Artificial Intelligence for Adenoma and Polyp Detection During Screening and Surveillance Colonoscopy: A Randomized-Controlled Trial
Abstract
1. Introduction
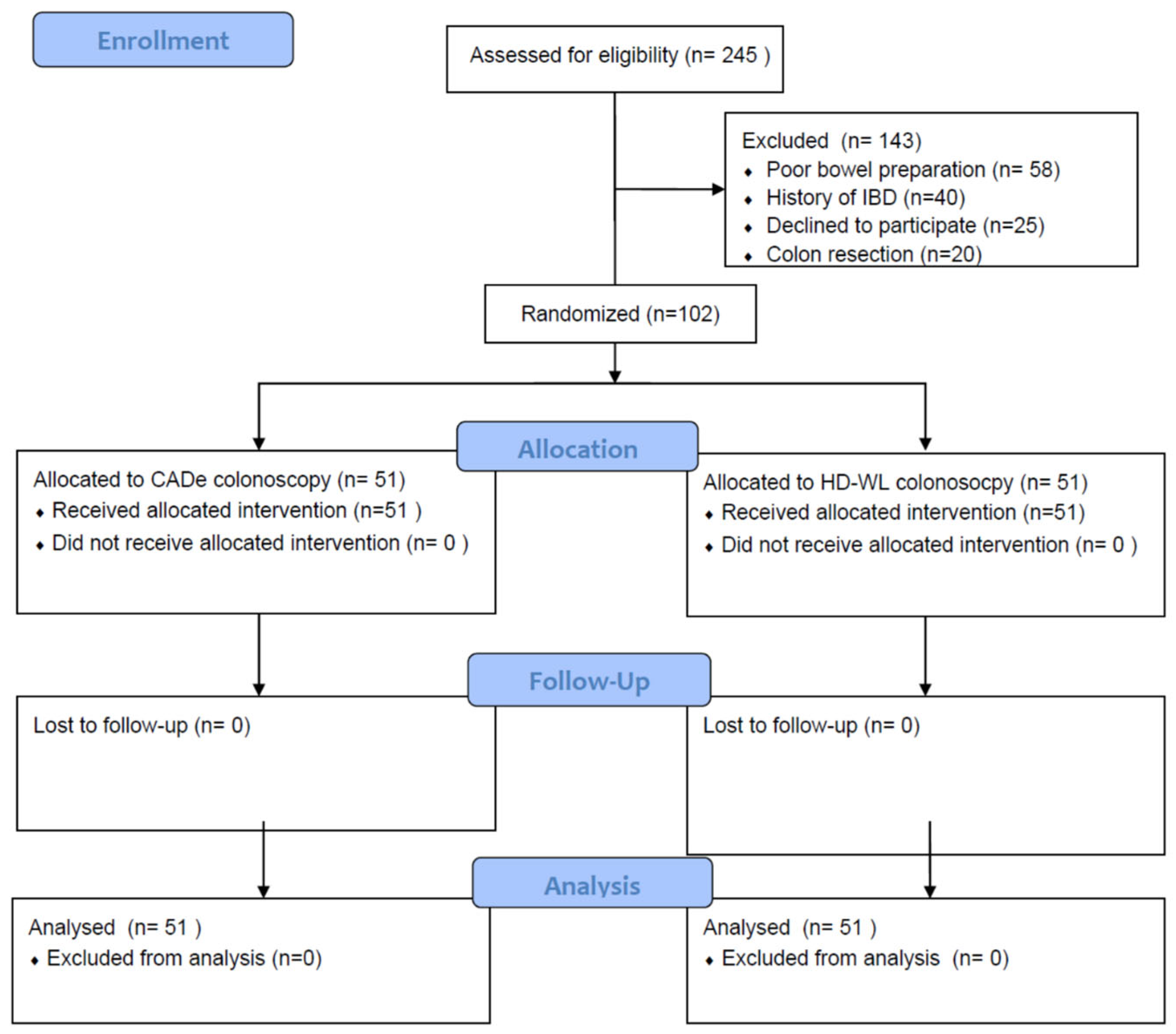
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | Adenoma detection rate |
| AI | Artificial intelligence |
| APC | Adenoma per colonoscopy |
| CADe | Computer-aided detection |
| CRC | Colorectal cancer |
| HD | High definition |
| PDR | Polyp detection rate |
| PPC | Polyp per colonoscopy |
| WL | White light |
References
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; Kubik, M.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Burr, N.E.; Valori, R. Causes of Post-Colonoscopy Colorectal Cancers Based on World Endoscopy Organization System of Analysis. Gastroenterology 2020, 158, 1287–1299.e1282. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.F.; Regula, J.; Kraszewska, E.; Polkowski, M.; Wojciechowska, U.; Didkowska, J.; Zwierko, M.; Rupinski, M.; Nowacki, M.P.; Butruk, E. Quality indicators for colonoscopy and the risk of interval cancer. N. Engl. J. Med. 2010, 362, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kim, A.S.; Church, T.R.; Perdue, D.G.; Shaukat, A. Adenomas per colonoscopy and adenoma per positive participant as quality indicators for screening colonoscopy. Endosc. Int. Open 2020, 8, E1560–E1565. [Google Scholar] [CrossRef]
- Rondonotti, E.; Di Paolo, D.; Rizzotto, E.R.; Alvisi, C.; Buscarini, E.; Spadaccini, M.; Tamanini, G.; Paggi, S.; Amato, A.; Scardino, G.; et al. Efficacy of a computer-aided detection system in a fecal immunochemical test-based organized colorectal cancer screening program: A randomized controlled trial (AIFIT study). Endoscopy 2022, 54, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Aniwan, S.; Mekritthikrai, K.; Kerr, S.J.; Tiankanon, K.; Vandaungden, K.; Sritunyarat, Y.; Piyachaturawat, P.; Luangsukrerk, T.; Kulpatcharapong, S.; Wisedopas, N.; et al. Computer-aided detection, mucosal exposure device, their combination, and standard colonoscopy for adenoma detection: A randomized controlled trial. Gastrointest. Endosc. 2023, 97, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Lichtenstein, D.R.; Somers, S.C.; Chung, D.C.; Perdue, D.G.; Gopal, M.; Colucci, D.R.; Phillips, S.A.; Marka, N.A.; Church, T.R.; et al. Computer-Aided Detection Improves Adenomas per Colonoscopy for Screening and Surveillance Colonoscopy: A Randomized Trial. Gastroenterology 2022, 163, 732–741. [Google Scholar] [CrossRef]
- Mangas-Sanjuan, C.; de-Castro, L.; Cubiella, J.; Díez-Redondo, P.; Suárez, A.; Pellisé, M.; Fernández, N.; Zarraquiños, S.; Núñez-Rodríguez, H.; Álvarez-García, V.; et al. Role of Artificial Intelligence in Colonoscopy Detection of Advanced Neoplasias: A Randomized Trial. Ann. Intern. Med. 2023, 176, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Spadaccini, M.; Mori, Y.; Foroutan, F.; Facciorusso, A.; Gkolfakis, P.; Tziatzios, G.; Triantafyllou, K.; Antonelli, G.; Khalaf, K.; et al. Real-Time Computer-Aided Detection of Colorectal Neoplasia During Colonoscopy: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2023, 176, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Spadaccini, M.; Iannone, A.; Maselli, R.; Badalamenti, M.; Desai, M.; Chandrasekar, V.T.; Patel, H.K.; Fugazza, A.; Pellegatta, G.; Galtieri, P.A.; et al. Computer-aided detection versus advanced imaging for detection of colorectal neoplasia: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Du, F.; Song, W.; Xia, Y.; Yue, X.; Yang, D.; Cui, B.; Liu, Y.; Han, P. Artificial intelligence for colorectal neoplasia detection during colonoscopy: A systematic review and meta-analysis of randomized clinical trials. EClinicalMedicine 2023, 66, 102341. [Google Scholar] [CrossRef]
