IgM Antiphospholipid Antibodies in Antiphospholipid Syndrome: Prevalence, Clinical Associations, and Diagnostic Implications—A Scoping Review
Abstract
1. Introduction
2. Methodology
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Charting
2.5. Synthesis of Results
2.6. Methodological Shortcuts and Implications
3. Results
3.1. Prevalence of aPL IgM Isotype Across Clinical Manifestations
3.1.1. Obstetric Manifestations
| Prevalence of LA, aCL, aβ2GPI, and aPS/PT Across Obstetric Manifestations [%] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Year | Cohort | Assay Type | Persistency † | Cut-Off | N | LA | aCL | aβ2GPI | aPS/PT | |||
| IgG | IgM | IgG | IgM | IgG | IgM | ||||||||
| Chen et al. [28] | 2024 | aPL+ patients with obstetric complications | NA | NA | ≥20–40 GPL/MPL/AU | 92 | 3.3 | 10.9 | 80.4 | 3.3 | 14.1 | ||
| Chen et al. [29] | 2024 | 118 aPL+ patients, with 124 pregnancies | NA | NA | Low: ≥20–40 GPL/MPL/AU | 92 | 3.8 | 9.8 | 81.5 | 3.3 | 14.1 | ||
| Medium–high: ≥40–80 GPL/MPL/AU | 32 | 7.4 | 21.9 | 37.5 | 40.6 | 31.3 | |||||||
| Long et al. [33] | 2023 | OAPS patients (nPAPS = 123) | Commercial ELISA | Yes | 40 AU | 209 | 50.2 | 35.9 | 11.0 | 34.0 | 24.4 | ||
| Liu et al. [31] | 2022 | APS patients | Commercial ELISA | Yes | 99th percentile | 240 ‡ | 15.0 | 6.7 | 13.3 | 2.1 | 62.9 | ||
| Alijotas-Reig et al. [34] | 2019 | OAPS patients | Commercial/In-house ELISA | Yes | 99th percentile | 706 # | 50.4 | 21.1 | 15.3 | 11.5 | 8.9 | ||
| Žigon et al. [35] | 2015 | Patients with OAPS clinical manifestations | In-house ELISA | NA | 99th percentile (222 HD) | 169 § | 8.7 | 10.1 | 3.6 | 5.9 | 2.4 | 9.5 | 7.1 |
| Pereira et al. [32] | 2015 | OAPS patients | In-house ELISA | Yes | 99th percentile | 71 | 16.9 | 39.4 | 49.7 | 29.6 | 23.9 | ||
| Bouvier et al. [30] | 2014 | OAPS patients | In-house ELISA | Yes | >42.1 GPL/MPL/AU | 517 | 61.7 | 47.2 | 71.9 | 22.1 | 40.6 | ||
| Roye-Green et al. [36] | 2011 | Women with a history of 2 or more consecutive spontaneous abortions | In-house ELISA | NA | aCL (Low): 10–20 GPL/MPL aβ2GPI: >20 AU | 50 | 2 | 2 | 10 | 6 | 2 | ||
| aCL (Medium): 21–80 GPL/MPL | 2 | 16 | |||||||||||
3.1.2. Combined Thrombotic and Obstetric Manifestations
| Prevalence of LA, aCL, aβ2GPI and aPS/PT Across Combined Thrombotic and Obstetric Manifestations [%] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Year | Cohort | Assay Type | Persistency † | Cut-Off | N | LA | aCL | aβ2GPI | aPS/PT | |||
| IgG | IgM | IgG | IgM | IgG | IgM | ||||||||
| Zhao et al. [38] | 2024 | APS patients meeting ACR/EULAR criteria | Commercial ELISA | Yes | Medium-high: 40–79 GPL/MPL/AU | 335 | 97.3 | 11.9 | 4.8 | 8.1 | 3.9 | ||
| High: 80 GPL/MPL/AU | 28.1 | 4.8 | 23.0 | 3.9 | |||||||||
| Egri et al. [39] | 2021 | APS patients (nPAPS = 17, nSAPS = 8) | aCL, aβ2GPI: CLIA aPS/PT: Commercial ELISA | Yes | aCL, aβ2GPI: 20 GPL/MPL/AU aPS/PT: 30 AU | 25 § | 58 | 64 | 25 | 76 | 32 | 28 | 72 |
| Liu et al. [40] | 2020 | APS patients (nPAPS = 88, nSAPS = 104) | Commercial ELISA | Yes | aPS/PT: 30 AU | 192 | 69 | 71 | 73 | ||||
| Volkov et al. [41] | 2020 | APS patients (nPAPS = 63, nSAPS = 67) | LIA strip | Yes | 95th percentile | 130 | 84 | 59 | 26 | 66 | 32 | ||
| Heikal et al. [37] | 2019 | APS patients (nPAPS = 51, nSAPS with associated SLE = 20) | Commercial ELISA | Yes | aCL, aβ2GPI: 20 GPL/MPL/AU | 71 § | 71 | 25 | 34 | 25 | 28 | 31 | 42 |
| aPS/PT: 30 AU | |||||||||||||
| Shi et al. [42] | 2018 | APS patients | aPS/PT: Commercial ELISA | Yes | aPS/PT: 30 AU | 67 (PAPS) | 70.1 | 79.1 | |||||
| 119 (SAPS) | 73.1 | 60.5 | |||||||||||
| Hoxha et al. [43] | 2017 | PAPS patients | aCL, aβ2GPI: In-house ELISA | Yes | aCL, aβ2GPI: 99th Percentile (100 HD) | 197 | 57.7 | 69.0 | 40.1 | 71.6 | 44.7 | 29.9 | 48.2 |
| aPS/PT: Commercial ELISA | aPS/PT IgG/IgM: 61.4 AU/56.3 AU | ||||||||||||
| Shi et al. [44] | 2017 | APS patients | Commercial ELISA | Yes | aCL: 40 GPL/MPL aβ2GPI: 99th Percentile | 252 # | 32.9 | 56.3 | 25.0 | 37.3 | 27.8 | ||
| Hoxha et al. [25] | 2015 | PAPS patients | aCL, aβ2GPI: In-house ELISA | Yes | aCL, aβ2GPI: 99th Percentile aPS/PT: 30 AU | 160 | 46.8 | 66.3 | 38.1 | 67.5 | 44.4 | 30 | 47.5 |
| aPS/PT: Commercial ELISA | |||||||||||||
| Vlagea et al. [45] | 2013 | APS patients | In-house ELISA | Yes | aPS/PT IgG/IgM: 10 AU/15 AU | 95 (PAPS) | 35.7 | 32.6 | |||||
| 45 (SAPS) | 40.0 | 31.1 | |||||||||||
