FEV1/FEV6 Cutoff Points to Avoid False Negatives When Using Portable Devices, PICO-6® and COPD-6®, in COPD Detection in Primary Healthcare Services
Abstract
1. Introduction
2. Methods
2.1. Sample Size and Inclusion Criteria
2.2. Development of Study Procedures
2.3. Statistical Analysis
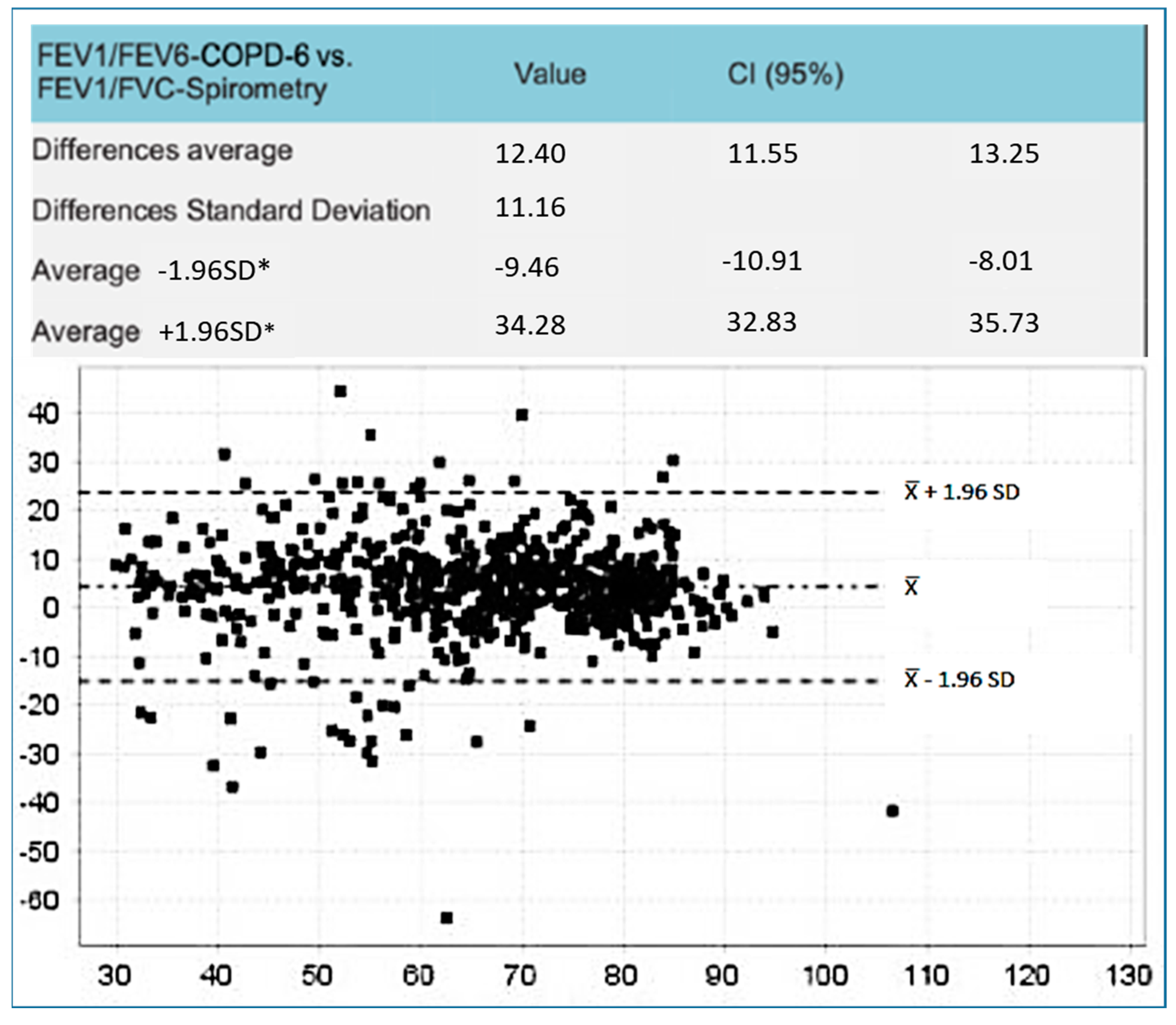
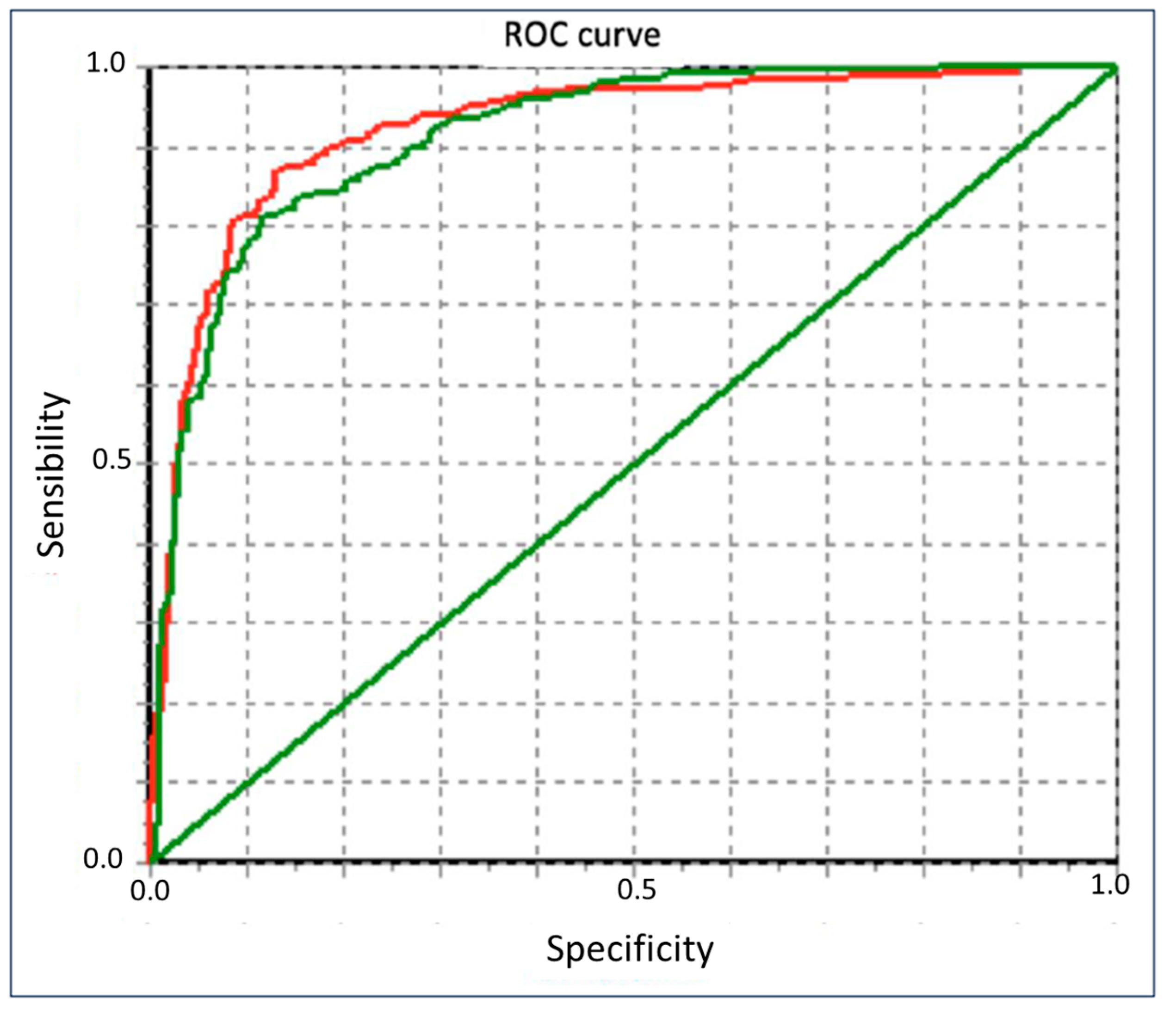
3. Results
Sample Studied and Main Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldomero, A.K.; Kunisaki, K.M.; Bangerter, A.; Nelson, D.B.; Wendt, C.H.; Fortis, S.; Hagedorn, H.; Dudley, R.A. Beyond Access: Factors Associated with Spirometry Underutilization Among Patients with a Diagnosis of COPD in Urban Tertiary Care Centers. Chronic Obstr. Pulm. Dis. 2022, 9, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Montes de Oca, M.; López, M.V.; Aguirre, C.; Schiavi, E.; Jardim, J.R.; Team, P. COPD Underdiagnosis and Misdiagnosis in a High-Risk Primary Care Population in Four Latin American Countries. A Key to Enhance Disease Diagnosis: The PUMA Study. PLoS ONE 2016, 11, e0152266. [Google Scholar] [CrossRef]
- Fukahori, S.; Matsuse, H.; Takamura, N.; Hirose, H.; Tsuchida, T.; Kawano, T.; Fukushima, C.; Mizuta, Y.; Kohno, S. Prevalence of chronic obstructive pulmonary diseases in general clinics in terms FEV1/FVC. Int. J. Clin. Pract. 2009, 63, 269–274. [Google Scholar] [CrossRef]
- Heffler, E.; Crimi, C.; Mancuso, S.; Campisi, R.; Puggioni, F.; Brussino, L.; Crimi, N. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir. Med. 2018, 142, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, M.J.; Gallo, H.M.; Wilson, E.L. Accuracy and Quality of Spirometry in Primary Care Offices. Ann. Am. Thorac. Soc. 2016, 13, 2119–2124. [Google Scholar] [CrossRef]
- Bednarek, M.; Maciejewski, J.; Wozniak, M.; Kuca, P.; Zielinski, J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008, 63, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Ragaišienė, G.; Kibarskytė, R.; Gauronskaitė, R.; Giedraitytė, M.; Dapšauskaitė, A.; Kasiulevičius, V.; Danila, E. Diagnosing COPD in primary care: What has real life practice got to do with guidelines? Multidiscip. Respir. Med. 2019, 14, 28. [Google Scholar] [CrossRef]
- Jones, R.C.; Price, D.; Ryan, D.; Sims, E.J.; von Ziegenweidt, J.; Mascarenhas, L.; Burden, A.; Halpin, D.M.G.; Winter, R.; Hill, S.; et al. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: A retrospective study of a clinical cohort. Lancet Respir. Med. 2014, 2, 267–276. [Google Scholar] [CrossRef]
