Abstract
Background/Objectives: In patients with malignancy, fluid electrolyte imbalance and renal dysfunction have been demonstrated to increase mortality and morbidity. The objective of this study was to evaluate body composition, and clinical and laboratory tests at baseline and at 3 months during chemotherapy and standard fluid therapy in patients with malignancy. Methods: This study included patients with an ECOG performance status of 0–1 who did not have clinically evident organ failure, brain tumors, or a need for intensive care treatment. All received standard fluid therapy and chemotherapy. Examinations, routine laboratory tests, and body composition measurements were performed at the beginning of chemotherapy and again after three months. Results: The number of hypervolemic patients increased. Although the body mass index (BMI) did not change compared to the baseline, serum levels of B-type natriuretic peptide (BNP), calcium, albumin, total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides increased at the second measurement. By month three, the frequency of overhydration (OH) increased. There was a significant, positive, moderate correlation between the difference in OH and the difference in BNP (p = 0.001). The leukocyte, neutrophil, neutrophil-to-lymphocyte ratio, and neutrophil-to-albumin ratio decreased (p < 0.05 for all). Body composition monitor (BCM) measurements revealed that the extracellular fluid/intracellular fluid ratio (E/I) increased at the second measurement (p = 0.001). Conclusions: The frequency of OH and BNP levels increased at three months. An initial fluid deficit or OH was associated with mortality. OH may mask sarcopenia in patients. Therefore, objective assessment of body composition is important for patient management to avoid OH and predict mortality. However, more studies with larger patient populations and long-term follow-up are needed.
1. Introduction
Cancer is defined as the uncontrolled proliferation of cells as a result of damage to some genetic and metabolic pathways. The prevalence of malnutrition in adult cancer patients ranges from 20% to 87%, contingent on the stage, type, or treatment of cancer [1,2].
Cytotoxic chemotherapy agents are prescribed for both curative and palliative purposes, and their dosage is typically determined using the body surface area (BSA) calculation. However, this method does not accurately reflect the patient’s actual body composition [3,4].
Malnutrition and cachexia, which are frequently seen in patients with malignancy, are important prognostic factors that negatively affect response to treatment [5]. These conditions have been shown to increase treatment side effects and negatively affect survival. Early recognition of malnutrition plays a critical role in improving prognosis [5,6].
Fluid–electrolyte balance disorders may develop in cancer patients due to their treatments and may worsen their clinical course by affecting hemodynamic balance. Therefore, accurately and objectively assessing body composition is important for monitoring patients’ clinical status [7]. A body composition monitor (BCM) is a non-invasive device that can determine the amount of fat, muscle, and lean muscle tissue, as well as intracellular and extracellular fluid distribution. It is useful in clinical settings. BCMs are used to assess fluid balance in dialysis patients, intensive care patients, and, more recently, oncology patients [8,9].
In this context, the aim of this study was to evaluate body composition, clinical parameters and laboratory findings in patients with malignancy before chemotherapy and standard fluid therapies and in the third month of treatment to reveal the changes in the treatment process. Monitoring the changes in muscle and adipose tissue as well as fluid distribution will contribute to the directing of patients to individualized supportive treatment approaches [10].
2. Materials and Methods
2.1. Study Design and Participants
Between 1 June 2023 and 1 December 2023, all 77 patients over the age of 18 who would be treated for the first time in the Medical Oncology chemotherapy unit of Çukurova University Faculty of Medicine, Feyyaz Etiz Oncology-Hematology Hospital were included in the study.
All patients were informed about the study and their written informed consent was obtained. The study was conducted with the approval of the Cukurova University Clinical Research Ethics Committee.
The demographic data, physical examination findings, and routine laboratory test results requested for the purpose of ensuring compliance with chemotherapy were meticulously documented. Body composition analysis was conducted using the BCM (Fresenius Medical Care, Bad Homburg vor der Höhe, Germany) device in accordance with the standard protocols recommended by the manufacturer. The measurements were taken prior to the initiation of chemotherapy and three months following the initial administration. The second measurement, which was independent of the completion time of chemotherapy, was performed at the same time interval for all patients.
