Tandem Detethering: A Novel One-Stage Approach Combining Cervicothoracic Cord Release Followed by Filum Terminale Sectioning
Abstract
1. Introduction
2. Materials and Methods
3. Results
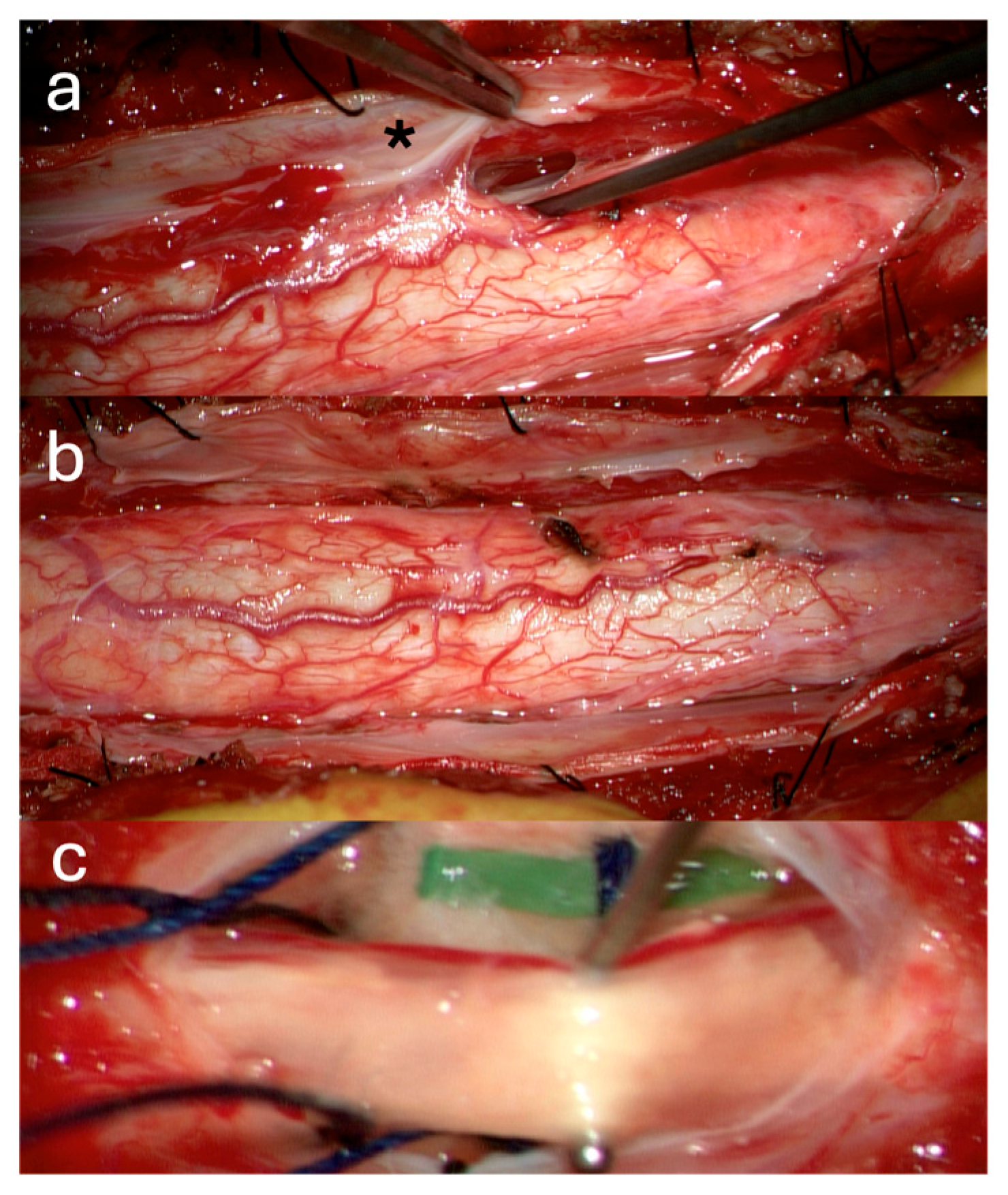
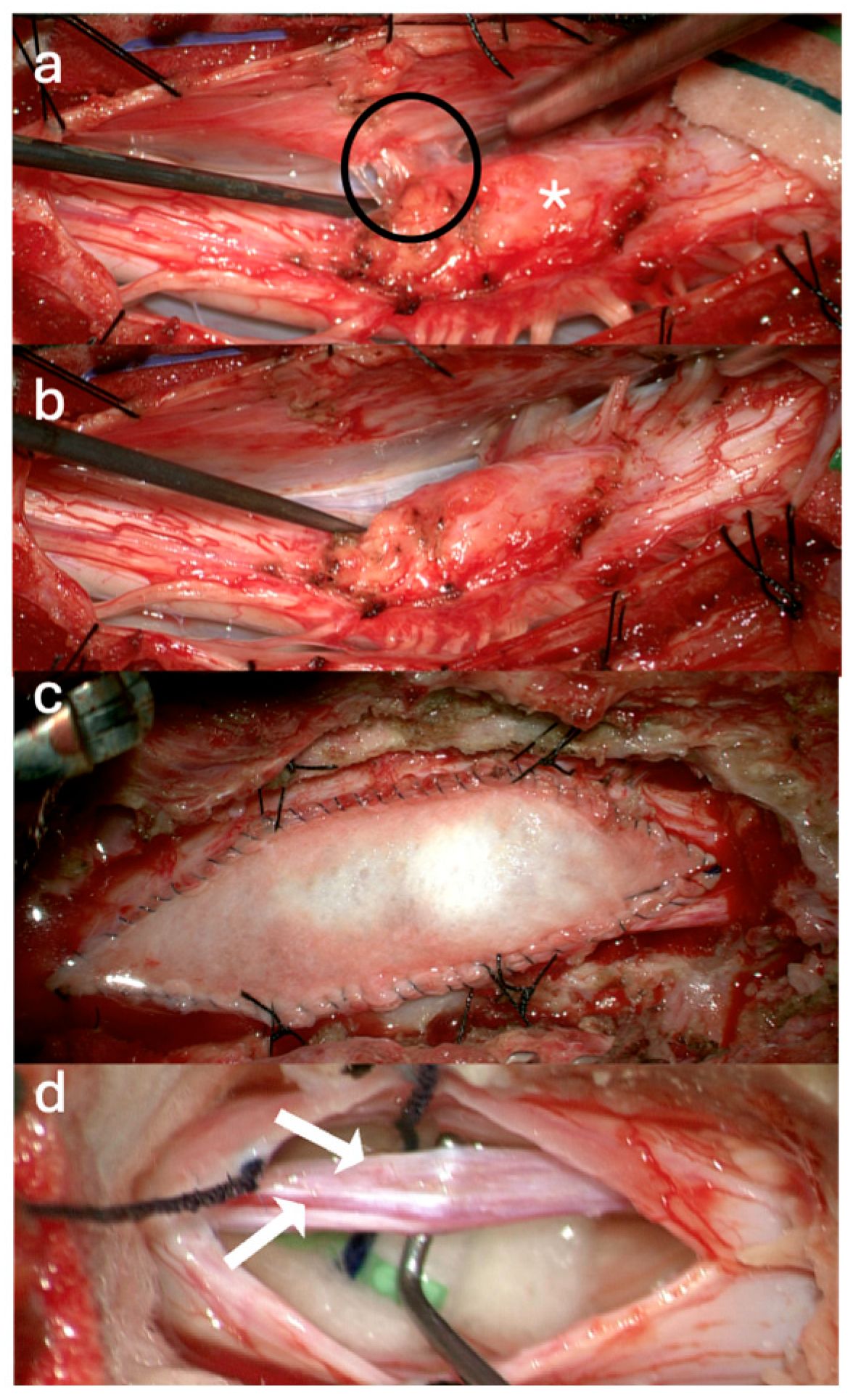
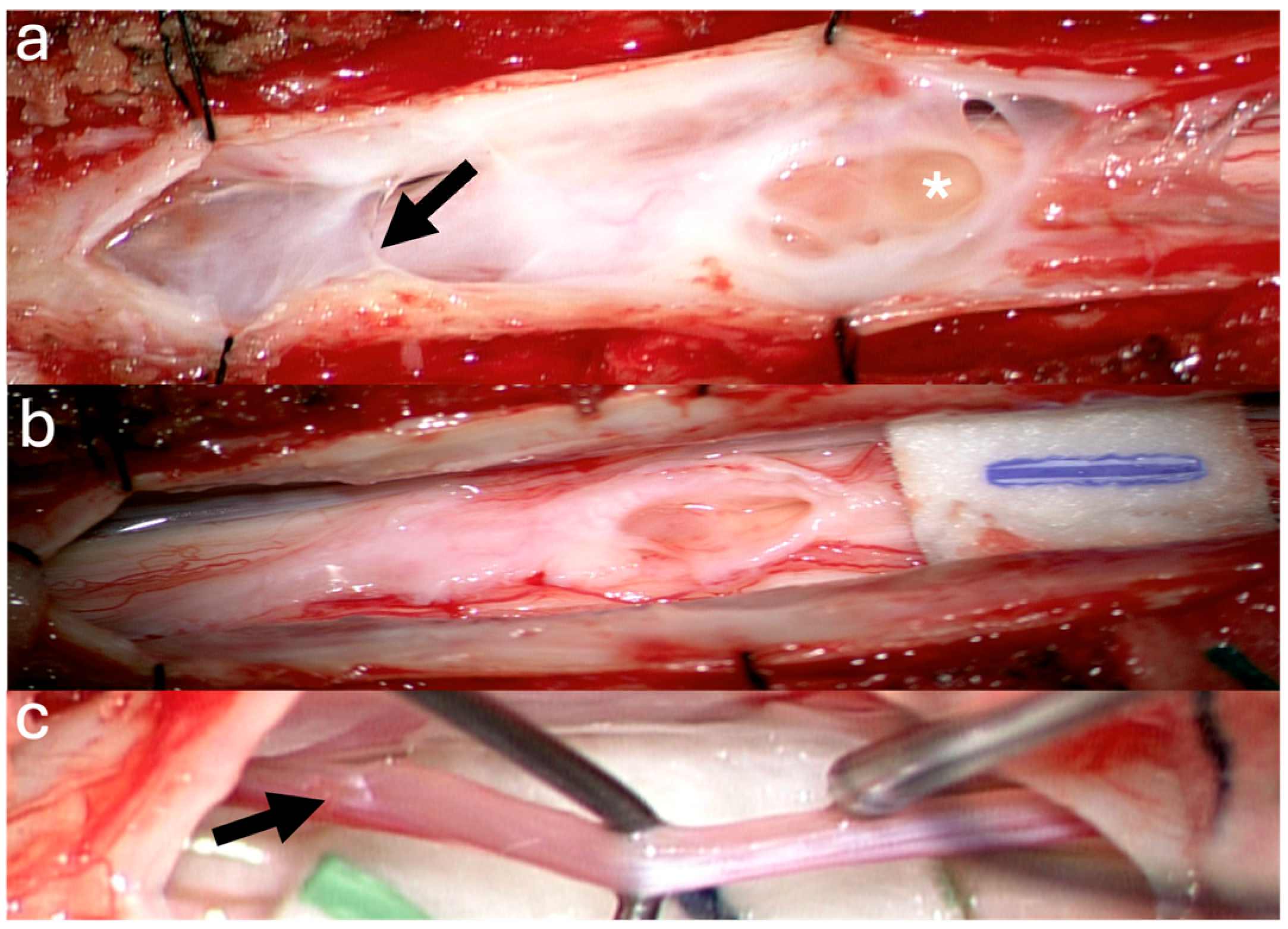
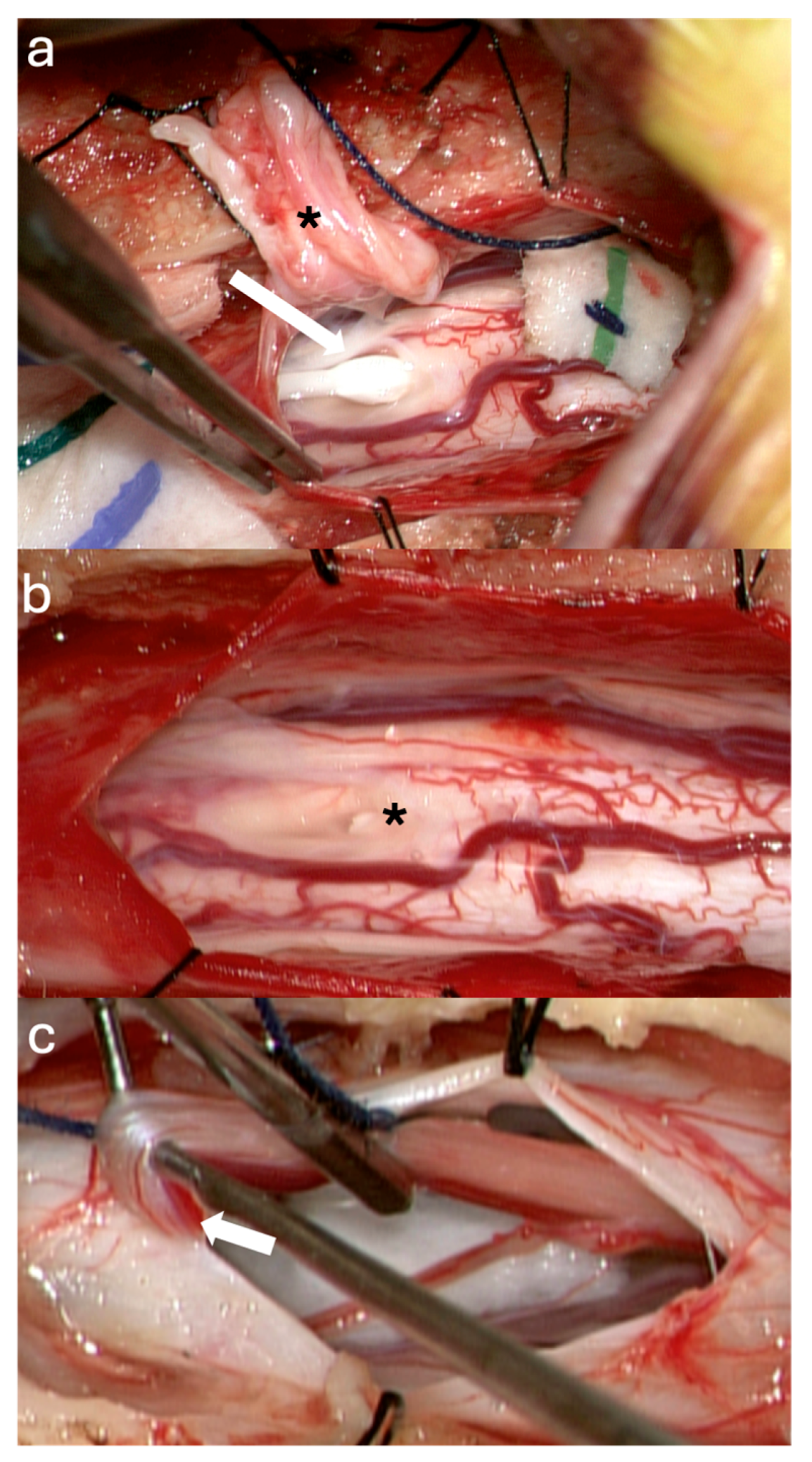
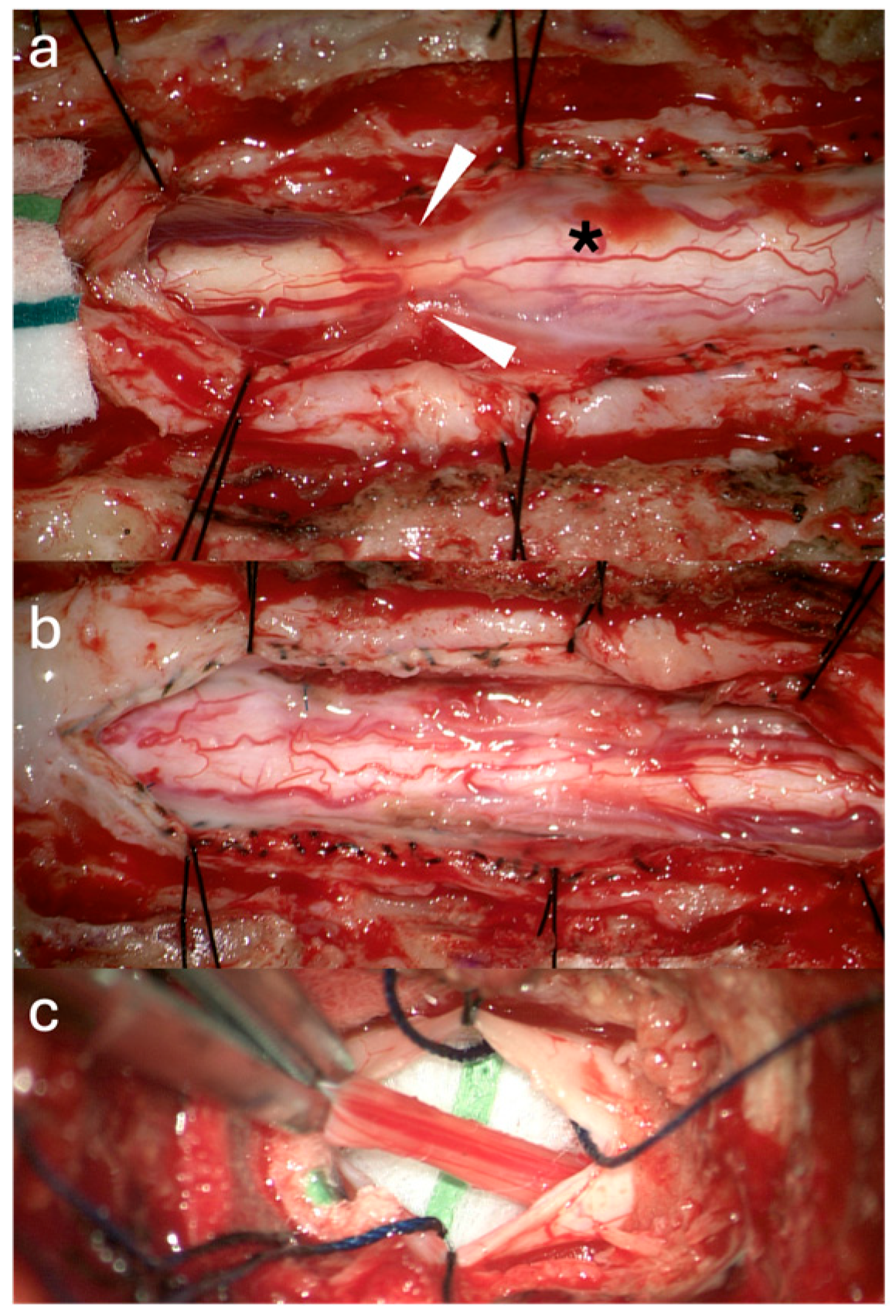
3.1. Surgical Technique
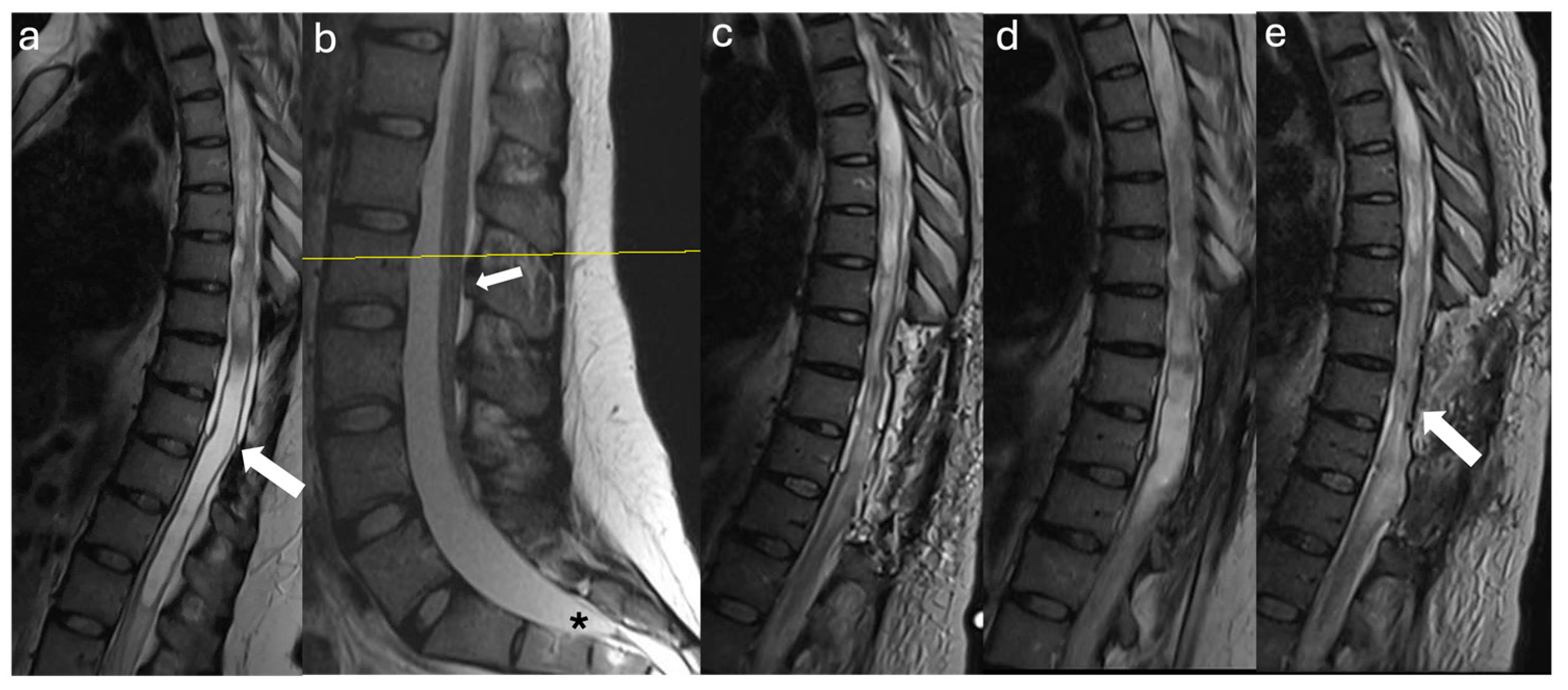
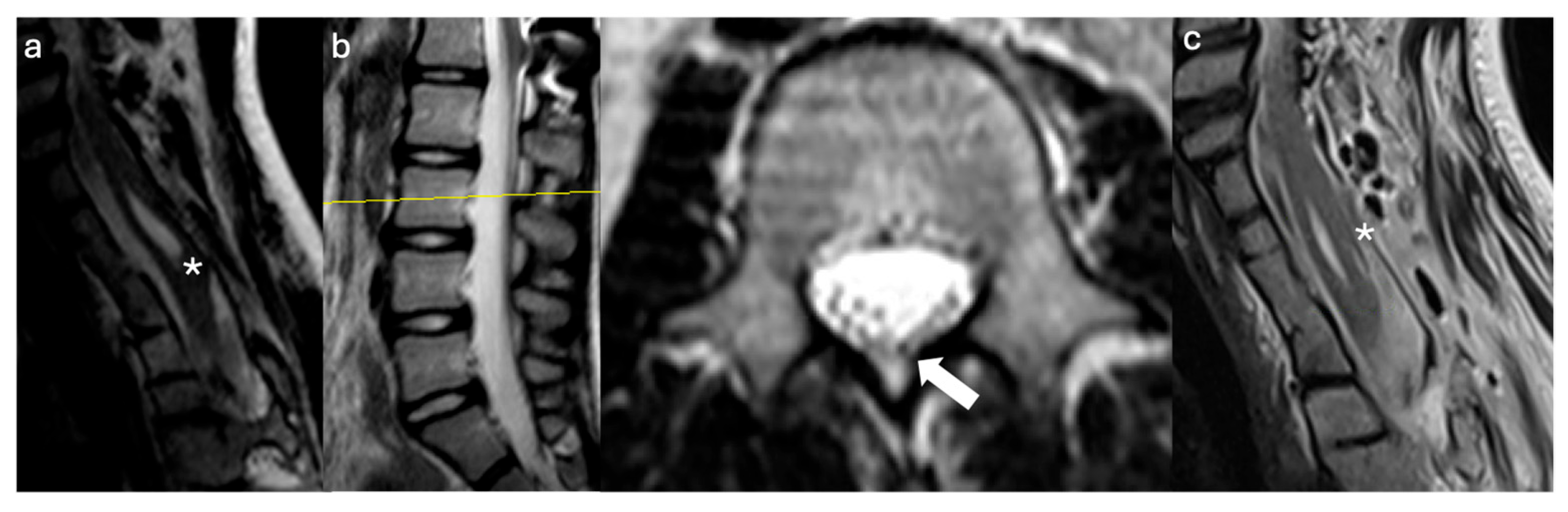
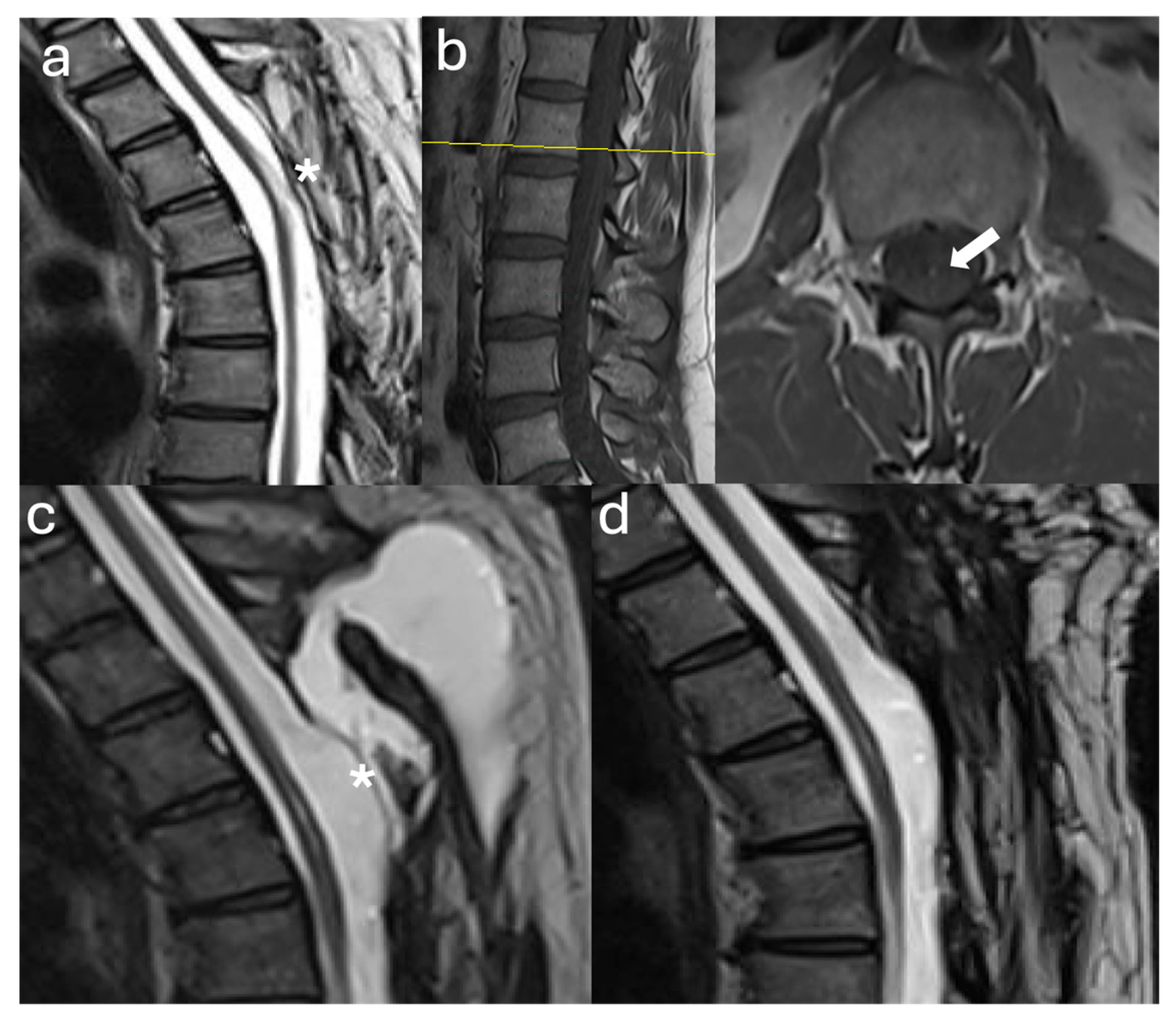
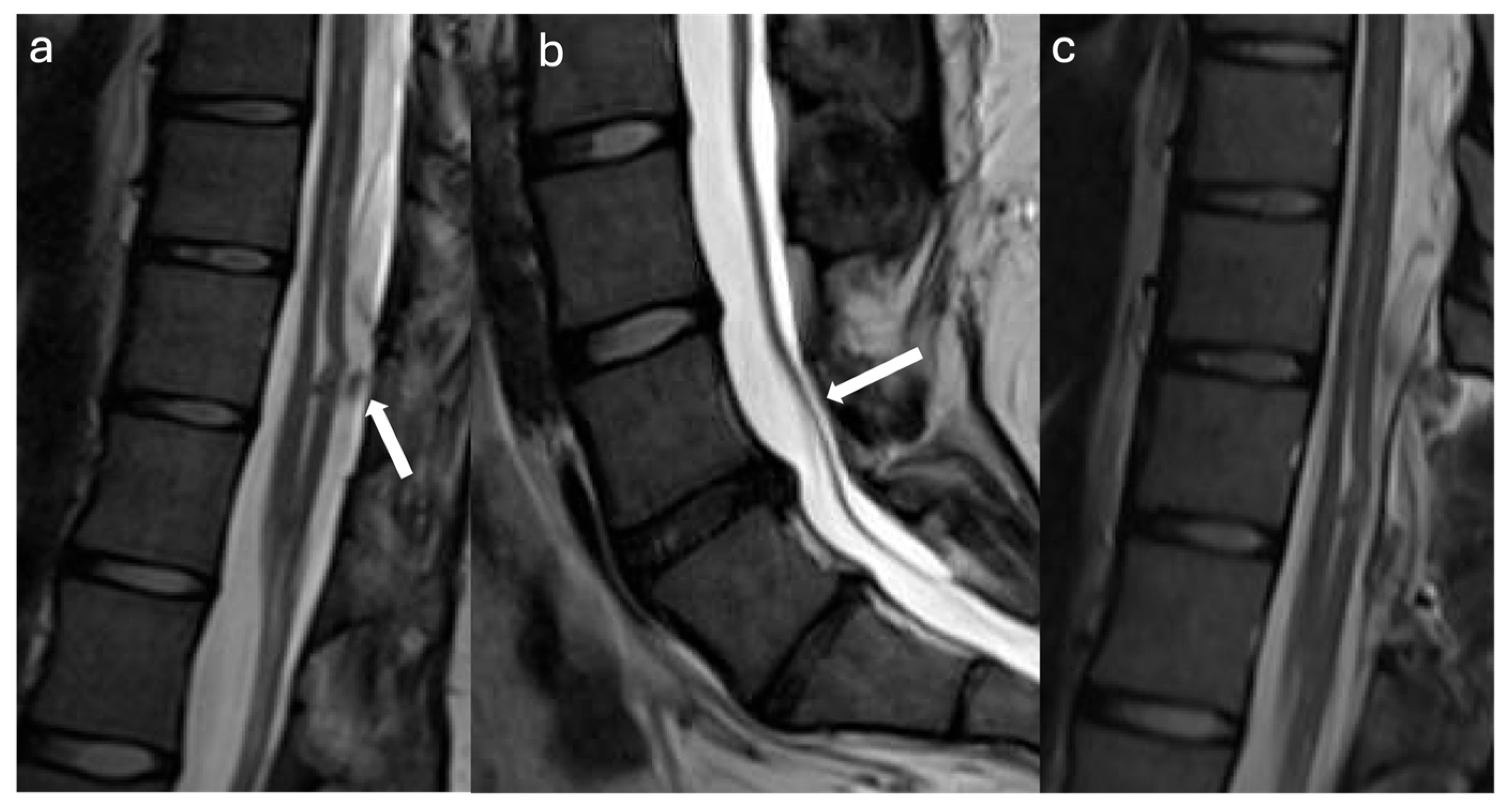
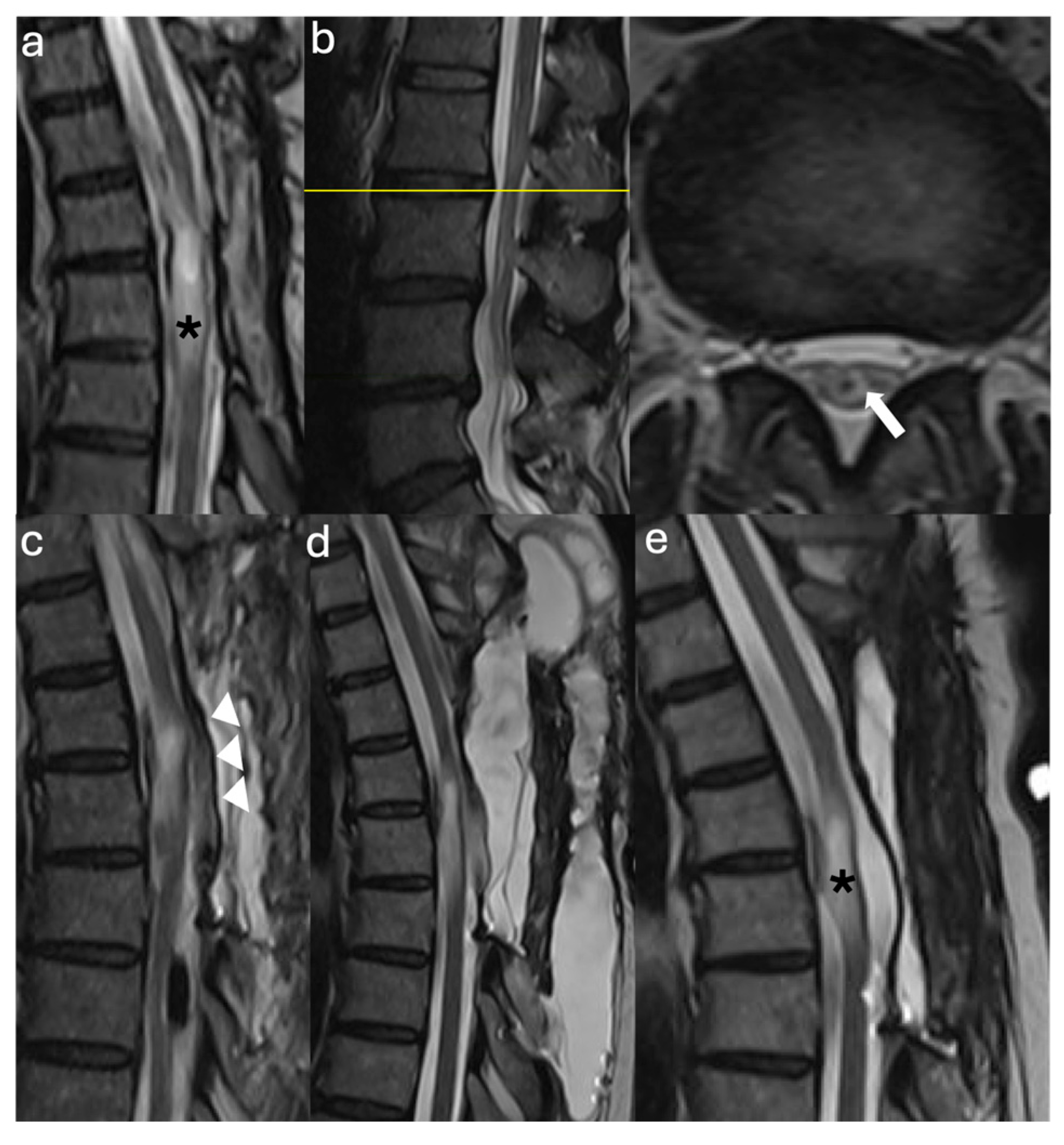
3.2. Case Illustrations
3.2.1. Case #1
3.2.2. Case #2
3.2.3. Case #3
3.2.4. Case #4
3.2.5. Case #5
4. Discussion
4.1. Pathophysiology and Histopathological Evidence Supporting Filum Terminale Resection
4.2. Relationship to Split Cord Malformation and Surgical Approaches
4.3. Surgical Risks and Complications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FT | Filum terminale |
| TCS | Tethered Cord Syndrome |
| MRI | Magnetic Resonance Imaging |
| CSF | Cerebrospinal Fluid |
| SCM | Split Cord Malformation |
References
- Yamada, S.; Won, D.J.; Yamada, S.M. Pathophysiology of tethered cord syndrome: Correlation with symptomatology. Neurosurg. Focus 2004, 16, 1–5. [Google Scholar] [CrossRef]