| Boffa et al. [46] | 2009 | APS patients | In-house and commercial ELISA | Yes | 99th Percentile | 109 | 37.5 | 71.6 | 18.3 | 33.0 | 23.9 | ||
3.1.3. Purely Thrombotic Manifestations
| Prevalence of LA, aCL, aβ2GPI and aPS/PT Across Thrombotic Manifestations [%] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Year | Cohort | Assay Type | Persistency † | Cut-Off | N | LA | aCL | aβ2GPI | aPS/PT | |||
| IgG | IgM | IgG | IgM | IgG | IgM | ||||||||
| Zen et al. [18] | 2025 | PAPS Patients | In-house ELISA | Yes | aCL IgG/IgM (Medium): 21.1 GPL/27.2 MPL | 171 (ACR/EULAR PAPS) / 34 (only Sydney PAPS) | 81.3 / 5.9 | 0 / 20.5 | 1.75 / 5.9 | 0.58 / 17.2 | 0 / 2.9 | ||
| Medium-to-high: 99th percentile | 81.3 / 0 | 42.7/ 70.5 | 78.4 / 0 | 35.7 / 58.8 | |||||||||
| Uludağ et al. [49] | 2023 | TAPS Patients | Commercial ELISA | Yes | aCL: 40 GPL/MPL aβ2GPI: 20 AU aPS/PT: 30 AU | 105 | 84.8 | 60.0 | 26.7 | 44.8 | 30.5 | 55.2 | 55.2 |
| Vandevelde et al. [50] | 2022 | TAPS Patients | Commercial ELISA | Yes | aCL, aβ2GPI: 20 GPL/MPL/AU | 197 | 94.9 | 43.1 | 27.4 | 35.0 | 26.4 | 48.7 | 61.9 |
| aPS/PT: 30 AU | |||||||||||||
| Masson et al. [48] | 2022 | APS Patients | ELISA | Yes | 40 AU or 99th percentile | 127 (<65 years old) § | 66.9 | 38.5 | 18.1 | 35.0 | 17.5 | ||
| 57 (≥65 years old) § | 52.8 | 16.4 | 33.9 | 14.3 | 31.4 | ||||||||
| Pérez et al. [47] | 2018 | APS Patients (nPAPS = 35, nSAPS with SLE = 22) | Commercial ELISA | Yes | 20 GPL/MPL/AU | 57 | 66.7 | 42.1 | 43.9 | 45.6 | 50.9 | ||
| Del Ross et al. [51] | 2015 | TAPS Patients | In-house ELISA | Yes | 99th percentile | 106 | 71.7 | 77.4 | 39.6 | 73.6 | 40.6 | ||
3.1.4. Thrombotic and Other Manifestations
| Prevalence of LA, aCL, aβ2GPI and aPS/PT Across Thrombotic and Other Manifestations [%] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Year | Cohort | Assay Type | Persistency † | Cut-Off | N | LA | aCL | aβ2GPI | aPS/PT | |||
| IgG | IgM | IgG | IgM | IgG | IgM | ||||||||
| Sloan et al. [56] | 2024 | Paediatric APS patients (nPAPS = 11, nSAPS = 8) | Commercial ELISA | Yes | aCL, aβ2GPI: 20 GPL/MPL/AU aPS/PT: 30 AU | 19 | 84 | 37 | 26 | 42 | 47 | 58 | 68 |
| 40 GPL/MPL/AU | 26 | 16 | 26 | 26 | 58 | 68 | |||||||
| Shi et al. [52] | 2023 | PAPS patients | Commercial ELISA | Yes | 40 GPL/MPL/AU | 74 (TP +) | 90.5 | 64.9 | 27.0 | 64.9 | 40.5 | ||
| 144 (TP −) | 67.4 | 40.3 | 23.6 | 49.3 | 40.3 | ||||||||
| Djokovic et al. [53] | 2022 | PAPS patients | Commercial ELISA | Yes | 41 GPL/MPL/AU | 360 | 55.0 | 29.7 | 47.8 | 30.3 | 40.8 | ||
| Morán-Álvarez et al. [57] | 2022 | aPL+ paediatric patients | ELISA, ECLIA | Yes | aCL: 18 GPL/MPL aβ2GPI: 10 AU | 82 | 77.8 | 30.5 | 23.5 | 31.7 | 20.7 | ||
| Stojanovich et al. [54] | 2013 | APS patients | Commercial ELISA | Yes | 11 GPL/MPL/AU | 260 (PAPS) | 51.2 | 36.5 | 54.2 | 31.9 | 37.7 | ||
| 114 (SAPS) | 49.1 | 59.6 | 64.0 | 43.0 | 44.7 | ||||||||
| Stojanovich et al. [55] | 2012 | APS patients | Commercial ELISA | No, only 6 weeks | 10 GPL/MPL/AU | 214 (PAPS) | 60.2 | 28.0 | 50.5 | 33.6 | 31.8 | ||
| 115 (SAPS) | 48.2 | 40.0 | 49.6 | 41.7 | 39.1 | ||||||||
3.1.5. Cerebrovascular Manifestations
| Prevalence of LA, aCL, aβ2GPI and aPS/PT Across Cerebrovascular Manifestations [%] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Year | Cohort | Assay Type | Persistency † | Cut-Off | N | LA | aCL | aβ2GPI | aPS/PT | |||
| IgG | IgM | IgG | IgM | IgG | IgM | ||||||||
| Radin et al. [58] | 2021 | Patients with a suspicion for APS or SLE negative for aCL and aβ2GPI | Commercial ELISA | Yes | 40 AU | 22 (LA +) | 100 | 0 | 0 | 0 | 0 | 36.4 | 25.0 |
| 20 (LA −) | 0 | 90.4 | 85.0 | ||||||||||
| Urbanski et al. [59] | 2018 | APS patients | Commercial ELISA | Yes | 12 GPL/MPL/AU | 144 | 78.5 | 80.6 | 24.3 | 53.5 | 26.4 | ||
| 24 (Isolated IgM) | 0 | 0 | 91.7 | 0 | 62.5 | ||||||||
| Camargo et al. [60] | 2012 | APS patients | In-house ELISA | Yes | 40 GPL/MPL/AU | 5 (APS-RF) | 40 | 40 | 60 | 60 | 0 | ||
| 68 (PAPS) | 79 | 38 | 53 | 25 | 10 | ||||||||
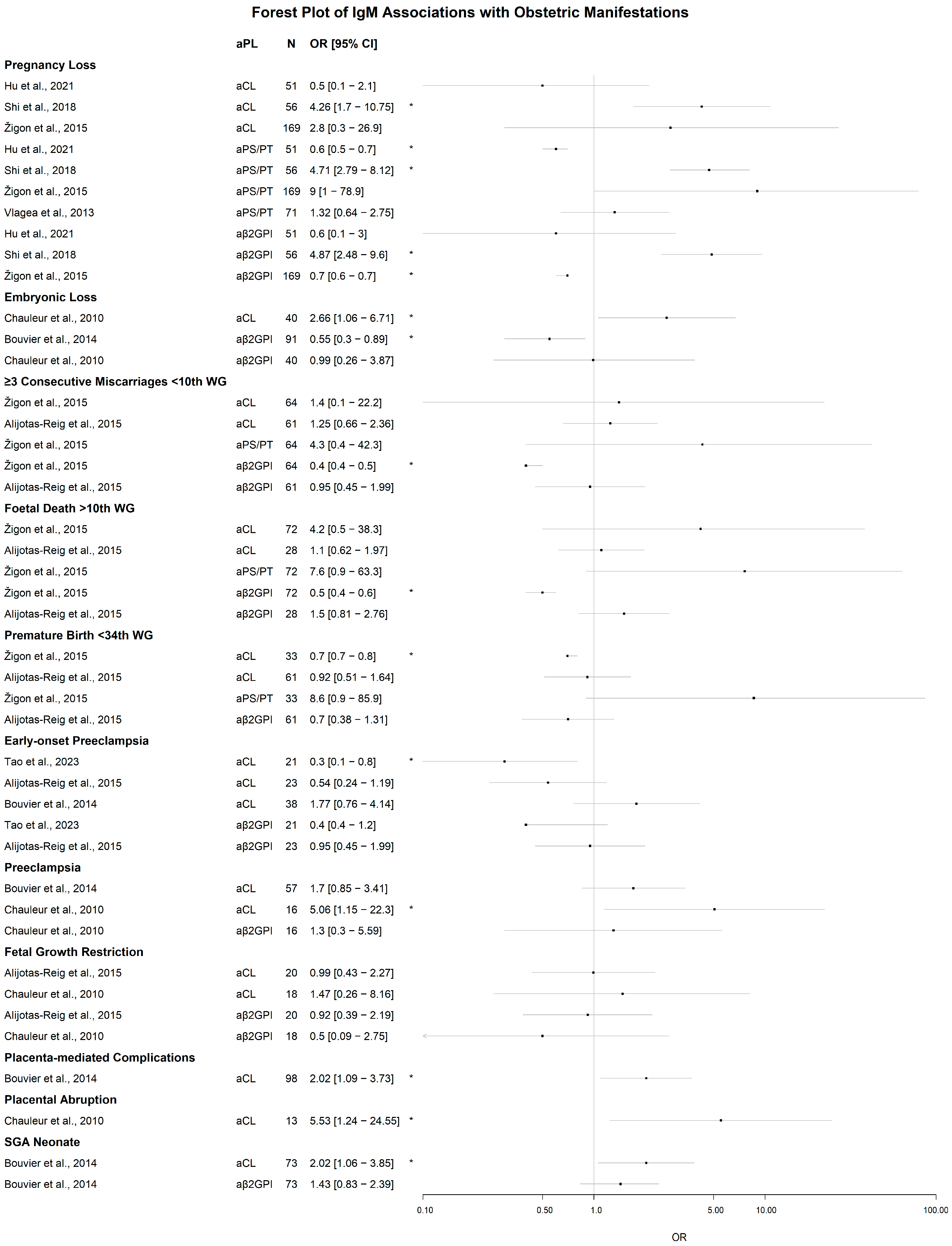
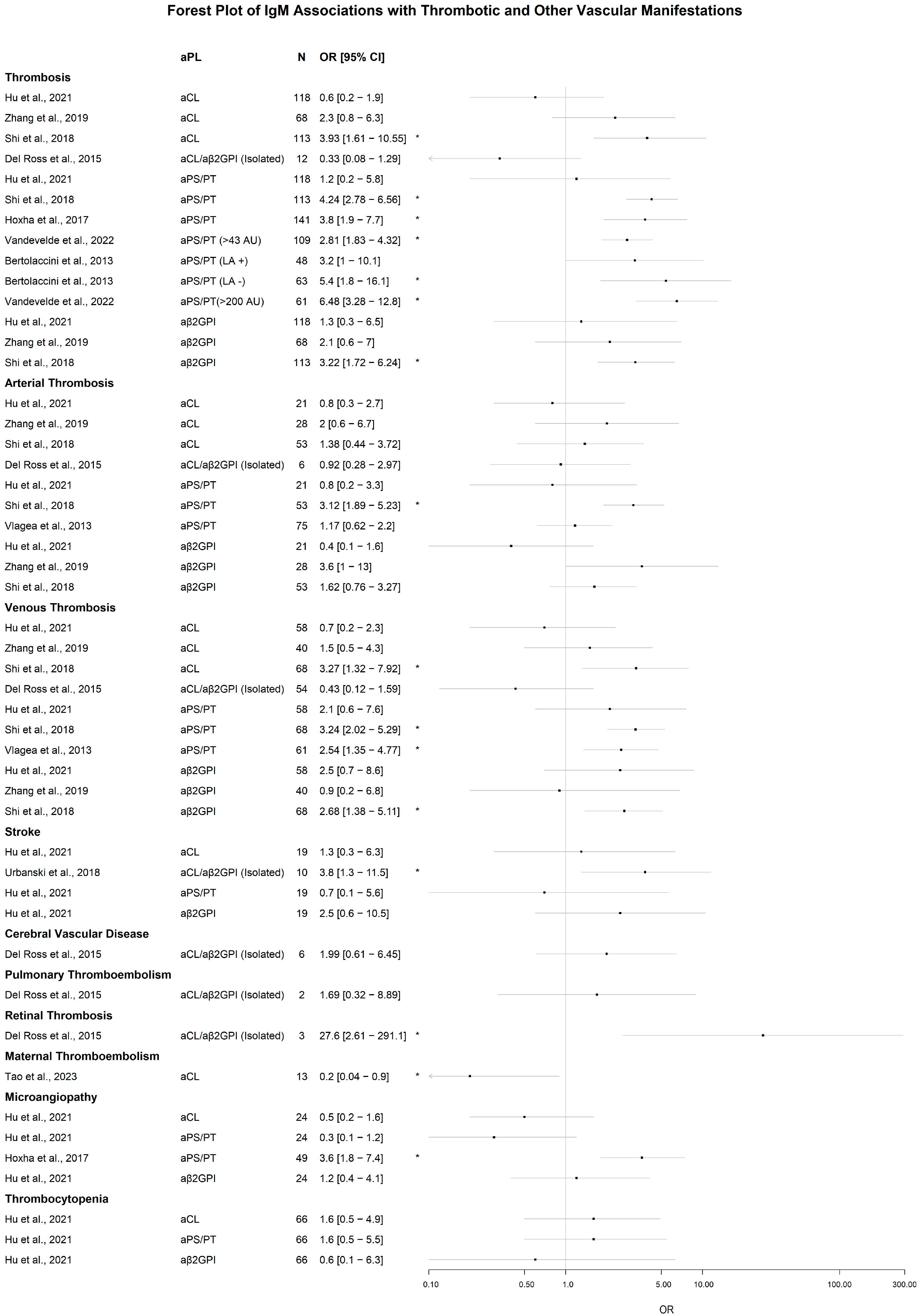
3.2. Associations of IgM aPL with Clinical Manifestations
3.2.1. Obstetric Manifestations
Significant Associations of IgM aPL
Lack of Significant Associations of IgM aPL
3.2.2. Thrombotic and Vascular Manifestations
Significant Associations of IgM aPL
Lack of Significant Associations of IgM aPL