In the third-month follow-up, eight patients died. Three patients were transferred to another center due to the earthquake. Three patients could not be reached, and one patient was referred for bone marrow transplantation. Consequently, the third-month measurements were conducted on a total of 62 patients.
2.2. Inclusion and Exclusion Criteria
The selection of patients for inclusion in the study was predicated on the fulfillment of specific criteria. Accordingly, patients who agreed to participate in the study and gave informed consent, were 18 years of age or older, had an ECOG performance status of 0–1, did not actively use alcohol, did not have a diagnosis of significant heart failure, impaired consciousness or brain tumor, and did not use drugs or psychoactive substances without physician approval were included in the study. Conversely, patients exhibiting signs of active infection, substantial organ dysfunction, or clinically significant heart failure necessitating intensive care treatment, hyperbilirubinemia or uremia, or who declined to participate in the study were excluded from the study.
2.3. Body Composition Monitor Measurements
Body composition measurements were performed with the BCM device (Fresenius Medical Care, Germany). The measurements were performed by the same trained researcher in each patient, in accordance with the manufacturer’s instructions. Prior to the procedure, the patients’ skin surfaces were meticulously cleansed, and the electrodes were strategically positioned on the wrists and ankles of the patients on the same side. Patient information such as height (centimeters), weight (kilograms), gender, and arterial blood pressure (systolic/diastolic, millimeters of mercury) were entered into the device.
During the measurement procedures with BCM, patients were evaluated in a supine position, having fasted and been given a sufficient amount of time to rest. Subsequent to the successful establishment of a connection to the device, the measurement commenced and was concluded within approximately two minutes. The data obtained were recorded on the patient card and transferred to the computer Via the Fluid Management Tool (FMT) of the BCM (Figure 1).
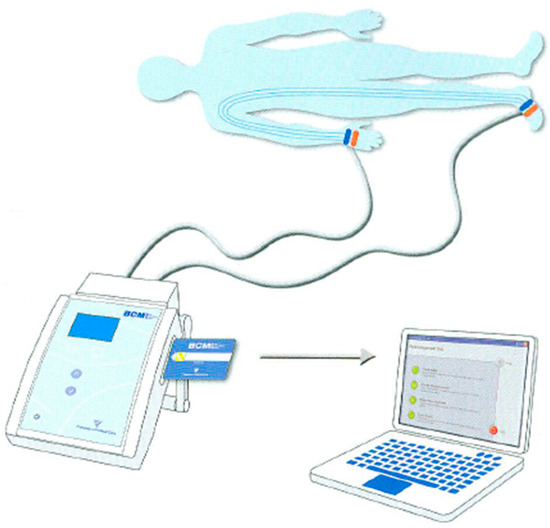
Figure 1.
Patient connection diagram and the data transfer process in measurement with the BCM.
The BCM system is a bioimpedance spectroscopy (BIS)-based apparatus that measures 50 distinct frequencies ranging from 5 kHz to 1000 kHz. It utilizes this data to calculate a variety of physiological parameters, including total body fluid (TBF), intracellular fluid (ICW), extracellular fluid (ECW), lean tissue index (LTI), fat tissue index (FTI), overhydration (OH), and body mass. The measurement time was approximately two minutes and was performed noninvasively.
Overhydration (OH) was defined according to the manufacturer’s validated BCM thresholds as an absolute fluid overload > 1.1 L. Patients were further classified as normovolemic, hypervolemic, or hypovolemic based on extracellular water (ECW) and intracellular water (ICW) distributions. These cut-offs were consistent with previously published studies validating BCM against isotope dilution and clinical fluid status assessment.
