Enhancing Postoperative Evaluation of Presbyopia Corrections: Correlation of Visual Curve Indices with Vision-Related Quality of Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Participants
2.3. Data Collection
2.4. AoC Estimation
2.5. Statistical Analysis
3. Results
3.1. Normality Test
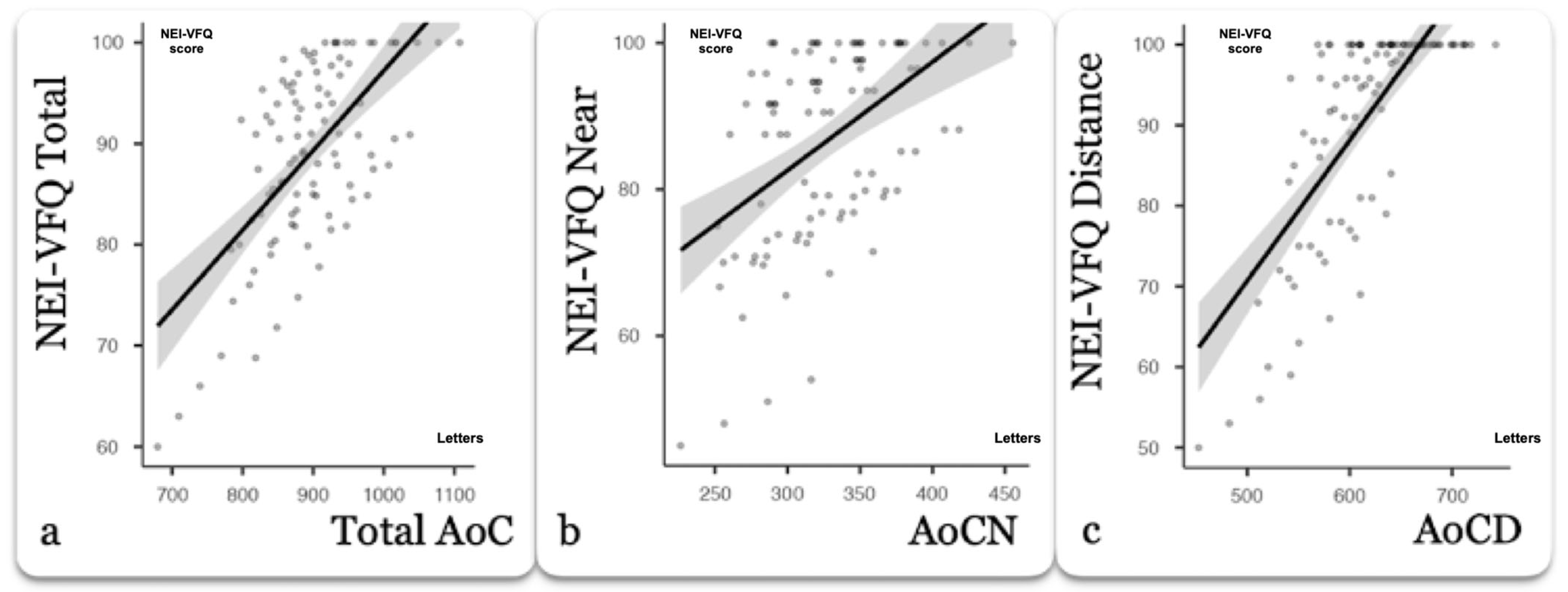
3.2. Correlation Coefficient
3.3. Lower Vision-Related QoL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VA | Visual Acuity |
| ViC | Visual Curve |
| AoC | Area of the Curve |
| DDART | Democritus Digital Acuity and Reading Test |
| NEI-VFQ 25 | National Eye Institute Visual Functioning Questionnaire 25 |
| IOL | Intraocular Lens |
| logMAR | Logarithm of the Minimum Angle of Resolution |
| AoCN | Near Vision Area of the Curve |
| AoCD | Distance Vision Area of the Curve |
| NA | Near Activities |
| DA | Distance Activities |
| DCT | Defocus Curve Testing |
| CI | Confidence Interval |
| PROMs | Patient-Reported Outcome Measures |
References
- Wolffsohn, J.S.; Davies, L.N.; Sheppard, A.L. New Insights in Presbyopia: Impact of Correction Strategies. BMJ Open Ophthalmol. 2023, 8, e001122. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Davies, L.N. Presbyopia: Effectiveness of Correction Strategies. Prog. Retin. Eye Res. 2019, 68, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N.; Reddy, P.A.; Mackenzie, G.; Golgate, P.; Wen, Q.; Clarke, M. Presbyopia and the Sustainable Development Goals. Lancet Glob. Health 2018, 6, e1067. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Sriganesh, S.S. Laser Refractive Correction of Presbyopia. Indian J. Ophthalmol. 2024, 72, 1236–1243. [Google Scholar] [CrossRef]
- McDonald, M.B.; Barnett, M.; Gaddie, I.B.; Karpecki, P.; Mah, F.; Nichols, K.K.; Trattler, W.B. Classification of Presbyopia by Severity. Ophthalmol. Ther. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Davidson, R.S.; Dhaliwal, D.; Hamilton, D.R.; Jackson, M.; Patterson, L.; Stonecipher, K.; Yoo, S.H.; Braga-Mele, R.; Donaldson, K. Surgical Correction of Presbyopia. J. Cataract. Refract. Surg. 2016, 42, 920–930. [Google Scholar] [CrossRef]
- Rodríguez-Vallejo, M.; Burguera, N.; Rocha-de-Lossada, C.; Aramberri, J.; Fernández, J. Refraction and Defocus Curves in Eyes with Monofocal and Multifocal Intraocular Lenses. J. Optom. 2023, 16, 236–243. [Google Scholar] [CrossRef]