3.3. New Approach to aPL Associations: Cluster Analysis
3.4. Factors Contributing to Thrombosis: Neutrophil Activation and NETosis
4. Discussion
5. Conclusions
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aβ2GPI | Anti-β2 glycoprotein I antibodies |
| aCL | Anti-cardiolipin antibodies |
| ACR/EULAR | American College of Rheumatology/European Alliance of Associations for Rheumatology |
| aPL | Antiphospholipid antibodies |
| aPS/PT | Anti-phosphatidylserine/prothrombin antibodies |
| APOs | Adverse pregnancy outcomes |
| APS | Antiphospholipid syndrome |
| AU | Arbitrary units |
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| CLIA | Chemiluminescent immunoassay |
| ECLIA | Electrochemiluminescence immunoassay |
| ELISA | Enzyme-linked immunosorbent assay |
| GAPSS | Global Antiphospholipid Syndrome Score |
| GPL | aCL IgG phospholipid level |
| HD | Healthy donor |
| LA | Lupus anticoagulant |
| LIA | Line immune assay |
| MPL | aCL IgM phospholipid level |
| N or n | Cohort size |
| NETs | Neutrophil extracellular traps |
| NEUT-RI | Neutrophil-Reactive Intensity |
| OAPS | Obstetric antiphospholipid syndrome |
| OR | Odds ratio |
| PAPS | Primary antiphospholipid syndrome |
| PE | Preeclampsia |
| PI | Placental insufficiency |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews |
| RF | Rheumatic fever |
| SAPS | Secondary antiphospholipid syndrome |
| SGA | Small-for-gestational-age |
| SLE | Systemic lupus erythematosus |
| TAPS | Thrombotic antiphospholipid syndrome |
| TP | Thrombocytopenia |
| VTE | Venous thromboembolism |
| WG | Weeks of gestation |
References
- Ruiz-Irastorza, G.; Crowther, M.; Branch, W.; Khamashta, M.A. Antiphospholipid Syndrome. Lancet 2010, 376, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Leal Rato, M.; Bandeira, M.; Romão, V.C.; Aguiar de Sousa, D. Neurologic Manifestations of the Antiphospholipid Syndrome—An Update. Curr. Neurol. Neurosci. Rep. 2021, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- El Hasbani, G.; Saliba, A.N.; Uthman, I.; Taher, A.T. Hematological Manifestations of Antiphospholipid Syndrome: Going beyond Thrombosis. Blood Rev. 2023, 58, 101015. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef]
- Schreiber, K.; Sciascia, S.; De Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastroza, G.; Salmon, J.E.; Shoenfeld, Y.; Shovman, O.; Hunt, B.J. Antiphospholipid Syndrome. Nat. Rev. Dis. Prim. 2018, 4, 17103. [Google Scholar] [CrossRef]
- Duarte-García, A.; Pham, M.M.; Crowson, C.S.; Amin, S.; Moder, K.G.; Pruthi, R.K.; Warrington, K.J.; Matteson, E.L. The Epidemiology of Antiphospholipid Syndrome: A Population-Based Study. Arthritis Rheumatol. 2019, 71, 1545–1552. [Google Scholar] [CrossRef]
- Dabit, J.Y.; Valenzuela-Almada, M.O.; Vallejo-Ramos, S.; Duarte-García, A. Epidemiology of Antiphospholipid Syndrome in the General Population. Curr. Rheumatol. Rep. 2022, 23, 85. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derkesen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International Consensus Statement on an Update of the Classification Criteria for Definite Antiphospholipid Syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Ann. Rheum. Dis. 2023, 82, 1258–1270. [Google Scholar] [CrossRef]
- Da Silva, F.F.; Levy, R.A.; De Carvalho, J.F. Cardiovascular Risk Factors in the Antiphospholipid Syndrome. J. Immunol. Res. 2014, 2014, 621270. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR Recommendations for the Management of Antiphospholipid Syndrome in Adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Vandevelde, A.; Gris, J.C.; Moore, G.W.; Musiał, J.; Zuily, S.; Wahl, D.; Devreese, K.M.J. Toward Harmonized Interpretation of Anticardiolipin and Anti-Β2-Glycoprotein I Antibody Detection for Diagnosis of Antiphospholipid Syndrome Using Defined Level Intervals and Likelihood Ratios: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2024, 22, 2345–2362. [Google Scholar] [CrossRef] [PubMed]
- Arachchillage, D.J.; Platton, S.; Hickey, K.; Chu, J.; Pickering, M.; Sommerville, P.; MacCallum, P.; Breen, K. Guidelines on the Investigation and Management of Antiphospholipid Syndrome. Br. J. Haematol. 2024, 205, 855–880. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, D.; Rossmann, H.; Schulz, A.; Zeller, T.; Pfeiffer, N.; Binder, H.; Münzel, T.; Beutel, M.E.; Müller-Calleja, N.; Wild, P.S.; et al. Distribution of Antiphospholipid Antibodies in a Large Population-Based German Cohort. Clin. Chem. Lab. Med. 2016, 54, 1663–1670. [Google Scholar] [CrossRef]