2.4. Laboratory Tests
Complete blood counts were performed with a Beckman Coulter DXH device (Beckman Coulter, Inc., Brea, CA, USA) and biochemical tests were performed with a Beckman Coulter AU5800 analyzer (Beckman Coulter, Inc., Brea, CA, USA). N-terminal pro B-type natriuretic peptide (NT-proBNP) levels were determined with the Elecsys proBNP II test kit using a Roche Diagnostics Cobas E411 device (Roche Diagnostics International Ltd., Rotkreuz, Switzerland).
2.5. Statistical Analysis
All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 22.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics are presented as mean ± standard deviation, median (minimum-maximum), frequency and percentage.
For normally distributed continuous variables, we applied paired samples t-test, as it is appropriate for comparing means between two related measurements. For non-normally distributed continuous variables, we used the Wilcoxon signed-rank test to compare paired medians. For categorical variables, we employed the Chi-square test to evaluate changes in proportions between baseline and month 3. A value of p < 0.05 was considered statistically significant in all tests.
3. Results
3.1. Patient Characteristics
The mean age of the 77 patients included in the study was 56.18 ± 12.15 years and the age range was 23–87 years. 59.7% of the participants were female (n = 46) and 40.3% were male (n = 31).
The most prevalent malignancies identified in the study were lung cancer (35%) and breast cancer (32.4%). Hypertension (19.5%) and diabetes mellitus (18.2%) were the most prevalent comorbid diseases.
3.2. Clinical and Laboratory Findings
The BMI was 27.21 ± 4.58 kg/m2 at the beginning of chemotherapy and 26.68 ± 4.64 kg/m2 at month 3; the change was not statistically significant (p = 0.056).
Significant changes were observed in serum albumin levels, total protein, potassium, magnesium and chlorine values by the third month. In particular, the proportion of patients with albumin levels below 40 g/L decreased from 59.7% at baseline to 41.9% in the third month (p = 0.037). No significant difference was found in the sodium level (p = 0.131).
An analysis was conducted for the inflammatory biomarker ratios obtained prior to chemotherapy and at the conclusion of the third month. The median value of the platelet/albumin ratio was determined to be 12.4 (5.4–14.9) at baseline and 11.45 (6.3–14.8) at the third month. This decrease was found to be statistically significant (p = 0.012). In a similar manner, the neutrophil/albumin ratio was 149.91 (32.54–2130.14) at baseline and 87.49 (11.9–325.43) at the third month, therefore having significantly decreased (p = 0.001). However, the neutrophil/lymphocyte ratio was 213.85 (5.07–1034) at baseline and 195.92 (79.47–2040) in the third month, with no significant difference observed between the groups (p = 0.814). A subsequent analysis of serum ferritin levels revealed that while the median baseline value was 54.5 (5.3–1198.9) ng/mL, it underwent a significant increase to 109.7 (6.5–762.3) ng/mL in the third month (p = 0.001).
A substantial decline was evident in the following parameters: hemoglobin, haematocrit, WBC, neutrophil, and platelet counts. A significant increase in ferritin levels was observed (p = 0.001). A substantial increase was identified in MCV (p = 0.001). A significant increase was observed in total cholesterol, HDL, and triglyceride levels. A significant decrease in systolic blood pressure was observed (p = 0.019).
The data obtained were analyzed statistically, and the significance levels are presented in Table 1.

Table 1.
Hematological and blood pressure changes between baseline and month 3.
Biochemical parameters (Table 2) showed significant improvements in serum albumin levels and reductions in total protein, potassium, magnesium, and BUN. Total bilirubin levels increased slightly but significantly. Lipid profile changes included increases in total cholesterol, HDL, and triglycerides. No significant changes were observed in sodium, renal function (creatinine, GFR), or most liver enzymes.

Table 2.
Biochemical and Electrolyte Parameters: Baseline Vs. Month 3.