- Law, E.M.; Buckhurst, H.D.; Aggarwal, R.K.; El-Kasaby, H.; Marsden, J.; Shum, G.L.; Buckhurst, P.J. Optimising Curve Fitting Techniques to Look for Standardisation of the Analysis of Defocus Curves Derived from Multifocal Intraocular Lenses. Ophthalmic Physiol. Opt. 2022, 42, 887–896. [Google Scholar] [CrossRef]
- Shafer, B.M.; Puls-Boever, K.; Berdahl, J.P.; Thompson, V.; Ibach, M.J.; Zimprich, L.L.; Schweitzer, J.A. Defocus Curve of Emerging Presbyopic Patients. Clin. Ophthalmol. 2023, 17, 843–847. [Google Scholar] [CrossRef]
- Labiris, G.; Panagis, C.; Ntonti, P.; Konstantinidis, A.; Bakirtzis, M. Mix-and-Match vs Bilateral Trifocal and Bilateral EDOF Intraocular Lens Implantation: The Spline Curve Battle. J. Cataract. Refract. Surg. 2024, 50, 167–173. [Google Scholar] [CrossRef]
- Kohnen, T.; Lemp-Hull, J.; Suryakumar, R. Defocus Curves: Focusing on Factors Influencing Assessment. J. Cataract. Refract. Surg. 2022, 48, 961–968. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Jinabhai, A.N.; Kingsnorth, A.; Sheppard, A.L.; Naroo, S.A.; Shah, S.; Buckhurst, P.; Hall, L.A.; Young, G. Exploring the Optimum Step Size for Defocus Curves. J. Cataract. Refract. Surg. 2013, 39, 873–880. [Google Scholar] [CrossRef]
- Gupta, N.; Wolffsohn, J.S.; Naroo, S.A. Optimizing Measurement of Subjective Amplitude of Accommodation with Defocus Curves. J. Cataract. Refract. Surg. 2008, 34, 1329–1338. [Google Scholar] [CrossRef]
- Böhm, M.; Petermann, K.; Hemkeppler, E.; Kohnen, T. Defocus Curves of 4 Presbyopia-Correcting IOL Designs: Diffractive Panfocal, Diffractive Trifocal, Segmental Refractive, and Extended-Depth-of-Focus. J. Cataract. Refract. Surg. 2019, 45, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Labiris, G.; Panagiotopoulou, E.K.; Delibasis, K.; Duzha, E.; Bakirtzis, M.; Panagis, C.; Boboridis, K.; Mokka, A.; Balidis, M.; Damtsi, C.; et al. Validation of a Web-Based Distance Visual Acuity Test. J. Cataract. Refract. Surg. 2023, 49, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Labiris, G.; Bakirtzis, M.; Panagis, C.; Mitsi, C.; Vorgiazidou, E.; Konstantinidis, A.; Delibasis, K.K. Revisiting the Visual Acuity Curves: A Proposed Methodology for the Evaluation of Postoperative Visual Acuity in Presbyopia. Clin. Ophthalmol. 2024, 18, 3935–3947. [Google Scholar] [CrossRef] [PubMed]
- Labiris, G.; Katsanos, A.; Fanariotis, M.; Tsirouki, T.; Pefkianaki, M.; Chatzoulis, D.; Tsironi, E. Psychometric Properties of the Greek Version of the NEI-VFQ 25. BMC Ophthalmol. 2008, 8, 4. [Google Scholar] [CrossRef]