- Filippidis, A.S.; Kalani, M.Y.; Theodore, N.; Rekate, H.L. Spinal cord traction, vascular compromise, hypoxia, and metabolic derangements in the pathophysiology of tethered cord syndrome. Neurosurg. Focus 2010, 29, E9. [Google Scholar] [CrossRef]
- Hertzler, D.A., 2nd; DePowell, J.J.; Stevenson, C.B.; Mangano, F.T. Tethered cord syndrome: A review of the literature from embryology to adult presentation. Neurosurg. Focus 2010, 29, E1. [Google Scholar] [CrossRef]
- Patel, K.V.; Amtmann, D.; Jensen, M.P.; Smith, S.M.; Veasley, C.; Turk, D.C. Clinical outcome assessment in clinical trials of chronic pain treatments. Pain Rep. 2021, 6, e784. [Google Scholar] [CrossRef] [PubMed]
- Klinge, P.M.; Leary, O.P.; Allen, P.A.; Svokos, K.; Sullivan, P.; Brinker, T.; Gokaslan, Z.L. Clinical criteria for filum terminale resection in occult tethered cord syndrome. J. Neurosurg. Spine 2024, 40, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazeq, H.; Shao, B.; Sastry, R.A.; Klinge, P.M. Microsurgical approach for resection of the filum terminale internum in tethered cord syndrome: A case demonstration of technical nuances and vignettes. Acta Neurochir. 2023, 165, 3505–3509. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.P.; Smitherman, A.D.; Milton, C.K.; Palejwala, A.H.; Lu, V.M.; Johnston, S.E.; Homburg, H.; Zhao, D.; Martin, M.D. Surgical treatment of tethered cord syndrome in adults: A systematic review and meta-analysis. World Neurosurg. 2020, 137, e221–e241. [Google Scholar] [CrossRef] [PubMed]