3.3. BCM Findings
A comparison of body composition measurements obtained at the onset of chemotherapy and at the third month of treatment revealed a median initial Qualification value of 92% (52–98) and a third-month value of 95% (75–99). This increase was found to be statistically significant (p = 0.001). The median ECW value was recorded as 15 (9–27.5) L at baseline and 15.5 (9.3–22.7) L at the third month. The difference between these values was not found to be statistically significant (p = 0.058). The median ICW (intracellular fluid) levels at baseline were found to be 15.95 (10.3–43.2) L, and these levels decreased to 15.6 (10.2–26.5) L by the third month. A subsequent analysis revealed that there was no significant difference between the groups (p = 0.069). Conversely, the E/I ratio (extracellular/intracellular fluid ratio) exhibited a significant increase in the third month (p = 0.001), signifying an augmentation in extracellular fluid.
Subsequent to the analysis of muscle and adipose tissue parameters, it was found that there was no statistically significant difference between the third month and baseline values of LTI (lean tissue index), FTI (fat tissue index), FAT (total fat mass), LTM (lean tissue mass), ATM (adipose tissue mass) and BCM (body cell mass) measurements (p > 0.05 for all).
The overhydration (OH) rate was 61% (47/77) at the onset of chemotherapy and increased to 79% (49/62) at the third month. While the number of normovolemic individuals was limited to only four, the number of normovolemic patients reached 13 in the third month, and no hypovolemic individuals were observed. A substantial rise in the proportion of hypervolaemic patients was observed in the third month. Of the eight patients who exited, six had overhydration and two had hypovolaemia. Baseline overhydration was more frequent among patients who died compared with survivors (62.5% vs. 30.0%), although the difference did not reach statistical significance due to the small sample size (p = 0.08). No significant association was observed between electrolyte disturbances and mortality.
Patients lost to follow-up by month 3 (n = 15) were excluded from paired comparisons, and no imputation was performed for missing data.
The median baseline OH (EC-EB) was 0.45 (interquartile range: −12.6–18.7) L, and the median 3rd month OH (EC-EB) was 0.51 (interquartile range: −1.1–11.1) L. A significant difference was found between the groups in terms of OH values (p = 0.001). The third-month OH value was higher than the baseline OH value.
When the distribution of overhydration difference according to gender was analyzed, the median value was 0.65 L (−1.5–2.4) in females and 1.3 L (−6.4–9.9) in males, and no significant difference was observed between the groups (p = 0.132). When evaluated according to chemotherapy regimen, the difference in OH was 1.25 L (−6.4–9.9) in patients treated with cisplatin or carboplatin and 0.65 L (−1.1–2.9) in patients treated with other agents. The difference was not statistically significant (p = 0.325). The disparity in mean oxygen levels (OH) among patients with distinct tumor subtypes was examined, revealing a mean difference of 0.75 L (0–2.9) in cases of lymphoma and 0.75 L (−6.4–9.9) in patients without lymphoma. Subsequent analysis revealed no statistically significant difference between these groups (p = 0.807) (Table 3).

Table 3.
BCM Parameters: Comparison Between Baseline and Month 3.
An evaluation of the correlations between overhydration difference and several clinical parameters yielded a significant, negative, and low-level correlation between the OH difference and the baseline Qualification Value (p = 0.002). Conversely, a significant positive yet low correlation was observed between the third month Qualification value and OH difference (p = 0.017). No statistically significant correlation was observed between the OH difference and the baseline KxT/V value (p = 0.727). A significant negative moderate correlation was identified between the OH difference and the baseline E/I ratio (p = 0.001). Furthermore, a significant positive and moderate correlation was observed between the OH difference and the BNP difference (p = 0.001). A significant, positive, low-level correlation was also identified between OH difference and baseline calcium level (p = 0.016). Conversely, no substantial correlation was identified between baseline and third-month OH levels and systolic and diastolic blood pressure, glucose, sodium, total bilirubin, direct bilirubin, ALP, AST, ALT, GGT, LDH, potassium, chlorine, magnesium, phosphorus, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol and ferritin levels (p > 0.05 for all, Spearman’s Rho Correlation Test used) (Table 4).