- Khokhar, S. Pearls on Choosing Presbyopia Correction IOLs. Indian J. Ophthalmol. 2024, 72, 1233–1235. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhong, Y.; Fu, Y. The Effects of Premium Intraocular Lenses on Presbyopia Treatments. Adv. Ophthalmol. Pract. Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Yoo, S.H.; Zein, M. Vision Restoration: Cataract Surgery and Surgical Correction of Myopia, Hyperopia, and Presbyopia. Med. Clin. N. Am. 2021, 105, 445–454. [Google Scholar] [CrossRef]
- Cho, J.Y.; Won, Y.K.; Park, J.; Nam, J.H.; Hong, J.Y.; Min, S.; Kim, N.; Chung, T.Y.; Lee, E.K.; Kwon, S.H.; et al. Visual Outcomes and Optical Quality of Accommodative, Multifocal, Extended Depth-of-Focus, and Monofocal Intraocular Lenses in Presbyopia-Correcting Cataract Surgery: A Systematic Review and Bayesian Network Meta-Analysis. JAMA Ophthalmol. 2022, 140, 1045–1053. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Y.; Yu, J.; Ren, X.; Li, Y.; Qiu, W.; Li, X. Comparison of Dynamic Defocus Curve on Cataract Patients Implanting Extended Depth of Focus and Monofocal Intraocular Lens. Eye Vis. 2023, 10, 5. [Google Scholar] [CrossRef]
- Lan, Q.; Xu, F.; Sun, T.; Zeng, S.; Liu, Y.; Yang, T.; Li, Y.; Yao, G.; Ma, B.; Tao, L.; et al. Comparison of Binocular Visual Quality in Six Treatment Protocols for Bilateral Cataract Surgery with Presbyopia Correction: A Prospective Two-Center Single-Blinded Cohort Study. Ann. Med. 2023, 55, 2258894. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Yan, W.; Baur, I.D.; Khoramnia, R.; Auffarth, G.U. Comparison of Five Presbyopia-Correcting Intraocular Lenses: Optical-Bench Assessment with Visual-Quality Simulation. J. Clin. Med. 2023, 12, 2523. [Google Scholar] [CrossRef] [PubMed]
- Buckhurst, P.J.; Wolffsohn, J.S.; Naroo, S.A.; Davies, L.N.; Bhogal, G.K.; Kipioti, A.; Shah, S. Multifocal Intraocular Lens Differentiation Using Defocus Curves. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3920–3926. [Google Scholar] [CrossRef]
- Terauchi, R.; Horiguchi, H.; Ogawa, S.; Sano, K.; Ogawa, T.; Shiba, T.; Nakano, T. Age-Related Visual Outcomes in Eyes with Diffractive Multifocal Intraocular Lenses. Eye 2022, 36, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Lapid-Gortzak, R.; Bala, C.; Schwiegerling, J.; Suryakumar, R. New Methodology for Measuring Intraocular Lens Performance Using Acuity Reserve. J. Cataract. Refract. Surg. 2021, 47, 1006–1010. [Google Scholar] [CrossRef]
| Feature | p-Value |
|---|---|
| Total ViC’s AoC | 0.7608 |
| ViC’s AoCN | 0.5349 |
| ViC’s AoCD | 0.9086 |
| Total DCT’s AoC | 0.261 |