- Dixon, M.F. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002, 51, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Al-Enezi, S.A.; Alsurayei, S.A.; Ismail, A.E.; Aly, N.Y.; Ismail, W.A.; Abou-Bakr, A.A. Adenomatous colorectal polyps in patients referred for colonoscopy in a regional hospital in Kuwait. Saudi J. Gastroenterol. 2010, 16, 188–193. [Google Scholar] [CrossRef]
- Liem, B.; Gupta, N. Adenoma detection rate: The perfect colonoscopy quality measure or is there more? Transl. Gastroenterol. Hepatol. 2018, 3, 19. [Google Scholar] [CrossRef]
- Dharwadkar, P.; Zaki, T.A.; Murphy, C.C. Colorectal Cancer in Younger Adults. Hematol. Oncol. Clin. N. Am. 2022, 36, 449–470. [Google Scholar] [CrossRef]
- Khan, R.; Ruan, Y.; Yuan, Y.; Khalaf, K.; Sabrie, N.S.; Gimpaya, N.; Scaffidi, M.A.; Bansal, R.; Vaska, M.; Brenner, D.R.; et al. Relative Efficacies of Interventions to Improve the Quality of Screening-Related Colonoscopy: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Gastroenterology 2024, 167, 560–590. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.T.; Shankar, U.; Parvin, R.; Abbas, S.H.; Chaudhary, S.; Friedlander, Y.; Friedland, S. Evaluation of Computer-Aided Detection During Colonoscopy in the Community (AI-SEE): A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2023, 118, 1841–1847. [Google Scholar] [CrossRef]
- Wallace, M.B.; Sharma, P.; Bhandari, P.; East, J.; Antonelli, G.; Lorenzetti, R.; Vieth, M.; Speranza, I.; Spadaccini, M.; Desai, M.; et al. Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia. Gastroenterology 2022, 163, 295–304.e295. [Google Scholar] [CrossRef] [PubMed]