- El Hasbani, G.; Viola, M.; Sciascia, S.; Taher, A.T.; Uthman, I. Antiphospholipid Antibodies in Inflammatory and Autoimmune Rheumatic and Musculoskeletal Diseases Beyond Lupus: A Systematic Review of the Available Evidence. Rheumatol. Ther. 2021, 8, 81. [Google Scholar] [CrossRef]
- Ruiz, J.T.; Blank, M.; Zandman-Goddard, G.; Sherer, Y.; Shoenfeld, Y. Antiphospholipid Antibodies and Their Relationship with Infections, Vaccines, and Drugs. Handb. Syst. Autoimmune Dis. 2017, 12, 167–179. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Tayar, J.H.; Fa’ak, F.; Sharma, G.; Lopez-Olivo, M.A.; Yousif, A.; Shagroni, T.; Al-Hawamdeh, S.; Rojas-Hernandez, C.M.; Suarez-Almazor, M.E. Systematic Review of Observational Studies Reporting Antiphospholipid Antibodies in Patients with Solid Tumors. Blood Adv. 2020, 4, 1746–1755. [Google Scholar] [CrossRef]
- Zen, M.; Tonello, M.; Carta, F.; Calligaro, A.; Favaro, M.; Del Ross, T.; Hulej, G.; Rahmé, Z.; Ruffatti, A.; Doria, A. Insights into the 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria: Findings from a Cohort of 205 Patients with Primary APS. Rheumatology 2025, 64, 4325–4330. [Google Scholar] [CrossRef]
- Sciascia, S.; Sanna, G.; Murru, V.; Roccatello, D.; Khamashta, M.A.; Bertolaccini, M.L. GAPSS: The Global Anti-Phospholipid Syndrome Score. Rheumatology 2013, 52, 1397–1403. [Google Scholar] [CrossRef]
- Pregnolato, F.; Gerosa, M.; Raimondo, M.G.; Comerio, C.; Bartoli, F.; Lonati, P.A.; Borghi, M.O.; Acaia, B.; Ossola, M.W.; Ferrazzi, E.; et al. EUREKA Algorithm Predicts Obstetric Risk and Response to Treatment in Women with Different Subsets of Anti-Phospholipid Antibodies. Rheumatology 2021, 60, 1114–1124. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, L.; Liu, H.; Cai, Q.; Yun, Z.; Sun, F.; Jia, Y.; Guo, J.; Li, C. Non-Criteria Antiphospholipid Antibodies in Antiphospholipid Syndrome: Diagnostic Value Added. Front. Immunol. 2022, 13, 972012. [Google Scholar] [CrossRef]
- Devreese, K.M.J. Noncriteria Antiphospholipid Antibodies in Antiphospholipid Syndrome. Int. J. Lab. Hematol. 2024, 46, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Pan, H.; Yang, Z.; Yang, C.; Shi, H. Beyond the Classics: The Emerging Value of Anti-Phosphatidylserine/Prothrombin Antibodies in Antiphospholipid Syndrome. Clin. Immunol. 2023, 256, 109804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Cheng, C.Y.; Yang, Y.; Denas, G.; Pengo, V. Prevalence of APhosphatidylserine/Prothrombin Antibodies and Association with Antiphospholipid Antibody Profiles in Patients with Antiphospholipid Syndrome: A Systematic Review and Meta-Analysis. Thromb. Res. 2022, 214, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, A.; Ruffatti, A.; Mattia, E.; Meneghel, L.; Tonello, M.; Salvan, E.; Pengo, V.; Punzi, L. Relationship between Antiphosphatidylserine/Prothrombin and Conventional Antiphospholipid Antibodies in Primary Antiphospholipid Syndrome. Clin. Chem. Lab. Med. 2015, 53, 1265–1270. [Google Scholar] [CrossRef]
- Pham, M.; Orsolini, G.; Crowson, C.; Snyder, M.; Pruthi, R.; Moder, K. Anti-Phosphatidylserine Prothrombin Antibodies as a Predictor of the Lupus Anticoagulant in an All-Comer Population. J. Thromb. Haemost. 2022, 20, 2070–2074. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Huang, S.; Li, J.; Li, T.; Chen, J.; Lu, Y.; Zhang, J. Analysis of Pregnancy Outcomes in Patients Exhibiting Recurrent Miscarriage with Concurrent Low-Titer Antiphospholipid Antibodies. Am. J. Reprod. Immunol. 2024, 92, e13940. [Google Scholar] [CrossRef]
- Chen, J.; Yue, J.; Lu, Y.; Li, T.; Li, X.; Zhang, J.Y. Recurrent Miscarriage and Low-Titer Antiphospholipid Antibodies. Clin. Rheumatol. 2024, 43, 1327. [Google Scholar] [CrossRef]
- Bouvier, S.; Cochery-Nouvellon, É.; Lavigne-Lissalde, G.; Mercier, É.; Marchetti, T.; Balducchi, J.P.; Marès, P.; Gris, J.C. Comparative Incidence of Pregnancy Outcomes in Treated Obstetric Antiphospholipid Syndrome: The NOH-APS Observational Study. Blood 2014, 123, 404–413. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, S.; Xu, H.; Zhang, X.; Chen, C.; Fu, R.; Li, C.; Guo, F.; Zhao, A. Prognostic Analysis of Antibody Typing and Treatment for Antiphospholipid Syndrome-Related Recurrent Spontaneous Abortion. Int. J. Gynecol. Obstet. 2022, 156, 107–111. [Google Scholar] [CrossRef]