| DCT’s AoCN | 0.183 |
| DCT’s AoCD | 0.465 |
| NEI-VFQ 25 Score | 0.3555 |
| Near Activities Score | 0.1685 |
| Distance Activities Score | 0.0051 |
| VA at 40 cm | 0.9423 |
| VA at 300 cm | 0.1726 |
| AoC | Num. of VA Actual Measurements for AoC Calculation | NEI-VFQ 25 | Correlation Method | Correlation Coefficient [95% CI] |
|---|---|---|---|---|
| Total AoC | 4 | Total Score | Pearson | 0.668 *** [0.471, 0.841] |
| 5 | Pearson | 0.670 *** [0.473, 0.842] | ||
| 6 | Pearson | 0.684 *** [0.496, 0.849] | ||
| 9 | Pearson | 0.682 *** [0.539, 0.763] | ||
| Conventional DCT | Pearson | 0.664 *** [0.38, 0.834] | ||
| AoCN | 4 | Near Activities Score | Pearson | 0.686 *** 0.399, 0.850] |
| 5 | Pearson | 0.686 *** [0.399, 0.851] | ||
| 6 | Pearson | 0.656 *** [0.355, 0.836] | ||
| 9 | Pearson | 0.686 *** [0.486, 0.868] | ||
| Conventional DCT | Pearson | 0.652 *** [0.362, 0.827] | ||
| 1 (40 cm) | Pearson | 0.596 ** [0.389, 0.737] | ||
| AoCD | 4 | Distance Activities Score | Spearman | 0.733 *** [0.535, 0.892] |
| 5 | Spearman | 0.753 *** [0.645, 0.922] | ||
| 6 | Spearman | 0.744 *** [0.639,0.921] | ||
| 9 | Spearman | 0.758 *** [0.735, 0.952] | ||
| Conventional DCT | Spearman | 0.734 *** [0.669, 0.923] | ||
| 1 (300 cm) | Spearman | 0.621 *** [0.642, 0.916] |
| Group | Mean ± SD | |
|---|---|---|
| NEI-VFQ Total | Lowest quartile | 76.7 ± 6.69 |
| Highest quartiles | 94 ± 5.21 | |
| NEI-VFQ Near | Lowest quartile | 70.8 ± 8.96 |
| Highest quartiles | 93.5 ± 7.31 | |
| NEI-VFQ Distance | Lowest quartile | 73 ± 8.96 |
| Highest quartiles | 93.5 ± 7.31 |
| Indices | NEI-VFQ Scores | Mean ± SD | p Value |
|---|---|---|---|
| Total AoC 9 (Letters/cm) | Lowest quartile | 830 ± 63.9 | <0.001 *** |
| Highest quartiles | 917 ± 63.0 | ||
| Total AoC 4 (Letters/cm) | Lowest quartile | 824 ± 75.0 | <0.001 *** |
| Highest quartiles | 877 ± 58.4 | ||
| Total AoC 5 (Letters/cm) | Lowest quartile | 823 ± 77.4 | <0.001 *** |
| Highest quartiles | 876 ± 59.0 | ||
| Total AoC 6 (Letters/cm) | Lowest quartile | 826 ± 67.6 | <0.001 *** |
| Highest quartiles | 875 ± 52.7 | ||
| Conventional Total AoC (Letters/cm) | Lowest quartile | 823 ± 71.0 | 0.06 |
| Highest quartiles | 874 ± 50.7 | ||
| AoCN 9 (Letters/cm) | Lowest quartile | 286 ± 31.2 | <0.001 *** |
| Highest quartiles | 338 ± 39.9 | ||
| AoCN 4 (Letters/cm) | Lowest quartile | 269 ± 24.1 | <0.001 *** |
| Highest quartiles | 292 ± 25.7 | ||
| AoCN 5 (Letters/cm) | Lowest quartile | 267 ± 23.9 | <0.001 *** |