Table 4.
Correlation of Overhydration Difference with Selected Clinical Parameters.
In subgroup analysis according to baseline serum sodium level, the median OH difference was 0.7 L (−4.8–3.2) in patients with <135 mEq/L and 0.8 L (−6.4–9.9) in patients with ≥135 mEq/L and there was no significant difference between them (p = 0.918). Similarly, in the analysis performed according to the baseline albumin level, the median OH difference was calculated as 0.8 L (−6.4–9.9) in patients <40 g/L and 0.75 L (−1.1–3) in patients ≥ 40 g/L and no statistically significant difference was found (p = 0.782).
4. Discussion
The incidence and mortality rates of cancer are increasing rapidly worldwide [11]. The aim of this study was to evaluate fluid status and electrolyte changes in patients with malignant disease at the beginning of treatment and after three months. A clinical evaluation was also performed at the time of body cell mass (BCM) measurement. There was a statistically significant decrease in hemoglobin and hematocrit values after three months, compared to the baseline, as well as an increase in the overhydration value, which may have been dilutional. Chemotherapy-related bone marrow toxicity is another possibility. The decrease in white blood cell and neutrophil counts, as well as the decrease in platelet values without a change in lymphocyte values, may have been due to an improvement in the initial inflammation or chemotherapy-related bone marrow toxicity. A significant increase in the MCV value at month three (although these values are within the normal range) may have been related to the improvement in initial inflammation or a deficiency of folic acid and vitamin B12 due to chemotherapy. In the third month, there may have been a relationship between increased overhydration and MCV; however, there was no increase in the ICW value or hyponatremia, though there was an increase in the E/I ratio.
OH increased in the third month of chemotherapy (p < 0.001). This may have been related with chemotherapy and the recommendations for standard fluid intake and excessive fluid intake in the follow-ups, indicating an increase in the total body fluid amount. The fluid deficiency we detected at baseline was not detected in the third month.
Cancer patients are generally at risk of developing cachexia as a result of progressive weight loss, loss of muscle and adipose tissue, impaired immune function, decreased food intake, systemic inflammation and abnormal metabolism [12,13]. Many studies have reported that muscle wasting is a strong predictor of the development of chemotherapy toxicity [14,15].
During the treatment and follow-up of our patients, we detected fluid overhydration in the third month. However, this did not adversely affect renal, cardiac, electrolyte, or liver function during the three-month follow-up period. Along with the overhydration, there was a slight increase in BNP values without hyponatremia; however, this increase was not statistically significant. However, there was a significant correlation between the difference in overhydration and the difference in BNP. Due to an insignificant decrease in ICW and an increase in ECW without a change in BMI, LTI, FTI, FAT, LTM, or ATM, it can be concluded that the patients did not develop sarcopenia within the three-month period.
In our study, the increase in OH frequency and slight elevation in BNP levels during chemotherapy, when evaluated in conjunction with the positive and significant correlation between the two variables, suggests that excessive fluid overload may contribute to cardiac stress. The fact that the increase in BNP is related to changes in fluid balance rather than being tumor-related is clinically important, especially in oncology patients with limited cardiac reserve. This situation suggests that early detection of excessive hydration in patients receiving routine fluid therapy during chemotherapy may contribute to the prevention of possible cardiac complications. Additionally, considering that OH may mask muscle mass loss relative to body weight, it was concluded that objective body composition measurements such as BCM could be a valuable tool both in preventing excessive hydration and in predicting mortality risk.
The prevalence of fluid electrolyte and acid-base disorders in cancer patients has been reported to be 58%. This rate is much higher than the rates seen in the general population, in patients admitted to the emergency department for any reason (13.7%) and in the elderly (22%). In a study, lower body surface area, presence of comorbidities such as hypertension, diabetes mellitus, renal failure, anemia, hypoalbuminemia, history of surgery and chemotherapy were found to be major risk factors for electrolyte and acid-base disturbances in cancer patients [16].
