilevel Racism and Discrimination and Cardiovascular Disease and Related Biopsychosocial Mechanisms: An Integrated Scoping and Literature Review and Future Research Agenda. Curr. Cardiol. Rep. 2025, 27, 91. [Google Scholar] [CrossRef]
- Machado, A.V.; Barreto, S.M.; Padilha dos Reis, R.C.; Giatti, L.; Alvim Matos, S.M.; Patrão, A.L.; Needham, B.L.; Camelo, L.V. Racial inequities in the incidence of major adverse cardiovascular events in ELSA-Brasil cohort: The mediating role of weathering. Soc. Sci. Med. 2025, 383, 118421. [Google Scholar] [CrossRef]
- Bidwell, J.T.; Fauer, A.J.; Howe, R.J.; Saylor, M.A.; Lee, C.S.; López, J.E.; Godden, M.; Hinton, L. Older Adults with Cardiovascular Disease and Their Care Partners: An Analysis of Care Needs, Care Activities, and Care Partner Stress and Mental Health. J. Cardiovasc. Nurs. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wagle, S.; Yang, S.; Osei, E.A.; Katare, B.; Lalani, N. Caregiving intensity, duration, and subjective financial well-being among rural informal caregivers of older adults with chronic illnesses or disabilities. Healthcare 2024, 12, 2260. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T. Women and ethnic minorities less likely to be treated after diagnosis of deadly heart disease in England, study finds; Research shows disparity in care after detection of aortic stenosis, also affecting those living in deprived areas. In The Guardian; Guardian News & Media: London, UK, 2025. [Google Scholar]
| Risk Factor | Chronic Effect Mechanism | Acute Trigger Mechanism | Estimated Relative Risk (RR) [1] | Population Attributable Risk (PAR) [2] | References |
|---|---|---|---|---|---|
| Hypertension | Sustained high arterial pressure → endothelial injury, left ventricular hypertrophy | Acute surges in BP raise shear stress and plaque rupture risk | ~1.8 per 20 mm Hg increase | ~20–25% in many populations | [70] |
| Hyperlipidemia | ApoB-containing lipoproteins drive plaque formation | Extremely high levels may precipitate plaque rupture | ~1.6 per mmol/L LDL-C increase | ~17–20% globally | [31] |
| Smoking | Chronic inflammation, oxidative stress, endothelial damage | Acute platelet activation, vasoconstriction | ~2–3× vs. non-smokers | ~15–25% in high-prevalence groups | [65] |
| Diabetes | Accelerated atherosclerosis via glycation, inflammation | Acute hyperglycemia impairs vascular function | ~2× risk of MI | ~10–15% in most populations | [71] |
| Emotional Stress | Chronic sympathetic activation, allostatic load, inflammation | Acute anger or grief triggers MSIMI and plaque rupture | RR ~2.0 for acute MI trigger | Estimated ~5–10% of acute MIs | [9,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eroume A Egom, E.; Lema, B.S. Integrating Emotional Stress and Lipid Lowering in Cardiovascular Disease Management: The Future of Precision Cardiovascular Prevention. J. Clin. Med. 2025, 14, 7208. https://doi.org/10.3390/jcm14207208
Eroume A Egom E, Lema BS. Integrating Emotional Stress and Lipid Lowering in Cardiovascular Disease Management: The Future of Precision Cardiovascular Prevention. Journal of Clinical Medicine. 2025; 14(20):7208. https://doi.org/10.3390/jcm14207208
Chicago/Turabian StyleEroume A Egom, Emmanuel, and Bernadette Sandrine Lema. 2025. "Integrating Emotional Stress and Lipid Lowering in Cardiovascular Disease Management: The Future of Precision Cardiovascular Prevention" Journal of Clinical Medicine 14, no. 20: 7208. https://doi.org/10.3390/jcm14207208
APA StyleEroume A Egom, E., & Lema, B. S. (2025). Integrating Emotional Stress and Lipid Lowering in Cardiovascular Disease Management: The Future of Precision Cardiovascular Prevention. Journal of Clinical Medicine, 14(20), 7208. https://doi.org/10.3390/jcm14207208