- Soriano, J.B.; Calle, M.; Montemayor, T.; Alvarez-Sala, J.L.; Ruiz-Manzano, J.; Miravitlles, M. The general public’s knowledge of chronic obstructive pulmonary disease and its determinants: Current situation and recent changes. Arch. Bronconeumol. 2012, 48, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Soriano, J.B.; Garcia-Rio, F.; Munoz, L.; Duran-Tauleria, E.; Sanchez, G.; Sobradillo, V.; Ancochea, J. Prevalence of COPD in Spain: Impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009, 64, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Brogger, J.; Eide, G.E.; Gulsvik, A. FEV6 as a surrogate for FVC: Authors should have included ROC-curve analyses. Am. J. Respir. Crit. Care Med. 2001, 163, 1759. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, J.W.; Foreman, M.G.; Jensen, R.L.; Stinson, D.S.; Wilson, C.G.; Lynch, D.A.; Make, B.J.; Dransfield, M.T. FEV(1)/FEV(6) to diagnose airflow obstruction. Comparisons with computed tomography and morbidity indices. Ann. Am. Thorac. Soc. 2014, 11, 335–341. [Google Scholar] [CrossRef]
- Jing, J.Y.; Huang, T.C.; Cui, W.; Xu, F.; Shen, H.H. Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest 2009, 135, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, P.; Dwivedi, D.P.; Govindraj, V. FEV1/FEV6 is effective as a surrogate for FEV1/FVC in the diagnosis of chronic obstructive pulmonary disease. Indian J. Tuber. 2021, 68, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gong, W.; Tian, Y.; Zhou, J. FEV1/FEV6 in Primary Care Is a Reliable Easy Method for the Diagnosis of COPD. Respir. Care 2016, 61, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Markovic, D.; ∙Buhr, R.G.; Christopher, B.; Fortis, S.; Arjomandi, M.; Couper, D.; Anderson, W.H.; Paine, R.; Woodruff, P.G.; et al. Significance of FEV3/FEV6 in Recognition of Early Airway Disease in Smokers at Risk of Development of COPD. Chest 2021, 161, 949–959. [Google Scholar] [CrossRef]
- Vandevoorde, J.; Verbanck, S.; Schuermans, D.; Broekaert, L.; Devroey, D.; Kartounian, J.; Vincken, W. Forced vital capacity and forced expiratory volume in six seconds as predictors of reduced total lung capacity. Eur. Resp. J. 2008, 31, 391–395. [Google Scholar] [CrossRef]