Cancer is one of the most common causes of hyponatremia in hospitalized patients. In a prospective observational study, the rate of hyponatremia was reported as 14% [17]. As is the case with the general population, lower sodium levels were found to be associated with a prolonged duration of hospitalization and an elevated 90-day mortality rate [18,19]. In the present study, hyponatraemia was identified as the most prevalent electrolyte imbalance at the commencement of treatment. Tas Et Al. reported that serum sodium concentration had no effect on survival in patients with small cell lung cancer, and therefore concluded that the presence and severity of hyponatraemia had no effect on the outcome of these patients [20].
Hypercalcaemia is a prevalent electrolyte disorder observed in cancer patients. Hypercalcaemia has been observed in 20–30% of cancer patients during the course of the disease [21]. Hypercalcaemia in cancer patients is generally indicative of a poor prognosis.
The decrease in BUN value in the third month of our patients, despite improvement in nutrition or steroid use during chemotherapy, may be related to clinical improvement or malnutrition. It is hypothesized that the absence of change in creatinine value may serve to reduce the likelihood of renal failure. However, it should be noted that malnutrition is a prevalent issue among cancer patients [22].
The prevalence of malnutrition in adult cancer patients ranges from 20% to 87%, contingent on the stage, type, or treatment of cancer [1,2]. Sarcopenia is a condition that invariably worsens with age. Consequently, it is imperative to detect and prevent sarcopenia and malnutrition at the onset of cancer treatment [23,24].
NT-proBNP and troponin T have been identified as valuable markers in the prognosis of oncological diseases, not only in terms of cardiac damage during chemotherapy, but also in terms of prognosis and prolonging the survival of cancer patients [25].
The presence of fluid deficiency or excess in patients was directly correlated with mortality. The presence of pathological findings in fat and muscle mass, as indicated by body composition analysis, is a significant component of a comprehensive clinical evaluation. In a multicentre study excess weight and obesity in 73% of patients, and body composition disorder and decreased muscle mass in the same group. Consequently, the increase in adipose tissue may obscure the decrease in muscle mass [26].
Limitations
This study has several limitations. First, its single-center design and modest sample size limit generalizability. The three-month observation period may be insufficient to detect long-term outcomes, such as mortality or sarcopenia. The absence of a control group (e.g., non-cancer or non-chemotherapy patients) limits our ability to distinguish chemotherapy-specific changes from other factors.
5. Conclusions
At the 3-month mark, signs of inflammation showed improvement in the surviving patients, while serum BNP levels and OH levels increased. It has been established that the presence of hypohypervolemia at the commencement of chemotherapy treatment is associated with an elevated mortality rate. Consequently, the evaluation of body composition by objective methods, such as BCM, to predict mortality and to avoid OH, may facilitate patient management. However, further research is required, involving a larger number of patients and long-term follow-up.
Author Contributions
Conceptualization, M.T.O.; methodology, M.T.O.; formal analysis, M.T.O. and E.B.; investigation, M.T.O.; data curation, M.T.O.; writing—original draft preparation, M.T.O.; writing—review and editing, M.T.O., E.B., S.P. (Saime Paydas), and S.P. (Semra Paydas); supervision, S.P. (Saime Paydas) and S.P. (Semra Paydas). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by Cukurova University Clinical Research Ethics Committee (Resolution No. 30 adopted at meeting No. 134 of 2 June 2023) and was conducted only with volunteer participants in accordance with the principles of the Declaration of Helsinki. The application was submitted well before the patient meeting, and the necessary permissions were obtained, but the ethics committee documents were issued late due to the committee’s late meeting.
Informed Consent Statement
Participants were informed in detail and signed written informed consent forms.
