Changes in Gut Microbiome According to Probiotic Intake in Rectal Cancer Patients Undergoing Diverting Stoma Repair: Study Protocol
Abstract
1. Background
2. Methods
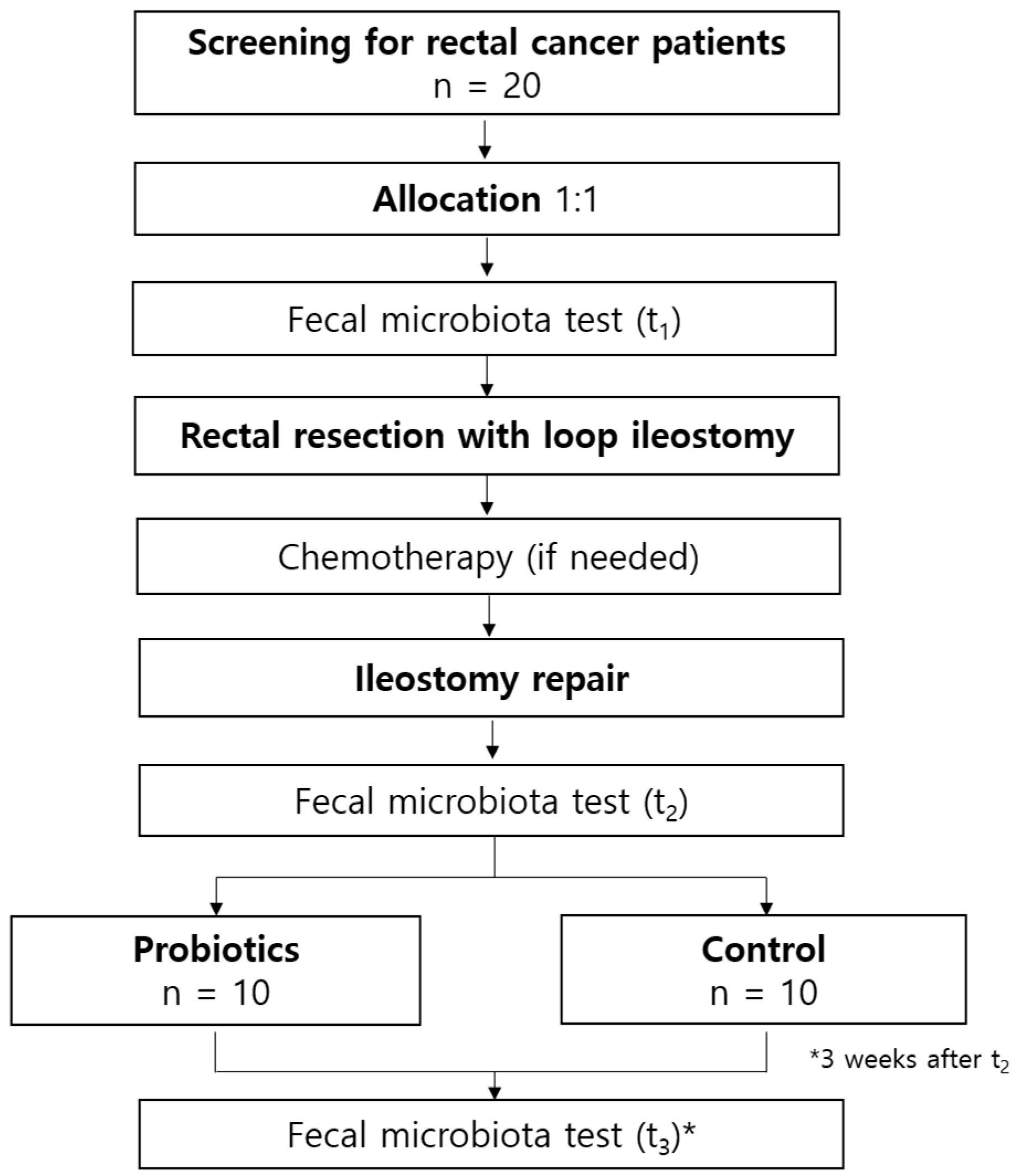
2.1. Trial Design
2.2. Eligibility
2.3. Participants
2.4. Interventions
2.5. Outcomes
2.6. Blinding
2.7. Data Collection and Management
2.8. Statistical Methods
2.9. Oversight and Monitoring
2.10. Dissemination Plans
3. Discussion
4. Trial Status
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRF | Case Report Form |
| TSC | Trial Steering Committee |
References
- Cresci, G.A.; Bawden, E. Gut Microbiome: What We Do and Don’t Know. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Marusch, F.; Koch, A.; Schmidt, U.; Geibetaler, S.; Dralle, H.; Saeger, H.D.; Wolff, S.; Nestler, G.; Pross, M.; Gastinger, I.; et al. Value of a protective stoma in low anterior resections for rectal cancer. Dis. Colon Rectum 2002, 45, 1164–1171. [Google Scholar] [CrossRef]
- Williams, L.; Armstrong, M.J.; Finan, P.; Sagar, P.; Burke, D. The effect of faecal diversion on human ileum. Gut 2007, 56, 796–801. [Google Scholar] [CrossRef]
- Dal Buono, A.; Carvello, M.; Sachar, D.B.; Spinelli, A.; Danese, S.; Roda, G. Diversion proctocolitis and the problem of the forgotten rectum in inflammatory bowel diseases: A systematic review. United Eur. Gastroenterol. J. 2021, 9, 1157–1167. [Google Scholar] [CrossRef]
- Kabir, S.I.; Kabir, S.A.; Richards, R.; Ahmed, J.; MacFie, J. Pathophysiology, clinical presentation and management of diversion colitis: A review of current literature. Int. J. Surg. 2014, 12, 1088–1092. [Google Scholar] [CrossRef]
- Tominaga, K.; Kamimura, K.; Takahashi, K.; Yokoyama, J.; Yamagiwa, S.; Terai, S. Diversion colitis and pouchitis: A mini-review. World J. Gastroenterol. 2018, 24, 1734–1747. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, H.M.; Kim, C.H.; Kim, H.R. Dysbiosis of gut microbiota during fecal stream diversion in patients with colorectal cancer. Gut Pathog. 2023, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Taminiau, B.; Rodriguez, C.; Daube, G. Gut Microbiota Composition Associated with Clostridioides difficile Colonization and Infection. Pathogens 2022, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Rombey, T.; Panagiotopoulou, I.G.; Hind, D.; Fearnhead, N.S. Preoperative bowel stimulation prior to ileostomy closure to restore bowel function more quickly and improve postoperative outcomes: A systematic review. Color. Dis. 2019, 21, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Chin, J. Modulating immune responses with probiotic bacteria. Immunol. Cell Biol. 2000, 78, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A review on prebiotics and probiotics for the control of dysbiosis: Present status and future perspectives. Animal 2015, 9, 43–48. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; McDonald, D.; Knight, R. Role of the microbiome, probiotics, and ‘dysbiosis therapy’ in critical illness. Curr. Opin. Crit. Care 2016, 22, 347–353. [Google Scholar] [CrossRef]