- Vandevoorde, J.; Verbanck, S.; Schuermans, D.; Kartounian, J.; Vincken, W. Obstructive and restrictive spirometric patterns: Fixed cut-offs for FEV1/FEV6 and FEV6. Eur. Respir. J. 2006, 27, 378–383. [Google Scholar] [CrossRef]
- Lundgren, F.L.; Cabral, M.M.; Climaco, D.C.; de Macedo, L.G.; Coelho Mde, A.; Dias, A.L. Determination of the efficacy of FEV6 as a surrogate for FVC in the diagnostic screening for chronic obstructive pulmonary disease through the comparison of FEV1/FVC and FEV1/FEV6 ratios. J. Bras. Pneumol. 2007, 33, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Perez-Padilla, R.; Wehrmeister, F.C.; Celli, B.R.; Lopez-Varela, M.V.; Montes de Oca, M.; Muiño, A.; Talamo, C.; Jardim, J.R.; Valdivia, G.; Lisboa, C.; et al. Reliability of FEV1/FEV6 to diagnose airflow obstruction compared with FEV1/FVC: The PLATINO longitudinal study. PLoS ONE 2013, 8, e67960. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.L.; Crapo, R.O.; Enright, P. A statistical rationale for the use of forced expired volume in 6 s. Chest 2006, 130, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Decramer, M.; Wedzicha, J.A.; Wilson, K.C.; Agusti, A.; Criner, G.J.; MacNee, W.; Make, B.J.; Rennard, S.I.; Stockley, R.A.; et al. An official Am Thoracic Society/Eur Resp Society statement: Research questions in COPD. Eur. Respir. Rev. 2015, 24, 159–172. [Google Scholar] [CrossRef]
- Miravitlles, M.; Soler-Cataluna, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Riesco, J.A.; Trigueros, J.A.; Piñera, P.; Simón, A.; et al. Spanish COPD Guidelines (GesEPOC): Update Pharmacological treatment of stable COPD. Arch. Bronconeumol. 2022, 58, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Heris, J.A.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.-A.; et al. Burden of Chronic Obstructive Pulmonary Disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e069679. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Ancochea, J.; Miravitlles, M.; Garcia-Rio, F.; Duran-Tauleria, E.; Munoz, L.; Jimenez-Ruiz, C.A.; Masa, J.F.; Viejo, J.L.; Villasante, C.; et al. Recent trends in COPD prevalence in Spain: A repeated cross-sectional survey 1997–2007. Eur. Respir. J. 2010, 36, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Peña, V.S.; Miravitlles, M.; Gabriel, R.; Jimenez-Ruiz, C.A.; Villasante, C.; Masa, J.F.; Viejo, J.L.; Fernández-Fau, L. Geographic variations in prevalence and underdiagnosis of COPD: Results of the IBERPOC multicentre epidemiological study. Chest 2000, 118, 981–989. [Google Scholar] [CrossRef]