Data Availability Statement
Data are available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| ATM | Adipose Tissue Mass |
| BCM | Body Composition Monitor |
| Bcm | Body Cell Mass |
| BIS | Bioimpedance Spectroscopy |
| BMI/VKİ | Body Mass Index |
| BNP | B-type Natriuretic Peptide |
| BUN | Blood Urea Nitrogen |
| EB | Maximum Value |
| ECOG | Eastern Cooperative Oncology Group |
| ECW/ES | Extracellular Water |
| EK | Minimum Value |
| E/I | Extracellular to Intracellular Water Ratio |
| FAT | Fat Mass |
| FMT | Fluid Management Tool |
| FTI | Fat Tissue Index |
| FTM | Fat Tissue Mass |
| GFR | Glomerular Filtration Rate |
| GGT | Gamma-Glutamyl Transferase |
| GLOBOCAN | Global Cancer Statistics |
| HDL | High-Density Lipoprotein |
| IARC | International Agency for Research on Cancer |
| ICW/IS | Intracellular Water |
| KKY | Congestive Heart Failure |
| KOAH | Chronic Obstructive Pulmonary Disease |
| LDH | Lactate Dehydrogenase |
| LDL | Low-Density Lipoprotein |
| LTI | Lean Tissue Index |
| LTM | Lean Tissue Mass |
| MCV | Mean Corpuscular Volume |
| OH | Overhydration |
| PC | Personal Computer |
| PDW | Platelet Distribution Width |
| PS | Performance Status |
| PTHrp | Parathyroid Hormone-related Peptide |
| SPSS | Statistical Package for the Social Sciences |
| TBW | Total Body Water |
| TNM | Tumor, Node, Metastasis Classification |
| TVS | Total Body Fluid |
| WBC | White Blood Cell Count |
References
- Beirer, A. Malnutrition and cancer, diagnosis and treatment. Memo—Mag. Eur. Med. Oncol. 2021, 14, 168–173. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Mehra, K.; Berkowitz, A.; Sanft, T. Diet, Physical Activity, and Body Weight in Cancer Survivorship. Med. Clin. N. Am. 2017, 101, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Stobäus, N.; Küpferling, S.; Lorenz, M.L.; Norman, K. Discrepancy between body surface area and body composition in cancer. Nutr. Cancer 2013, 65, 1151–1156. [Google Scholar] [CrossRef]
- Gangadharan, A.; Choi, S.E.; Hassan, A.; Ayoub, N.M.; Durante, G.; Balwani, S.; Kim, Y.H.; Pecora, A.; Goy, A.; Suh, K.S. Protein calorie malnutrition, nutritional intervention and personalized cancer care. Oncotarget 2017, 8, 24009–24030. [Google Scholar] [CrossRef]
- Mareschal, J.; Achamrah, N.; Norman, K.; Genton, L. Clinical Value of Muscle Mass Assessment in Clinical Conditions Associated with Malnutrition. J. Clin. Med. 2019, 8, 1040. [Google Scholar] [CrossRef]
- Latcha, S. Electrolyte Disorders in Cancer Patients. In Onconephrology: Cancer, Chemotherapy and the Kidney; Jhaveri, K.D., Salahudeen, A.K., Eds.; Springer: New York, NY, USA, 2015; pp. 131–162. [Google Scholar]
- Shah, U.A.; Ballinger, T.J.; Bhandari, R.; Dieli-Conwright, C.M.; Guertin, K.A.; Hibler, E.A.; Kalam, F.; Lohmann, A.E.; Ippolito, J.E. Imaging modalities for measuring body composition in patients with cancer: Opportunities and challenges. JNCI Monogr. 2023, 2023, 56–67. [Google Scholar] [CrossRef]
- Keane, D.; Chamney, P.; Heinke, S.; Lindley, E. Use of the Body Composition Monitor for Fluid Status Measurements in Subjects with High Body Mass Index. Nephron 2016, 133, 163–168. [Google Scholar] [CrossRef]