- Vleugels, J.L.A.; Hassan, C.; Senore, C.; Cassoni, P.; Baron, J.A.; Rex, D.K.; Ponugoti, P.L.; Pellise, M.; Parejo, S.; Bessa, X.; et al. Diminutive Polyps With Advanced Histologic Features Do Not Increase Risk for Metachronous Advanced Colon Neoplasia. Gastroenterology 2019, 156, 623–634.e623. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Pickhardt, P.J.; Rex, D.K. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2010, 8, 865–869.e3. [Google Scholar] [CrossRef] [PubMed]
- Ferlitsch, M.; Hassan, C.; Bisschops, R.; Bhandari, P.; Dinis-Ribeiro, M.; Risio, M.; Paspatis, G.A.; Moss, A.; Libânio, D.; Lorenzo-Zúñiga, V.; et al. Colorectal polypectomy and endoscopic mucosal resection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2024. Endoscopy 2024, 56, 516–545. [Google Scholar] [CrossRef]
- Yoshida, N.; Dohi, O.; Inoue, K.; Itoh, Y. The efficacy of polyp detection and tumor characterization of blue laser imaging, blue light imaging, and linked color imaging with light-emitted diode (LED) and LASER endoscope. Ann. Transl. Med. 2020, 8, 152. [Google Scholar] [CrossRef]
- Subramaniam, S.; Hayee, B.; Aepli, P.; Schoon, E.; Stefanovic, M.; Kandiah, K.; Thayalasekaran, S.; Alkandari, A.; Bassett, P.; Coron, E.; et al. Optical diagnosis of colorectal polyps with Blue Light Imaging using a new international classification. United Eur. Gastroenterol. J. 2019, 7, 316–325. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 102) | HD-WL Colonoscopy (n = 51) | CADe-Assisted Colonoscopy (n = 51) | p-Value | |
|---|---|---|---|---|
| Male, n (%) | 51 (50) | 21 (41.2) | 30 (58.8) | 0.08 |
| Age, mean (SD), years | 52.8 (8.2) | 54.5 (8.3) | 51.1 (7.7) | 0.03 |
| Weight (KG), mean (SD) | 79.5 (14.2) | 79.9 (16.3) | 79.0 (11.9) | 0.73 |