- Pereira, F.R.; Macri, F.; Jackowski, M.P.; Kostis, W.J.; Gris, J.C.; Beregi, J.P.; Mekkaoui, C. Diffusion Tensor Imaging in Patients with Obstetric Antiphospholipid Syndrome without Neuropsychiatric Symptoms. Eur. Radiol. 2015, 26, 959–968. [Google Scholar] [CrossRef]
- Long, Y.; Huang, C.; Cui, Y.; Xie, Z.; Zhou, Y.; Shi, X.; Song, Y.; Tian, X.; Li, M.; Liu, J.; et al. Cluster Analysis of Antiphospholipid Antibodies-Associated Adverse Pregnancy Outcome Patients: Based on a 13-Years Cohort Study. Clin. Exp. Med. 2023, 23, 5377–5388. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Ferrer-Oliveras, R.; Sáez-Comet, L.; Lefkou, E.; Mekinian, A.; Belizna, C.; Ruffatti, A.; Tincani, A.; Marozio, L.; et al. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): A Survey of 1000 Consecutive Cases. Autoimmun. Rev. 2019, 18, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Žigon, P.; Perdan Pirkmajer, K.; Tomšič, M.; Kveder, T.; Božič, B.; Sodin Šemrl, S.; Čučnik, S.; Ambrožič, A. Anti-Phosphatidylserine/Prothrombin Antibodies Are Associated with Adverse Pregnancy Outcomes. J. Immunol. Res. 2015, 2015, 975704. [Google Scholar] [CrossRef] [PubMed]
- Roye-Green, K.; Frederick, J.; Wharfe, G.; Choo- Kang, E.; Dacosta, V.; Fletcher, H.; Smikle, M. Antiphospholipid and Other Autoantibodies in a Cohort of Habitual Aborters and Healthy Multiparous Women in Jamaica. Hum. Antibodies 2011, 20, 1–5. [Google Scholar] [CrossRef]
- Heikal, N.; Martins, T.B.; White, S.K.; Willis, R.; Ware Branch, D.; Schmidt, R.L.; Tebo, A.E. Laboratory Evaluation of Antiphospholipid Syndrome: Is There a Role for Specific Noncriteria Antiphospholipid Antibody Tests? Am. J. Clin. Pathol. 2019, 152, 638–646. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, C.; Zhou, Y.; Qi, W.; Cai, B.; Hu, C.; Song, Y.; Zhu, T.; Shi, X.; Liu, X.; et al. Performance Validation of the 2023 American College of Rheumatology/European League Against Rheumatism Antiphospholipid Syndrome Classification Criteria in an Antiphospholipid Syndrome Cohort. J. Thromb. Haemost. 2024, 22, 1660–1674. [Google Scholar] [CrossRef]
- Egri, N.; Bentow, C.; Rubio, L.; Norman, G.L.; López-Sañudo, S.; Mahler, M.; Pérez-Isidro, A.; Cervera, R.; Viñas, O.; Espinosa, G.; et al. Anti-Phosphatidylserine/Prothrombin Antibodies at Two Points: Correlation with Lupus Anticoagulant and Thrombotic Risk. Front. Immunol. 2021, 12, 754469. [Google Scholar] [CrossRef]
- Liu, T.; Gu, J.; Wan, L.; Hu, Q.; Teng, J.; Liu, H.; Cheng, X.; Ye, J.; Su, Y.; Sun, Y.; et al. “Non-Criteria” Antiphospholipid Antibodies Add Value to Antiphospholipid Syndrome Diagnoses in a Large Chinese Cohort. Arthritis Res. Ther. 2020, 22, 33. [Google Scholar] [CrossRef]
- Volkov, I.; Seguro, L.; Leon, E.P.; Kovács, L.; Roggenbuck, D.; Schierack, P.; Gilburd, B.; Doria, A.; Tektonidou, M.G.; Agmon-Levin, N. Profiles of Criteria and Non-Criteria Anti-Phospholipid Autoantibodies Are Associated with Clinical Phenotypes of the Antiphospholipid Syndrome. Autoimmun. Highlights 2020, 11, 8. [Google Scholar] [CrossRef]
- Shi, H.; Zheng, H.; Yin, Y.F.; Hu, Q.Y.; Teng, J.L.; Sun, Y.; Liu, H.L.; Cheng, X.B.; Ye, J.N.; Su, Y.T.; et al. Antiphosphatidylserine/Prothrombin Antibodies (APS/PT) as Potential Diagnostic Markers and Risk Predictors of Venous Thrombosis and Obstetric Complications in Antiphospholipid Syndrome. Clin. Chem. Lab. Med. 2018, 56, 614–624. [Google Scholar] [CrossRef]
- Hoxha, A.; Mattia, E.; Tonello, M.; Grava, C.; Pengo, V.; Ruffatti, A. Antiphosphatidylserine/Prothrombin Antibodies as Biomarkers to Identify Severe Primary Antiphospholipid Syndrome. Clin. Chem. Lab. Med. 2017, 55, 890–898. [Google Scholar] [CrossRef]
- Shi, H.; Teng, J.-L.; Sun, Y.; Wu, X.-Y.; Hu, Q.-Y.; Liu, H.-L.; Cheng, X.-B.; Yin, Y.-F.; Ye, J.-N.; Chen, P.P.; et al. Clinical Characteristics and Laboratory Findings of 252 Chinese Patients with Anti-Phospholipid Syndrome: Comparison with Euro-Phospholipid Cohort. Clin. Rheumatol. 2017, 36, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Vlagea, A.; Gil, A.; Cuesta, M.V.; Arribas, F.; Diez, J.; Lavilla, P.; Pascual-Salcedo, D. Antiphosphatidylserine/Prothrombin Antibodies (APS/PT) as Potential Markers of Antiphospholipid Syndrome. Clin. Appl. Thromb. 2013, 19, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Boffa, M.C.; Boinot, C.; De Carolis, S.; Rovere-Querini, P.; Aurousseau, M.H.; Allegri, F.; Nicaise-Roland, P.; Barra, A.; Botta, A.; Ambrozic, A.; et al. Laboratory Criteria of the Obstetrical Antiphospholipid Syndrome: Data from a Multicentric Prospective European Women Cohort. Thromb. Haemost. 2009, 102, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.; Stojanovich, L.; Naranjo, L.; Stanisavljevic, N.; Bogdanovic, G.; Serrano, M.; Serrano, A. Presence of Immune Complexes of IgG/IgM Bound to B2-Glycoprotein i Is Associated with Non-Criteria Clinical Manifestations in Patients with Antiphospholipid Syndrome. Front. Immunol. 2018, 9, 2644. [Google Scholar] [CrossRef]