- Soriano, J.B.; Alfageme, I.; Miravitlles, M.; de Lucas, P.; Soler-Cataluña, J.J.; García-Río, F.; Casanova, C.; Rodríguez González-Moro, J.M.; Cosío, B.G.; Sánchez, G.; et al. Prevalence Determinants of COPD in Spain: EPISCAN II. Arch. Bronconeumol. 2021, 57, 61–69. [Google Scholar] [CrossRef]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; de Oca, M.M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef]
- Miravitlles, M.; Soler-Cataluna, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Trigueros, J.A.; Cosío, B.G.; Casanova, C.; Antonio Riesco, J.; et al. Spanish Guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch. Bronconeumol. 2017, 53, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, M.; Rodríguez-Blanco, T.; Parcet, J.; Penalver, N.; Rubio, C.; Ferrer, M.; Miravitlles, M. Variability in the performing of spirometry and its consequences in the treatment of COPD in primary care. Arch. Bronconeumol. 2011, 47, 226–233. [Google Scholar] [CrossRef]
- Akpinar-Elci, M.; Fedan, K.B.; Enright, P.L. FEV6 as a surrogate for FVC in detecting airways obstruction and restriction in the workplace. Eur. Respir. J. 2006, 27, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Frith, P.; Crockett, A.; Beilby, J.; Marshall, D.; Attewell, R.; Ratnanesan, A.; Gavagna, G. Simplified COPD screening: Validation of the Piko-6® in primary care. Prim. Care Respir. J. 2011, 20, 190–198. [Google Scholar] [CrossRef]
- Represas Represas, C.; Botana Rial, M.; Leiro Fernandez, V.; Gonzalez Silva, A.I.; del Campo Perez, V.; Fernandez-Villar, A. Assessment of the portable COPD-6® device for detecting obstructive airway diseases. Arch. Bronconeumol. 2010, 46, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Sichletidis, L.; Spyratos, D.; Papaioannou, M.; Chloros, D.; Tsiotsios, A.; Tsagaraki, V.; Haidich, A.-B. A combination of the IPAG questionnaire and Piko-6® flow meter is a valuable screening tool for COPD in the primary care setting. Prim. Care Respir. J. 2011, 20, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo Sierra, V.; Hernandez-Mezquita, M.A.; Palomo Cobos, L.; Garcia Sanchez, M.; Castellanos, R.D.; Jodra Sanchez, S.; Pérez, R.C.; Ferrero, M.B. Usefulness of The Piko-6® Portable Device for Early COPD Detection in Primary Care. Arch. Bronconeumol. 2018, 54, 445–454. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Wang, X.; Yu, N. Accuracy of portable spirometers in the diagnosis of chronic obstructive pulmonary disease. A meta-analysis. NPJ Prim. Care Respir. Med. 2022, 32, 2–15. [Google Scholar] [CrossRef]