| Indication, n (%) | 1.00 | |||
| - Screening | 96 (94.1) | 48 (94.1) | 48 (94.1) | |
| - Surveillance | 6 (5.9) | 3 (5.9) | 3 (5.9) | |
| Use of antithrombotics, n (%) | 5 (4.9) | 3 (5.9) | 2 (3.9) | 0.65 |
| BBPS colon cleansing scale, median (IQR) | 8 (6–9) | 8 (6–9) | 8 (6–9) | 1.00 |
| Insertion time, mean (SD), s | 341.2 (186.5) | 359.9 (136.8) | 322.5 (225.4) | 0.31 |
| Withdrawal time, mean (SD), s | 525.9 (115.0) | 509.4 (104.2) | 542.4 (123.8) | 0.15 |
| IC valve intubation, n (%) | 94 (92.2) | 44 (86.3) | 50 (98.0) | 0.03 |
| Adverse Events, n (%) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Overall (n = 121) | HD-WL Colonoscopy (n = 52) | CADe-Assisted Colonoscopy (n = 69) | p-Value | |
|---|---|---|---|---|
| Polyp size (mm), mean (SD) | 4.18 (5.1) | 4.19 (6.3) | 4.17 (4.0) | 0.98 |
| Polyp histology, n (%) | 0.81 | |||
| 56 (46.3) | 23 (44.2) | 33 (47.8) | |
| 57 (47.1) | 25 (48.1) | 32 (46.4) | |
| 0 (0) | 0 (0) | 0 (0) | |
| 3 (2.5) | 1 (1.9) | 2 (2.9) | |
| 1 (0.8) | 1 (1.9) | 0 (0) | |
| 4 (3.3) | 2 (3.9) | 2 (2.9) | |
| 0 (0) | 0 (0) | 0 (0) | |
| Paris classification, n (%) | 0.39 | |||
| 1 (0.8) | 0 (0) | 1 (1.45) | |
| 15 (12.4) | 6 (11.5) | 9 (13.0) | |
| 1 (0.8) | 0 (0) | 1 (1.45) | |
| 101 (83.5) | 46 (88.5) | 55 (79.7) | |
| 3 (2.5) | 0 (0) | 3 (4.4) | |
| 0 (0) | 0 (0) | 0 (0) | |
| 0 (0) | 0 (0) | 0 (0) | |
| Polyp location, n (%) | 0.27 | |||
| 15 (12.4) | 6 (11.5) | 9 (13.0) | |
| 20 (16.5) | 8 (15.4) | 12 (17.4) | |
| 26 (21.5) | 10 (19.2) | 16 (23.2) | |
| 32 (26.5) | 18 (34.6) | 14 (20.3) | |
| 23 (19.0) | 10 (19.2) | 13 (18.8) | |
| 5 (4.1) | 0 (0) | 5 (7.2) |
| Overall (n = 102) | HD-WL Colonoscopy (n = 51) | CADe-Assisted Colonoscopy (n = 51) | p-Value | Risk Ratio (95%CI) | |
|---|---|---|---|---|---|