- Selden, N.R. Occult tethered cord syndrome: The case for surgery. J. Neurosurg. 2006, 104, 302–304. [Google Scholar] [CrossRef]
- Michael, M.M.; Garton, A.L.A.; Kuzan-Fischer, C.M.; Uribe-Cardenas, R.; Greenfield, J.P. A critical analysis of surgery for occult tethered cord syndrome. Childs Nerv. Syst. 2021, 37, 3003–3011. [Google Scholar] [CrossRef]
- Abdulrazeq, H.; Leary, O.P.; Tang, O.Y.; Karimi, H.; McElroy, A.; Gokaslan, Z.; Punsoni, M.; Donahue, J.E.; Klinge, P.M. The surgical histopathology of the filum terminale: Findings from a large series of patients with tethered cord syndrome. J. Clin. Med. 2024, 13, 6. [Google Scholar] [CrossRef]
- Selçuki, M.; Vatansever, S.; Inan, S.; Erdemli, E.; Bağdatoğlu, C.; Polat, A. Is a filum terminale with a normal appearance really normal? Childs Nerv. Syst. 2003, 19, 3–10. [Google Scholar] [CrossRef]
- Klinge, P.M.; Srivastava, V.; McElroy, A.; Leary, O.P.; Ahmed, Z.; Donahue, J.E.; Brinker, T.; De Vloo, P.; Gokaslan, Z.L. Diseased filum terminale as a cause of tethered cord syndrome in Ehlers–Danlos syndrome: Histopathology, biomechanics, clinical presentation, and outcome of filum excision. World Neurosurg. 2022, 162, e492–e502. [Google Scholar] [CrossRef] [PubMed]