| Highest quartiles | 293 ± 26.0 | ||
| AoCN 6 (Letters/cm) | Lowest quartile | 271 ± 13.1 | <0.001 *** |
| Highest quartiles | 291 ± 24.1 | ||
| Conventional AoCN (Letters/cm) | Lowest quartile | 262 ± 17.0 | 0.002 ** |
| Highest quartiles | 294 ± 24.7 | ||
| AoCD 9 (Letters/cm) | Lowest quartile | 547 ± 36.7 | <0.001 *** |
| Highest quartiles | 567 ± 33.5 | ||
| AoCD 4 (Letters/cm) | Lowest quartile | 539 ± 39.1 | 0.001 ** |
| Highest quartiles | 588 ± 34.6 | ||
| AoCD 5(Letters/cm) | Lowest quartile | 528 ± 38.1 | <0.001 *** |
| Highest quartiles | 591 ± 36.0 | ||
| AoCD 6 (Letters/cm) | Lowest quartile | 531 ± 33.5 | <0.001 *** |
| Highest quartiles | 584 ± 30.8 | ||
| Conventional AoCD (Letters/cm) | Lowest quartile | 535 ± 33.7 | <0.001 *** |
| Highest quartiles | 584 ± 27.5 | ||
| Age (Years) | Lowest quartile | 66.2 ± 10.2 | 0.66 |
| Highest quartiles | 60.2 ± 6.73 | ||
| Gender | Lowest quartile | NA | 0.075 |
| Highest quartiles | NA | ||
| Preoperative spherical equivalent (D) | Lowest quartile | −0.57 ± 2.02 | 0.12 |
| Highest quartiles | −0.34 ± 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labiris, G.; Panagis, C.; Mitsi, C.; Panagiotopoulou, E.-K.; Vorgiazidou, E.; Delibasis, K.K.; Bakirtzis, M. Enhancing Postoperative Evaluation of Presbyopia Corrections: Correlation of Visual Curve Indices with Vision-Related Quality of Life. J. Clin. Med. 2025, 14, 7149. https://doi.org/10.3390/jcm14207149
Labiris G, Panagis C, Mitsi C, Panagiotopoulou E-K, Vorgiazidou E, Delibasis KK, Bakirtzis M. Enhancing Postoperative Evaluation of Presbyopia Corrections: Correlation of Visual Curve Indices with Vision-Related Quality of Life. Journal of Clinical Medicine. 2025; 14(20):7149. https://doi.org/10.3390/jcm14207149
Chicago/Turabian StyleLabiris, Georgios, Christos Panagis, Christina Mitsi, Eirini-Kanella Panagiotopoulou, Eleftheria Vorgiazidou, Konstantinos K. Delibasis, and Minas Bakirtzis. 2025. "Enhancing Postoperative Evaluation of Presbyopia Corrections: Correlation of Visual Curve Indices with Vision-Related Quality of Life" Journal of Clinical Medicine 14, no. 20: 7149. https://doi.org/10.3390/jcm14207149
APA StyleLabiris, G., Panagis, C., Mitsi, C., Panagiotopoulou, E.-K., Vorgiazidou, E., Delibasis, K. K., & Bakirtzis, M. (2025). Enhancing Postoperative Evaluation of Presbyopia Corrections: Correlation of Visual Curve Indices with Vision-Related Quality of Life. Journal of Clinical Medicine, 14(20), 7149. https://doi.org/10.3390/jcm14207149