- Aschard, H.; Laville, V.; Tchetgen, E.T.; Knights, D.; Imhann, F.; Seksik, P.; Zaitlen, N.; Silverberg, M.S.; Cosnes, J.; Weersma, R.K.; et al. Genetic effects on the commensal microbiota in inflammatory bowel disease patients. PLoS Genet. 2019, 15, e1008018. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, J.H.; Kye, B.H.; Oh, H.K.; Cho, Y.B.; Kim, Y.T.; Kim, J.Y.; Sung, N.Y.; Kang, S.B.; Seo, J.M.; et al. Effects of PrObiotics on the Symptoms and Surgical ouTComes after Anterior REsection of Colon Cancer (POSTCARE): A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 2181. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Xu, Q.; Yu, Y.; Yan, H.; Shao, W.; Zhang, F. Exploring the clinical value of probiotics in the perioperative management of colorectal cancer: Meta-analysis with trial sequential analysis. Oncol. Lett. 2025, 30, 515. [Google Scholar] [CrossRef]
- Acevedo-Román, A.; Pagán-Zayas, N.; Velázquez-Rivera, L.I.; Torres-Ventura, A.C.; Godoy-Vitorino, F. Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. Int. J. Mol. Sci. 2024, 25, 9715. [Google Scholar] [CrossRef]
- Sam, S.W.; Hafeez, B.; Ong, H.I.; Gill, S.; Smibert, O.; Lavelle, A.; Burgess, A.; Proud, D.; Mohan, H. The impact of faecal diversion on the gut microbiome: A systematic review. Gut Microbiome 2024, 5, e4. [Google Scholar] [CrossRef]
- Stephens, J.H.; Hewett, P.J. Clinical trial assessing VSL#3 for the treatment of anterior resection syndrome. ANZ J. Surg. 2012, 82, 420–427. [Google Scholar] [CrossRef]
- Yoon, B.J.; Oh, H.K.; Lee, J.; Cho, J.R.; Kim, M.J.; Kim, D.W.; Kang, S.B. Effects of probiotics on bowel function restoration following ileostomy closure in rectal cancer patients: A randomized controlled trial. Color. Dis. 2021, 23, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yeom, S.S.; Kim, C.H.; Kim, H.R. Effect of preoperative immunonutrition on outcomes of colon cancer surgery: Study protocol for a randomized controlled trial. Trials 2020, 21, 628. [Google Scholar] [CrossRef]
- Wlodarska, M.; Kostic, A.D.; Xavier, R.J. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe 2015, 17, 577–591. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef]
- Gérard, P. Gut microbiota and obesity. Cell Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The gut microbiome in human neurological disease: A review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Seekatz, A.M.; Young, V.B. Clostridium difficile and the microbiota. J. Clin. Investig. 2014, 124, 4182–4189. [Google Scholar] [CrossRef]
- Kwon, H.; Chae, S.H.; Jung, H.J.; Shin, H.M.; Ban, O.H.; Yang, J.; Kim, J.H.; Jeong, J.E.; Jeon, H.M.; Kang, Y.W.; et al. The effect of probiotics supplementation in postoperative cancer patients: A prospective pilot study. Ann. Surg. Treat. Res. 2021, 101, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Alander, M.; Korpela, R.; Saxelin, M.; Vilpponen-Salmela, T.; Mattila-Sandholm, T.; von Wright, A. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Lett. Appl. Microbiol. 1997, 24, 361–364. [Google Scholar] [CrossRef]