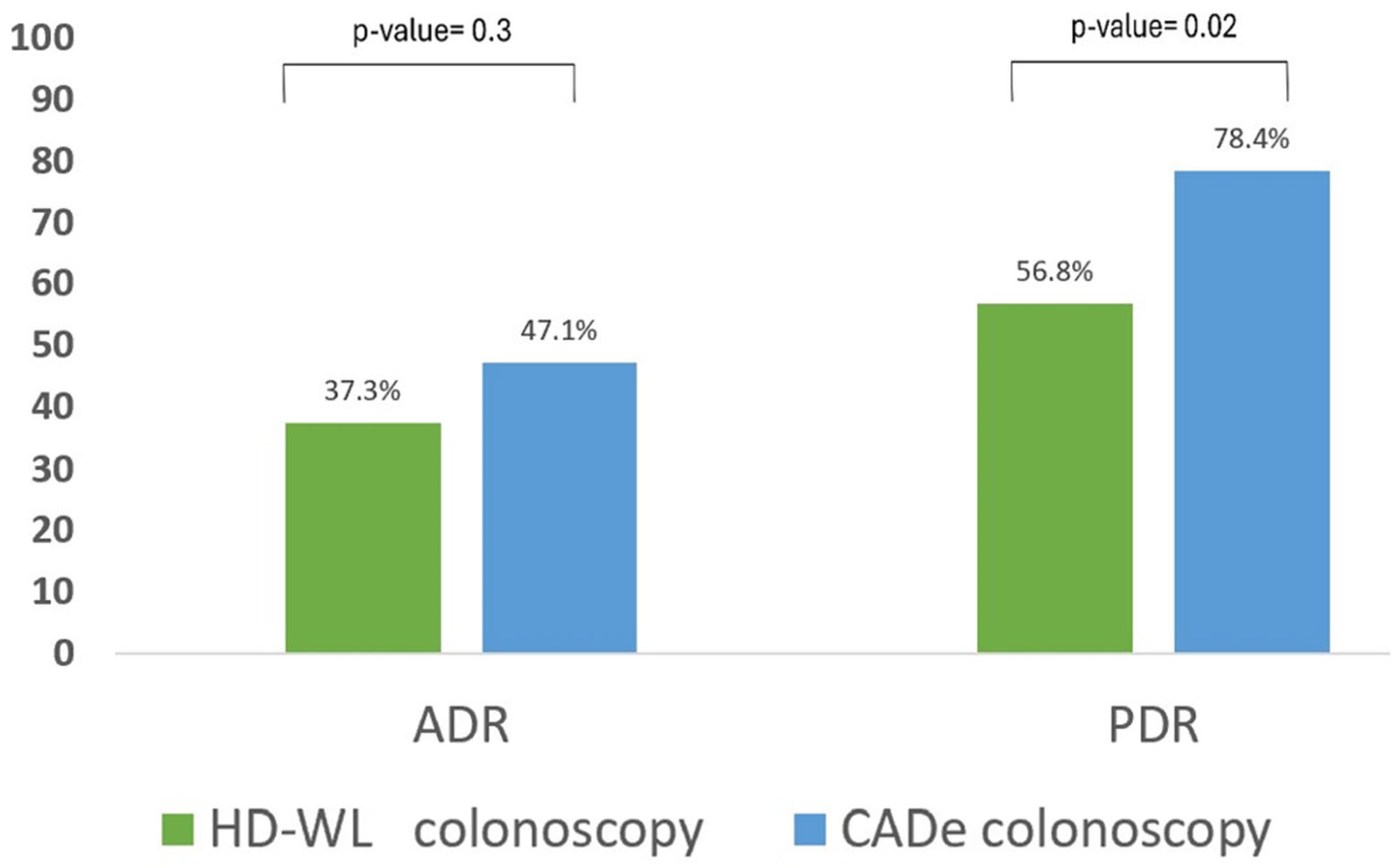
| Adenoma detection rate (ADR), n (%) | 43 (42.2) | 19 (37.3) | 24 (47.1) | 0.32 | 1.26 (0.80–2.00) |
| Polyp detection rate (PDR), n (%) | 69 (67.6) | 29 (56.8) | 40 (78.4) | 0.02 | 1.38 (1.04–1.82) |
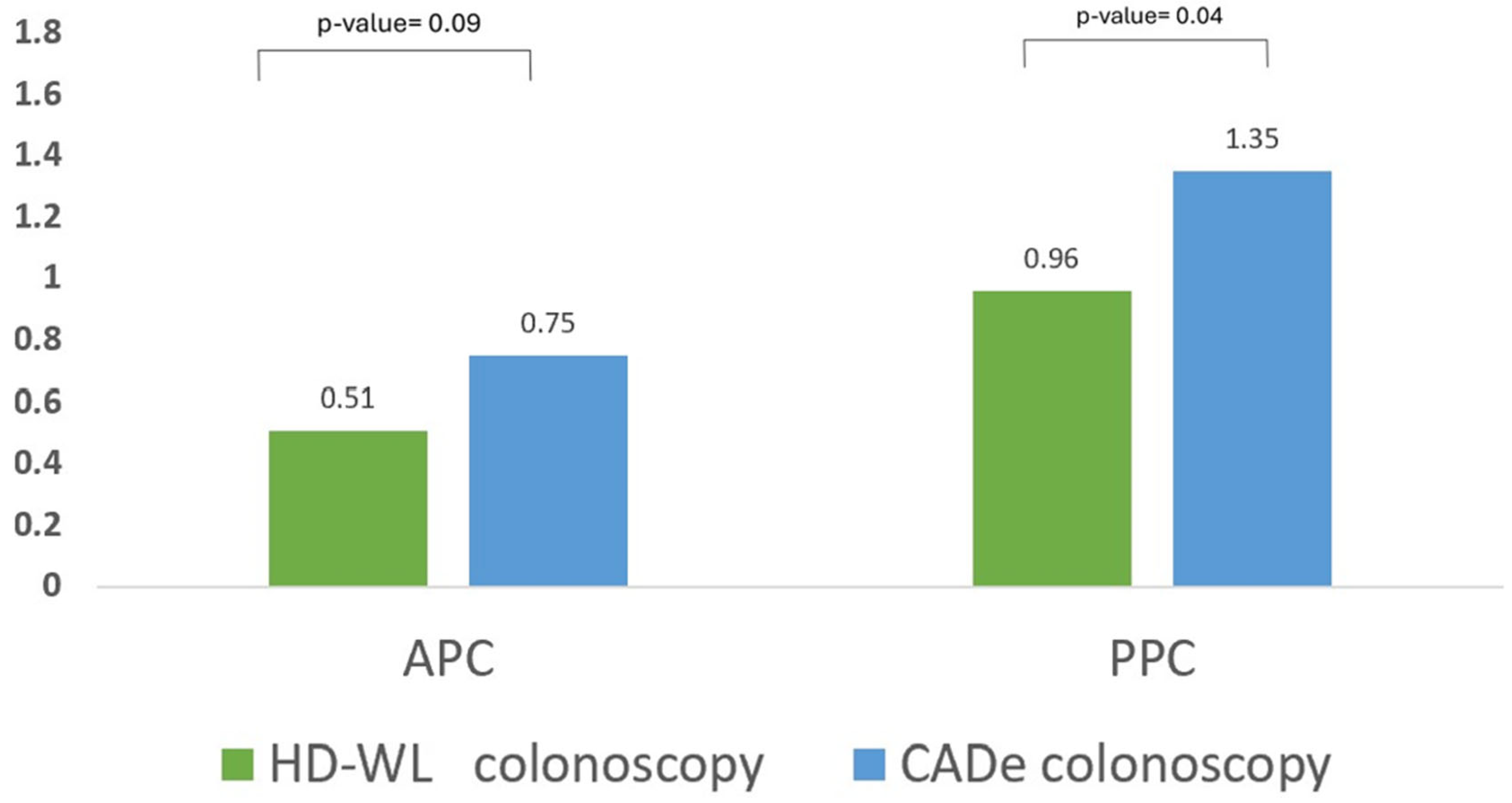
| Adenoma per colonoscopy, mean (SD) | 0.63 (0.89) | 0.51 (0.78) | 0.75 (0.99) | 0.09 | - |
| Polyp per colonoscopy, mean (SD) | 1.15 (1.15) | 0.96 (1.20) | 1.35 (1.07) | 0.04 | - |
| Accuracy in characterization, n (%) * | 95 (78.5) | 40 (76.9) | 55 (79.7) | 0.71 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alali, A.A.; Alhashmi, A.; Alotaibi, N.; Ali, N.; Alali, M.; Alfadhli, A. Artificial Intelligence for Adenoma and Polyp Detection During Screening and Surveillance Colonoscopy: A Randomized-Controlled Trial. J. Clin. Med. 2025, 14, 581. https://doi.org/10.3390/jcm14020581
Alali AA, Alhashmi A, Alotaibi N, Ali N, Alali M, Alfadhli A. Artificial Intelligence for Adenoma and Polyp Detection During Screening and Surveillance Colonoscopy: A Randomized-Controlled Trial. Journal of Clinical Medicine. 2025; 14(2):581. https://doi.org/10.3390/jcm14020581
Chicago/Turabian StyleAlali, Ali A., Ahmad Alhashmi, Nawal Alotaibi, Nargess Ali, Maryam Alali, and Ahmad Alfadhli. 2025. "Artificial Intelligence for Adenoma and Polyp Detection During Screening and Surveillance Colonoscopy: A Randomized-Controlled Trial" Journal of Clinical Medicine 14, no. 2: 581. https://doi.org/10.3390/jcm14020581
APA StyleAlali, A. A., Alhashmi, A., Alotaibi, N., Ali, N., Alali, M., & Alfadhli, A. (2025). Artificial Intelligence for Adenoma and Polyp Detection During Screening and Surveillance Colonoscopy: A Randomized-Controlled Trial. Journal of Clinical Medicine, 14(2), 581. https://doi.org/10.3390/jcm14020581