- Djindjian, M.; Ribeiro, A.; Ortega, E.; Gaston, A.; Poirier, J. The normal vascularization of the intradural filum terminale in man. Surg. Radiol. Anat. 1988, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Uhthoff, H.K.; Loehr, J.W. Calcific tendinopathy of the rotator cuff: Pathogenesis, diagnosis, and management. J. Am. Acad. Orthop. Surg. 1997, 5, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Sung, J.H.; Hong, J.T.; Lee, S.W. Split cord malformation combined with tethered cord syndrome in an adult. J. Korean Neurosurg. Soc. 2013, 54, 363–366. [Google Scholar] [CrossRef]
- Akay, K.M.; Izci, Y.; Baysefer, A.; Timurkaynak, E. Split cord malformation in adults. Neurosurg. Rev. 2004, 27, 99–105. [Google Scholar] [CrossRef]
- Quinones-Hinojosa, A.; Gadkary, C.A.; Mummaneni, P.V.; Rosenberg, W.S. Split spinal cord malformation in an elderly patient: Case report. Surg. Neurol. 2004, 61, 201–203. [Google Scholar] [CrossRef]
- Elmacı, İ.; Dağçinar, A.; Özgen, S.; Ekinci, G.; Pamir, M.N. Diastematomyelia and spinal teratoma in an adult: Case report. Neurosurg. Focus 2001, 10, 1–4. [Google Scholar] [CrossRef]
- Oh, T.; Avalos, L.N.; Burke, J.F.; Mummaneni, N.; Safaee, M.; Gupta, N.; Clark, A.J. A type II split cord malformation in an adult patient: An operative case report. Oper. Neurosurg. 2021, 20, E148–E151. [Google Scholar] [CrossRef]
- D’Agostino, E.N.; Calnan, D.R.; Makler, V.I.; Khan, I.; Kanter, J.H.; Bauer, D.F. Type I split cord malformation and tethered cord syndrome in an adult patient: A case report and literature review. Surg. Neurol. Int. 2019, 10, 90. [Google Scholar] [CrossRef]