- Wang, J.; Tan, S.; Gianotti, L.; Wu, G. Evaluation and management of body composition changes in cancer patients. Nutrition 2023, 114, 112132. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Rier, H.N.; Jager, A.; Sleijfer, S.; Maier, A.B.; Levin, M.-D. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist 2016, 21, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Fabbro, E.d.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef] [PubMed]
- Siff, T.; Parajuli, P.; Razzaque, M.S.; Atfi, A. Cancer-Mediated Muscle Cachexia: Etiology and Clinical Management. Trends Endocrinol. Metab. 2021, 32, 382–402. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Shen, Z.; Wang, Y.; Hu, J.; Xu, J.; Shen, B.; Ding, X. Electrolyte and acid-base disorders in cancer patients and its impact on clinical outcomes: Evidence from a real-world study in China. Ren. Fail. 2020, 42, 234–243. [Google Scholar] [CrossRef]
- Sangamesh, S.; BG, K. A Study on Incidence and Etiology of Hyponatremia in Hospitalised Patients in A Tertiary Care Hospital. Eur. J. Cardiovasc. Med. 2023, 13, 1830–1836. [Google Scholar]
- Doshi, S.M.; Shah, P.; Lei, X.; Lahoti, A.; Salahudeen, A.K. Hyponatremia in Hospitalized Cancer Patients and Its Impact on Clinical Outcomes. Am. J. Kidney Dis. 2012, 59, 222–228. [Google Scholar] [CrossRef]
- Rosner, M.H.; Jhaveri, K.D.; McMahon, B.A.; Perazella, M.A. Onconephrology: The intersections between the kidney and cancer. CA Cancer J. Clin. 2021, 71, 47–77. [Google Scholar] [CrossRef]
- Tas, F.; Ozturk, A.; Erturk, K. Neither the presence nor the severity of hyponatremia affected the outcome of the patients with small cell lung cancer. Ir. J. Med. Sci. 2023, 192, 1613–1619. [Google Scholar] [CrossRef]
- Sheehan, M.T.; Wermers, R.A.; Jatoi, A.; Loprinzi, C.L.; Onitilo, A.A. Oral cinacalcet responsiveness in non-parathyroid hormone mediated hypercalcemia of malignancy. Med. Hypotheses 2020, 143, 110149. [Google Scholar] [CrossRef]
- Shen, F.; Ma, Y.; Guo, W.; Li, F. Prognostic Value of Geriatric Nutritional Risk Index for Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Lung 2022, 200, 661–669. [Google Scholar] [CrossRef]
- Aytulu, T.; Selçukbiricik, F.; Çalikoğlu, F.; Yilmaz, M.; Ergene, G.; Işsever, H.; Satman, I. Evaluation of sarcopenia, malnutrition and nutritional status of patients with and without cancer treatment by age. Sağlık Bilim. İleri Araştırmalar Derg./J. Adv. Res. Health Sci. 2022, 5, 6–13. [Google Scholar]
- Bizzarri, F.P.; Nelson, A.W.; Colquhoun, A.J.; Lobo, N. Utility of Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Detecting Lymph Node Involvement in Comparison to Conventional Imaging in Patients with Bladder Cancer with Variant Histology. Eur. Urol. Oncol. 2025. [Google Scholar] [CrossRef]
- Chovanec, J.; Chovanec, M.; Mego, M. Levels of NT-proBNP and troponin T in cancer patients-mini-review. Klin. Onkol. 2020, 33, 171–176. [Google Scholar] [CrossRef]
- Sullivan, E.S.; Daly, L.E.; Scannell, C.; Ní Bhuachalla, É.B.; Cushen, S.; Power, D.G.; Ryan, A.M. A large, multi-centre prospective study demonstrating high prevalence of malnutrition associated with reduced survival in ambulatory systemic anti-cancer therapy patients. Clin. Nutr. ESPEN 2022, 52, 208–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).