- Masson, C.; An Nguyen, T.T.; Dufrost, V.; Audrain, M.; Hémont, C.; Agard, C.; Artifoni, M.; Connault, J.; Fouassier, M.; Hamidou, M.; et al. Antiphospholipid Syndrome in Patients over 65 Years: A Comparative Study of Clinical and Biological Features and Thrombotic Relapses. Lupus 2022, 31, 1816–1823. [Google Scholar] [CrossRef]
- Uludağ, Ö.; Çinar, S.; McDonnell, T.; Çene, E.; Yalçinkaya, Y.; Gül, A.; İnanç, M.; Artim Esen, B. Thrombotic Risk Assessment in Antiphospholipid Syndrome: Do Noncriteria Antibodies Contribute? Turkish J. Med. Sci. 2023, 53, 1067–1074. [Google Scholar] [CrossRef]
- Vandevelde, A.; Chayoua, W.; de Laat, B.; Moore, G.W.; Musiał, J.; Zuily, S.; Wahl, D.; Devreese, K.M.J. Added Value of Antiphosphatidylserine/Prothrombin Antibodies in the Workup of Thrombotic Antiphospholipid Syndrome: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2022, 20, 2136–2150. [Google Scholar] [CrossRef]
- Del Ross, T.; Ruffatti, A.; Cuffaro, S.; Tonello, M.; Calligaro, A.; Favaro, M.; Facchinetti, M.; Hoxha, A.; Punzi, L. The Clinical Relevance of the IgM Isotype of Antiphospholipid Antibodies in the Vascular Antiphospholipid Syndrome. Thromb. Res. 2015, 136, 883–886. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, J.; Jiang, H.; Huang, C.; Qi, W.; Song, Y.; Wang, Q.; Li, M.; Tian, X.; Zhao, Y.; et al. Thrombocytopenia in Primary Antiphospholipid Syndrome: Association with Prognosis and Clinical Implications. Rheumatology 2023, 62, 256–263. [Google Scholar] [CrossRef]
- Djokovic, A.; Stojanovich, L.; Stanisavljevic, N.; Djokic, S.; Filipovic, B.; Matic, P.; Milanovic, M.; Apostolovic, S.; Saponjski, J. Cardiac Manifestations in Primary Antiphospholipid Syndrome and Their Association to Antiphospholipid Antibodies’ Types and Titers—Cross-Sectional Study of Serbian Cohort. Clin. Rheumatol. 2022, 41, 1447–1455. [Google Scholar] [CrossRef]
- Stojanovich, L.; Kontic, M.; Djokovic, A.; Marisavljevic, D.; Ilijevski, N.; Stanisavljevic, N.; Mikovic, Z.; Petkovic, M.; Kovcin, V. Association between Systemic Non-Criteria Aps Manifestations and Antibody Type and Level: Results from the Serbian National Cohort Study. Clin. Exp. Rheumatol. 2013, 31, 0234–0242. [Google Scholar]
- Stojanovich, L.; Kontic, M.; Djokovic, A.; Ilijevski, N.; Stanisavljevic, N.; Marisavljevic, D. Pulmonary Events in Antiphospholipid Syndrome: Influence of Antiphospholipid Antibody Type and Levels. Scand. J. Rheumatol. 2012, 41, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.E.; Kmetova, K.; NaveenKumar, S.K.; Kluge, L.; Chong, E.; Hoy, C.K.; Yalavarthi, S.; Sarosh, C.; Baisch, J.; Walters, L.; et al. Non-Criteria Antiphospholipid Antibodies and Calprotectin as Potential Biomarkers in Pediatric Antiphospholipid Syndrome. Clin. Immunol. 2024, 261, 109926. [Google Scholar] [CrossRef] [PubMed]
- Morán-Álvarez, P.; Andreu-Suárez, Á.; Caballero-Mota, L.; Gassiot-Riu, S.; Berrueco-Moreno, R.; Calzada-Hernández, J.; Antón-López, J.; Vázquez-Díaz, M.; Boteanu, A. Non-Criteria Manifestations in the Presence of Antiphospholipid Antibodies in a Paediatric Cohort. Rheumatology 2022, 61, 4465–4471. [Google Scholar] [CrossRef]
- Radin, M.; Barinotti, A.; Foddai, S.G.; Cecchi, I.; Rubini, E.; Roccatello, D.; Menegatti, E.; Sciascia, S. Cerebrovascular Events in Patients with Isolated Anti-Phosphatidyl-Serine/Prothrombin Antibodies. Immunol. Res. 2021, 69, 372. [Google Scholar] [CrossRef]
- Urbanski, G.; Yelnik, C.M.; Maillard, H.; Launay, D.; Dubucquoi, S.; Hachulla, E.; Hatron, P.-Y.; Lambert, M. Antiphospholipid Syndrome with Isolated Isotype M Anticardiolipin and/or Anti-B2GPI Antibody Is Associated with Stroke. Stroke 2018, 49, 2770–2772. [Google Scholar] [CrossRef]