- Ahmed, K.; Howell, E.P.; Harward, S.; Sankey, E.W.; Ehresman, J.; Schilling, A.; Wang, T.; Pennington, Z.; Gray, L.; Sciubba, D.M.; et al. Split cord malformation in adults: Literature review and classification. Clin. Neurol. Neurosurg. 2020, 193, 105733. [Google Scholar] [CrossRef]
- Kobets, A.J.; Oliver, J.; Cohen, A.; Jallo, G.I.; Groves, M.L. Split cord malformation and tethered cord syndrome: Case series with long-term follow-up and literature review. Childs Nerv. Syst. 2021, 37, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Nasto, L.A.; Andaloro, A.; Piatelli, G. Double spinal cord tethering and congenital kyphosis in a 4-year-old boy. Spine Deform. 2023, 11, 501–506. [Google Scholar] [CrossRef]
- Alelyani, F.; Aronyk, K.; Alghamdi, H.; Alnaami, I. Split notochord syndrome with spinal column duplication and spinal cord lipoma: A case report. Children 2022, 9, 1138. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoon, S.H.; Cho, K.H.; Won, G.S. Tethered spinal cord with double spinal lipomas. J. Korean Med. Sci. 2006, 21, 1133–1136. [Google Scholar] [CrossRef]
- Ramdurg, S. Noncontiguous double spinal lipoma with tethered cord and polydactyly: Two different embryological events in one patient. J. Pediatr. Neurosci. 2017, 12, 43–46. [Google Scholar] [CrossRef]