- Hernández-Mezquita, M.A.; de los Santos-Ventura, I.; Hidalgo-Sierra, V.; Pérez-Trullen, A.; Barrueco-Otero, E. Accuracy of PIKO 6® and COPD6® Devices in COPD Screening. Arch. Bronconeumol. 2024, in press. [CrossRef]
- Topole, E.; Biondaro, S.; Montagna, I.; Corre, S.; Corradi, M.; Stanojevic, S.; Graham, B.; Das, N.; Ray, K.; Topalovic, M. Artificial intelligence-based software facilitates spirometry quality control in asthma and COPD clinical trials. ERJ Open Res. 2023, 9, 00292–2022. [Google Scholar] [CrossRef]
- Van den Bemt, L.; Wouters, B.C.; Grootens, J.; Denis, J.; Poels, P.J.; Schermer, T.R. Diagnostic accuracy of pre-bronchodilator FEV1/FEV6 from microspirometry to detect airflow obstruction in primary care: A randomised cross-sectional study. NPJ Prim. Care Respir. Med. 2014, 24, 14033. [Google Scholar] [CrossRef][Green Version]
- Kaufmann, M.; Hartl, S.; Geyer, K.; Breyer, M.K.; Burghuber, O.C. Measuring FEV(6) for detecting early airway obstruction in the primary care setting. Quality and utility of the new Piko-6® device. Respiration 2009, 78, 161–167. [Google Scholar] [CrossRef]
- Fathima, M.; Saini, B.; Foster, J.M.; Armour, C.L. Community pharmacy-based case finding for COPD in urban and rural settings is feasible and effective. Int. J. Chron. Obstruct Pulmon Dis. 2017, 12, 2753–2761. [Google Scholar] [CrossRef]
- Chen, C.Z.; Sheu, C.C.; Cheng, S.L.; Wang, H.C.; Lin, M.C.; Hsu, W.H.; Lee, K.Y.; Perng, D.W.; Lin, H.I.; Lin, M.S.; et al. Performance and Clinical Utility of Various Chronic Obstructive Pulmonary Disease Case-Finding Tools. Int. J. Chron. Obstruct Pulmon Dis. 2021, 16, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Doe, G.; El-Emir, E.; Edwards, G.D.; Topalovic, M.; Evans, R.A.; Russell, R.; Sylvester, K.P.; Van Orshoven, K.; Sunjaya, A.P.; Scott, D.; et al. Comparing performance of primary care clinicians in the interpretation of SPIROmetry with or without Artificial Intelligence Decision support software (SPIRO-AID): A protocol for a randomised controlled trial. BMJ Open 2024, 14, e086736. [Google Scholar] [CrossRef]


| Frequency | Percentage | Valid Percentage | Accumulated Percentage | ||
|---|---|---|---|---|---|
| Forced Spirometry | |||||