- Camargo, E.W.; Freire, P.V.; Silva, C.A.; Dos Santos, N.R.; Da Mota, L.M.H.; Pereira, R.M.R.; De Carvalho, J.F. Antiphospholipid Syndrome plus Rheumatic Fever: A Higher Risk Factor for Stroke? Rheumatol. Int. 2012, 32, 1721–1725. [Google Scholar] [CrossRef]
- Hu, C.; Li, S.; Xie, Z.; You, H.; Jiang, H.; Shi, Y.; Qi, W.; Zhao, J.; Wang, Q.; Tian, X.; et al. Evaluation of the Diagnostic Value of Non-Criteria Antibodies for Antiphospholipid Syndrome Patients in a Chinese Cohort. Front. Immunol. 2021, 12, 741369. [Google Scholar] [CrossRef]
- Chauleur, C.; Galanaud, J.P.; Alonso, S.; Cochery-Nouvellon, E.; Balducchi, J.P.; Marès, P.; Fabbro-Peray, P.; Gris, J.C. Observational Study of Pregnant Women with a Previous Spontaneous Abortion before the 10th Gestation Week with and without Antiphospholipid Antibodies. J. Thromb. Haemost. 2010, 8, 699–706. [Google Scholar] [CrossRef]
- Tao, J.J.; Adurty, S.; D’Angelo, D.; DeSancho, M.T. Management and Outcomes of Women with Antiphospholipid Syndrome during Pregnancy. J. Thromb. Thrombolysis 2023, 55, 751–759. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Ferrer-Oliveras, R.; Ruffatti, A.; Tincani, A.; Lefkou, E.; Bertero, M.T.; Coloma-Bazan, E.; de Carolis, S.; Espinosa, G.; Rovere-Querini, P.; et al. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): A Survey of 247 Consecutive Cases. Autoimmun. Rev. 2015, 14, 387–395. [Google Scholar] [CrossRef]
- Bertolaccini, M.L.; Sciascia, S.; Murru, V.; Garcia-Fernandez, C.; Sanna, G.; Khamashta, M.A. Prevalence of Antibodies to Prothrombin in Solid Phase (APT) and to Phosphatidylserine-Prothrombin Complex (APS/PT) in Patients with and without Lupus Anticoagulant. Thromb. Haemost. 2013, 109, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Z.; Zhang, W.; Zhang, F.; Li, Y.; Liu, Y. Clinical Performance of Non-Criteria Antibodies to Phospholipids in Chinese Patients with Antiphospholipid Syndrome. Clin. Chim. Acta 2019, 495, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Kanthi, Y. Mechanisms of Immunothrombosis and Vasculopathy in Antiphospholipid Syndrome. Semin. Immunopathol. 2022, 44, 347. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, S.; Fortier, M.; Nouvellon, E.; Olivan, A.; Chéa, M.; Gris, J.C. NEUT-RI, a Surrogate Marker of NETosis Is Lower in Patients with Strong IgM Antiphospholipid Antibodies. J. Thromb. Thrombolysis 2024, 57, 1051–1055. [Google Scholar] [CrossRef]
- Kwiecień, I.; Rutkowska, E.; Kulik, K.; Kłos, K.; Plewka, K.; Raniszewska, A.; Rzepecki, P.; Chciałowski, A. Neutrophil Maturation, Reactivity and Granularity Research Parameters to Characterize and Differentiate Convalescent Patients from Active SARS-CoV-2 Infection. Cells 2021, 10, 2332. [Google Scholar] [CrossRef]
- Chayoua, W.; Kelchtermans, H.; Gris, J.C.; Moore, G.W.; Musiał, J.; Wahl, D.; de Groot, P.G.; de Laat, B.; Devreese, K.M.J. The (Non-)Sense of Detecting Anti-Cardiolipin and Anti-Β2glycoprotein I IgM Antibodies in the Antiphospholipid Syndrome. J. Thromb. Haemost. 2020, 18, 169–179. [Google Scholar] [CrossRef]
- Viall, C.A.; Chamley, L.W. Histopathology in the Placentae of Women with Antiphospholipid Antibodies: A Systematic Review of the Literature. Autoimmun. Rev. 2015, 14, 446–471. [Google Scholar] [CrossRef] [PubMed]
- Fredi, M.; Andreoli, L.; Aggogeri, E.; Bettiga, E.; Lazzaroni, M.G.; Le Guern, V.; Lojacono, A.; Morel, N.; Piette, J.C.; Zatti, S.; et al. Risk Factors for Adverse Maternal and Fetal Outcomes in Women with Confirmed APL Positivity: Results from a Multicenter Study of 283 Pregnancies. Front. Immunol. 2018, 9, 353763. [Google Scholar] [CrossRef] [PubMed]
- Skeith, L.; Abou-Nassar, K.E.; Walker, M.; Ramsay, T.; Booth, R.; Wen, S.W.; Smith, G.N.; Rodger, M.A. Are Anti-Β2 Glycoprotein 1 Antibodies Associated with Placenta-Mediated Pregnancy Complications? A Nested Case-Control Study. Am. J. Perinatol. 2018, 35, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, G.; Chen, L.; Guo, X.; Fang, X.; Zhang, W.; Tu, Y.; Sun, K.; Cai, Z. The Relation of Anti-Phosphatidylserine/Prothrombin Antibodies and Premature Rupture of Membranes: A Retrospective Cohort Study. J. Reprod. Immunol. 2025, 167, 104421. [Google Scholar] [CrossRef]
- Kelchtermans, H.; Pelkmans, L.; de Laat, B.; Devreese, K.M. IgG/IgM Antiphospholipid Antibodies Present in the Classification Criteria for the Antiphospholipid Syndrome: A Critical Review of Their Association with Thrombosis. J. Thromb. Haemost. 2016, 14, 1530–1548. [Google Scholar] [CrossRef]