- Belfatmi, N.; Motawei, A.S.; Rezkallah, A.; Benarous, N.D.; Ahmed, A.H.; Ghozy, S.; Zaazoue, M.A.; Si Saber, M. Double lumbar localization of myelomeningocele: Case report. Pediatr. Neurosurg. 2023, 58, 97–104. [Google Scholar] [CrossRef]
- Xu, F.; Wang, X.; Li, L.; Guan, J.; Jian, F. Tethered cord syndrome caused by duplicated filum terminale in an adult with split cord malformation. World Neurosurg. 2020, 143, 7–10. [Google Scholar] [CrossRef]
- Barutcuoglu, M.; Selcuki, M.; Selcuki, D.; Umur, S.; Mete, M.; Gurgen, S.G.; Umur, Ö. Cutting filum terminale is very important in split cord malformation cases to achieve total release. Childs Nerv. Syst. 2015, 31, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Tahir, Z.; Craven, C. Gastrulation and split cord malformation. In Spinal Dysraphic Malformations; Pang, D., Wang, K.C., Eds.; Springer: Cham, Switzerland, 2023; Volume 47, pp. 1–23. [Google Scholar]
- Aufschnaiter, K.; Fellner, F.; Wurm, G. Surgery in adult onset tethered cord syndrome (ATCS): Review of literature on occasion of an exceptional case. Neurosurg. Rev. 2008, 31, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Khalfallah, M.; Perrouin-Verbe, B.; Caillon, F.; Deschamps, C.; Bord, E.; Robert, R. Arachnoiditis ossificans of the cauda equina: Case report and review of the literature. J. Neurosurg. Spine 2002, 97, 239–243. [Google Scholar] [CrossRef]