| Valid | ILL (COPD) | 411 | 61.9 | 61.9 | 61.9 |
| HEALTHY | 253 | 38.1 | 38.1 | 100.0 | |
| Total | 664 | 100.0 | 100.0 | ||
| Piko-6® | |||||
| Valid | ILL (COPD) | 340 | 51.2 | 51.2 | 51.2 |
| HEALTHY | 324 | 48.8 | 48.8 | 100.0 | |
| Total | 664 | 100.0 | 100.0 | ||
| COPD-6® | |||||
| Valid | ILL (COPD) | 209 | 31.5 | 31.5 | 31.5 |
| HEALTHY | 455 | 68.5 | 68.5 | 100.0 | |
| Total | 664 | 100.0 | 100.0 | ||
| Average | Standard Deviation | |
|---|---|---|
| FEV1 (%) by Piko-6® | 71.06 | 26.83 |
| FEV1 (%) by COPD-6® | 79.94 | 30.04 |
| FEV1 (%) by FS | 83.58 | 46.53 |
| FEV6 (%) by Piko-6® | 81.15 | 48.52 |
| FEV6 (%) by COPD-6® | 82.52 | 24.21 |
| FVC (%) by FS | 100.08 | 106.51 |
| FEV1 (cc) by Piko-6® | 2000.87 | 886.98 |
| FEV1 (cc) by COPD-6® | 2158.71 | 969.35 |
| FEV1 (cc) by FS | 2303.30 | 987.97 |
| FEV6 (cc) by Piko-6® | 2936.43 | 998.72 |
| FEV6 (cc) by COPD-6® | 2800.87 | 1025.12 |
| FVC (cc) by FS | 3577.03 | 1042.26 |
| Piko-6® vs. Spirometry | Correlation | CI (95%) | |
|---|---|---|---|
| FEV1, cc | 0.8916 | 0.8748 | 0.9062 |
| FEV6, cc (FVC, cc) | 0.6456 | 0.599 | 0.6879 |
| FEV1, % | 0.3756 | 0.3084 | 0.439 |
| FEV6, % (FVC, %) | 0.0133 | 0 | 0.0892 |
| FEV1/FVC | 0.7663 | 0.733 | 0.7959 |
| COPD-6® vs. Spirometry | |||
| FEV1, cc | 0.9599 | 0.9535 | 0.9655 |
| FEV6, cc (FVC, cc) | 0.6743 | 0.6306 | 0.7137 |
| FEV1, % | 0.4321 | 0.3682 | 0.492 |
| FEV6, % (FVC, %) | 0.0526 | 0 | 0.1281 |
| FEV1/FVC | 0.4936 | 0.4339 | 0.549 |
| PEARSON CORRELATION BETWEEN COPD-6®, PIKO-6®, AND FS | ||
|---|---|---|
| Piko-6® FEV1 (cc) | Pearson Correlation | 0.946 |
| Sig. (bilateral) | 0.000 | |
| COPD-6® FEV1 (cc) | Pearson Correlation | 0.971 |
| Sig. (bilateral) | 0.000 | |
| Piko-6® FEV1/FEV6 | Pearson Correlation | 0.796 |
| Sig. (bilateral) | 0.000 | |
| COPD-6® FEV1/FEV6 | Pearson Correlation | 0.737 |
| Sig. (bilateral) | 0.000 | |
| FEV1/FEV6 Piko-6® (%) | YI | S (%) | E (%) | PPV (%) | NPV− (%) |
|---|---|---|---|---|---|
| <68 | 0.63 | 72.99 | 90.32 | 91.23 | 70.79 |
| <69 | 0.64 | 73.7 | 90.42 | 92.24 | 69.01 |
| <70 | 0.71 | 78.35 | 92.89 | 94.71 | 72.53 |
| <71 | 0.69 | 75.63 | 92.89 | 95.4 | 66.14 |
| <72 | 0.73 | 77.29 | 96.12 | 97.79 | 65.56 |
| <73 | 0.74 | 78.21 | 95.41 | 97.6 | 64.71 |
| <74 | 0.72 | 79.34 | 92.78 | 96.73 | 62.55 |
| <75 | 0.74 | 81.91 | 92.44 | 96.88 | 64.11 |
| <76 | 0.72 | 83.04 | 88.54 | 95.9 | 61.78 |