- Gálvez-Sánchez, R.; Salmón González, Z.; Fernández-García, M.; Cerveró Varona, A.; González-Mesones, B.; López-Hoyos, M.; Martínez-Taboada, V.; Luis Hernández, J. Impact of the 2023 ACR/EULAR Antiphospholipid Syndrome Criteria on Retinal Vein Occlusion Patients. J. Clin. Med. 2025, 14, 2826. [Google Scholar] [CrossRef]
- Tang, Z.; Shi, H.; Liu, H.L.; Cheng, X.; Ye, J.; Su, Y.; Hu, Q.; Meng, J.; Pan, H.; Yang, C.; et al. Correspondence on “2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria”. Ann. Rheum. Dis. 2023, 83, E4. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, H.; Tang, Z.; Pan, H.; Liu, H.; Cheng, X.; Su, Y.; Ye, J.; Hu, Q.; Meng, J.; et al. Assessment of the 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria in a Chinese Cohort: Impact on Clinical Practice. J. Autoimmun. 2024, 146, 103237. [Google Scholar] [CrossRef]
- Foddai, S.G.; Radin, M.; Cecchi, I.; Rubini, E.; Barinotti, A.; Alba, P.; Alonso, C.G.; Rossi, D.; Roccatello, D.; Sciascia, S. 2023 ACR/EULAR Classification Criteria in Existing Research Cohorts: An International Study. Rheumatology 2024, 63, 2770–2775. [Google Scholar] [CrossRef]
- Aiello, A.; Sarti, L.; Sandri, G.; Poli, D.; Sivera, P.; Barcellona, D.; Prisco, D.; Pizzini, A.M.; Vercillo, G.; Antonucci, E.; et al. Impact of the 2023 ACR/EULAR Classification Criteria on START2 Antiphospholipid Registry. Int. J. Lab. Hematol. 2025, 47, 313–317. [Google Scholar] [CrossRef]
- Vasi, İ.; Kardaş, R.C.; Ekici, M.; Yıldırım, D.; Kaya, B.; Duran, R.; Karadeniz, H.; Güler, A.A.; Küçük, H.; Göker, B.; et al. Assessment and Comparison of the 2023 ACR/EULAR APS Criteria with the Revised Sapporo Criteria. Int. J. Rheum. Dis. 2024, 27, e15175. [Google Scholar] [CrossRef]
- Li, X.; Deng, X.; Duan, H.; Zeng, L.; Zhou, J.; Liu, C.; Guo, X.; Liu, X. Clinical Features Associated with Pregnancy Outcomes in Women with Positive Antiphospholipid Antibodies and Previous Adverse Pregnancy Outcomes: A Real-World Prospective Study. Clin. Rheumatol. 2021, 40, 193–204. [Google Scholar] [CrossRef]
- Di Simone, N.; Caliandro, D.; Castellani, R.; Ferrazzani, S.; De Carolis, S.; Caruso, A. Low-Molecular Weight Heparin Restores in-Vitro Trophoblast Invasiveness and Differentiation in Presence of Immunoglobulin G Fractions Obtained from Patients with Antiphospholipid Syndrome. Hum. Reprod. 1999, 14, 489–495. [Google Scholar] [CrossRef]
- Mulla, M.J.; Brosens, J.J.; Chamley, L.W.; Giles, I.; Pericleous, C.; Rahman, A.; Joyce, S.K.; Panda, B.; Paidas, M.J.; Abrahams, V.M. Antiphospholipid Antibodies Induce a Pro-Inflammatory Response in First Trimester Trophoblast via the TLR4/MyD88 Pathway. Am. J. Reprod. Immunol. 2009, 62, 96–111. [Google Scholar] [CrossRef]
- Kravvariti, E.; Koutsogianni, A.; Samoli, E.; Sfikakis, P.P.; Tektonidou, M.G. The Effect of Hydroxychloroquine on Thrombosis Prevention and Antiphospholipid Antibody Levels in Primary Antiphospholipid Syndrome: A Pilot Open Label Randomized Prospective Study. Autoimmun. Rev. 2020, 19, 102491. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Očková, M.; Anunciación-Llunell, A.; Andrada, C.; Esteve-Valverde, E.; Miró-Mur, F.; Alijotas-Reig, J. IgM Antiphospholipid Antibodies in Antiphospholipid Syndrome: Prevalence, Clinical Associations, and Diagnostic Implications—A Scoping Review. J. Clin. Med. 2025, 14, 7164. https://doi.org/10.3390/jcm14207164
Očková M, Anunciación-Llunell A, Andrada C, Esteve-Valverde E, Miró-Mur F, Alijotas-Reig J. IgM Antiphospholipid Antibodies in Antiphospholipid Syndrome: Prevalence, Clinical Associations, and Diagnostic Implications—A Scoping Review. Journal of Clinical Medicine. 2025; 14(20):7164. https://doi.org/10.3390/jcm14207164
Chicago/Turabian StyleOčková, Monika, Ariadna Anunciación-Llunell, Catalina Andrada, Enrique Esteve-Valverde, Francesc Miró-Mur, and Jaume Alijotas-Reig. 2025. "IgM Antiphospholipid Antibodies in Antiphospholipid Syndrome: Prevalence, Clinical Associations, and Diagnostic Implications—A Scoping Review" Journal of Clinical Medicine 14, no. 20: 7164. https://doi.org/10.3390/jcm14207164
APA StyleOčková, M., Anunciación-Llunell, A., Andrada, C., Esteve-Valverde, E., Miró-Mur, F., & Alijotas-Reig, J. (2025). IgM Antiphospholipid Antibodies in Antiphospholipid Syndrome: Prevalence, Clinical Associations, and Diagnostic Implications—A Scoping Review. Journal of Clinical Medicine, 14(20), 7164. https://doi.org/10.3390/jcm14207164