- Moskven, E.; McIntosh, G.; Nataraj, A.; Christie, S.D.; Kumar, R.; Phan, P.; Wang, Z.; Tarabay, B.; Weber, M.H.; Singh, S.; et al. Factors associated with increased length of stay in degenerative cervical spine surgery: A cohort analysis from the Canadian Spine Outcomes and Research Network. J. Neurosurg. Spine 2024, 41, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Boop, S.H.; Barber, J.K.; Susarla, S.M.; Durfy, S.; Ojemann, J.G.; Goldstein, H.E.; Lee, A.; Browd, S.; Ellenbogen, R.G.; et al. Perioperative complications and secondary retethering after pediatric tethered cord release surgery. J. Neurosurg. Pediatr. 2023, 32, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Maulana, A.F.; Nurikhwan, P.W.; Lahdimawan, A.; Ahsani, I.F.; Lahdimawan, M.R.R.; Jamila, A. Reducing complications in duraplasty with autologous dural graft material: A meta-analysis. J. Cerebrovasc. Endovasc. Neurosurg. 2025, 27, 103–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finger, T.; Schaumann, A.; Grillet, F.; Schulz, M.; Thomale, U.W. Retethering after transection of a tight filum terminale, postoperative MRI may help to identify patients at risk. Child’s Nerv. Syst. 2020, 36, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Klekamp, J.; Samii, M. Introduction of a score system for the clinical evaluation of patients with spinal processes. Acta Neurochir. 