- Tannock, G.W.; Munro, K.; Harmsen, H.J.; Welling, G.W.; Smart, J.; Gopal, P.K. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 2000, 66, 2578–2588. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Nutrition and Health through the Use of Probiotic Strains in Fermentation to Produce Non-Dairy Functional Beverage Products Supporting Gut Microbiota. Foods 2022, 11, 2760. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Antibiotic-Therapy-Induced Gut Dysbiosis Affecting Gut Microbiota-Brain Axis and Cognition: Restoration by Intake of Probiotics and Synbiotics. Int. J. Mol. Sci. 2023, 24, 3074. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Padilla, Á.; Morales-Martín, G.; Pérez-Quintero, R.; Rada-Morgades, R.; Gómez-Salgado, J.; Ruiz-Frutos, C. Diversion Colitis and Probiotic Stimulation: Effects of Bowel Stimulation Prior to Ileostomy Closure. Front. Med. 2021, 8, 654573. [Google Scholar] [CrossRef] [PubMed]
- Mulita, F.; Tepetes, K.; Verras, G.I.; Liolis, E.; Tchabashvili, L.; Kaplanis, C.; Perdikaris, I.; Velissaris, D.; Maroulis, I. Perineal colostomy: Advantages and disadvantages. Prz. Gastroenterol. 2022, 17, 89–95. [Google Scholar] [CrossRef]
- Verras, G.I.; Mulita, F. Butyrylcholinesterase levels correlate with surgical site infection risk and severity after colorectal surgery: A prospective single-center study. Front. Surg. 2024, 11, 1379410. [Google Scholar] [CrossRef]
| Study Period | ||||
|---|---|---|---|---|
| Enrolment | Post-Allocation | Close-Out | ||
| Time Point | t1 | t2 (Before Rectal Resection) | t3 (After Ileostomy Repair) | t4 (Outpatient Visit) |
| Enrolment: | ||||
| Eligibility screen | X | |||
| Informed consent | X | |||
| Allocation | X | |||
| Interventions: | ||||
| Probiotic intake |  | |||
| Standard care |  | |||
| Assessments: | ||||
| Fecal testing | X | X | X | |
| Serum laboratory test * | X | X | X | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-m.; Lee, J.; Lee, S.Y.; Kim, C.H.; Kim, H.R. Changes in Gut Microbiome According to Probiotic Intake in Rectal Cancer Patients Undergoing Diverting Stoma Repair: Study Protocol. J. Clin. Med. 2025, 14, 7190. https://doi.org/10.3390/jcm14207190
Park H-m, Lee J, Lee SY, Kim CH, Kim HR. Changes in Gut Microbiome According to Probiotic Intake in Rectal Cancer Patients Undergoing Diverting Stoma Repair: Study Protocol. Journal of Clinical Medicine. 2025; 14(20):7190. https://doi.org/10.3390/jcm14207190
Chicago/Turabian StylePark, Hyeung-min, Jaram Lee, Soo Young Lee, Chang Hyun Kim, and Hyeong Rok Kim. 2025. "Changes in Gut Microbiome According to Probiotic Intake in Rectal Cancer Patients Undergoing Diverting Stoma Repair: Study Protocol" Journal of Clinical Medicine 14, no. 20: 7190. https://doi.org/10.3390/jcm14207190
APA StylePark, H.-m., Lee, J., Lee, S. Y., Kim, C. H., & Kim, H. R. (2025). Changes in Gut Microbiome According to Probiotic Intake in Rectal Cancer Patients Undergoing Diverting Stoma Repair: Study Protocol. Journal of Clinical Medicine, 14(20), 7190. https://doi.org/10.3390/jcm14207190