| <77 | 0.74 | 85.06 | 88.73 | 96.52 | 61.76 |
| <78 | 0.69 | 84.87 | 84.43 | 96.03 | 55.68 |
| <79 | 0.68 | 84.55 | 83.17 | 96.55 | 49.12 |
| <80 | 0.63 | 85.52 | 77.38 | 96.31 | 43.62 |
| FEV1/FEV6 COPD-6® (%) | |||||
| <68 | 0.44 | 46.49 | 97.85 | 96.76 | 56.99 |
| <69 | 0.44 | 47.15 | 96.93 | 95.96 | 54.29 |
| <70 | 0.46 | 47.78 | 97.89 | 97.61 | 50.99 |
| <71 | 0.46 | 48.52 | 97.78 | 97.71 | 49.33 |
| <72 | 0.49 | 50.22 | 99.03 | 99.14 | 47.22 |
| <73 | 0.5 | 51.5 | 98.47 | 98.77 | 45.95 |
| <74 | 0.5 | 52.69 | 97.78 | 98.46 | 43.46 |
| <75 | 0.55 | 56.3 | 98.26 | 98.93 | 44.01 |
| <76 | 0.56 | 57.79 | 98.09 | 98.99 | 41.85 |
| <77 | 0.57 | 58.81 | 98.59 | 99.35 | 39.44 |
| <78 | 0.58 | 59.23 | 98.36 | 99.38 | 35.19 |
| <79 | 0.58 | 61.46 | 97.03 | 99.14 | 31.11 |
| <80 | 0.59 | 64.14 | 95.24 | 98.94 | 27.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Mezquita, M.A.; Santos-Ventura, I.D.l.; Hidalgo-Sierra, V.; Pérez-Trullen, A.; Garcia, R.G.; Clavero-Sánchez, T.; Barrueco-Otero, E. FEV1/FEV6 Cutoff Points to Avoid False Negatives When Using Portable Devices, PICO-6® and COPD-6®, in COPD Detection in Primary Healthcare Services. J. Clin. Med. 2025, 14, 576. https://doi.org/10.3390/jcm14020576
Hernandez-Mezquita MA, Santos-Ventura IDl, Hidalgo-Sierra V, Pérez-Trullen A, Garcia RG, Clavero-Sánchez T, Barrueco-Otero E. FEV1/FEV6 Cutoff Points to Avoid False Negatives When Using Portable Devices, PICO-6® and COPD-6®, in COPD Detection in Primary Healthcare Services. Journal of Clinical Medicine. 2025; 14(2):576. https://doi.org/10.3390/jcm14020576
Chicago/Turabian StyleHernandez-Mezquita, Miguel A., Idania De los Santos-Ventura, Vanesa Hidalgo-Sierra, Alfonso Pérez-Trullen, Ruth García Garcia, Tamara Clavero-Sánchez, and Enrique Barrueco-Otero. 2025. "FEV1/FEV6 Cutoff Points to Avoid False Negatives When Using Portable Devices, PICO-6® and COPD-6®, in COPD Detection in Primary Healthcare Services" Journal of Clinical Medicine 14, no. 2: 576. https://doi.org/10.3390/jcm14020576
APA StyleHernandez-Mezquita, M. A., Santos-Ventura, I. D. l., Hidalgo-Sierra, V., Pérez-Trullen, A., Garcia, R. G., Clavero-Sánchez, T., & Barrueco-Otero, E. (2025). FEV1/FEV6 Cutoff Points to Avoid False Negatives When Using Portable Devices, PICO-6® and COPD-6®, in COPD Detection in Primary Healthcare Services. Journal of Clinical Medicine, 14(2), 576. https://doi.org/10.3390/jcm14020576