1993, 123, 221–223. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral-Nieves, N.; Sagaityte, E.; Shao, B.; Sampath, S.; Sastry, R.; Sampath, P.; Klinge, P.M.; Cielo, D. Tandem Detethering: A Novel One-Stage Approach Combining Cervicothoracic Cord Release Followed by Filum Terminale Sectioning. J. Clin. Med. 2025, 14, 7169. https://doi.org/10.3390/jcm14207169
Amaral-Nieves N, Sagaityte E, Shao B, Sampath S, Sastry R, Sampath P, Klinge PM, Cielo D. Tandem Detethering: A Novel One-Stage Approach Combining Cervicothoracic Cord Release Followed by Filum Terminale Sectioning. Journal of Clinical Medicine. 2025; 14(20):7169. https://doi.org/10.3390/jcm14207169
Chicago/Turabian StyleAmaral-Nieves, Natalie, Emilija Sagaityte, Belinda Shao, Shailen Sampath, Rahul Sastry, Prakash Sampath, Petra M. Klinge, and Deus Cielo. 2025. "Tandem Detethering: A Novel One-Stage Approach Combining Cervicothoracic Cord Release Followed by Filum Terminale Sectioning" Journal of Clinical Medicine 14, no. 20: 7169. https://doi.org/10.3390/jcm14207169
APA StyleAmaral-Nieves, N., Sagaityte, E., Shao, B., Sampath, S., Sastry, R., Sampath, P., Klinge, P. M., & Cielo, D. (2025). Tandem Detethering: A Novel One-Stage Approach Combining Cervicothoracic Cord Release Followed by Filum Terminale Sectioning. Journal of Clinical Medicine, 14(20), 7169. https://doi.org/10.3390/jcm14207169





