Fluorescence Cholangiography for Extrahepatic Bile Duct Visualization in Urgent Mild and Moderate Acute Cholecystitis Patients Undergoing Laparoscopic Cholecystectomy: A Prospective Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Surgical Procedure
2.3. Assessment of CVS Principles
2.4. Intraoperative Assessment
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
3.1. Intraoperative Findings
3.2. Number of CVS Steps Performed
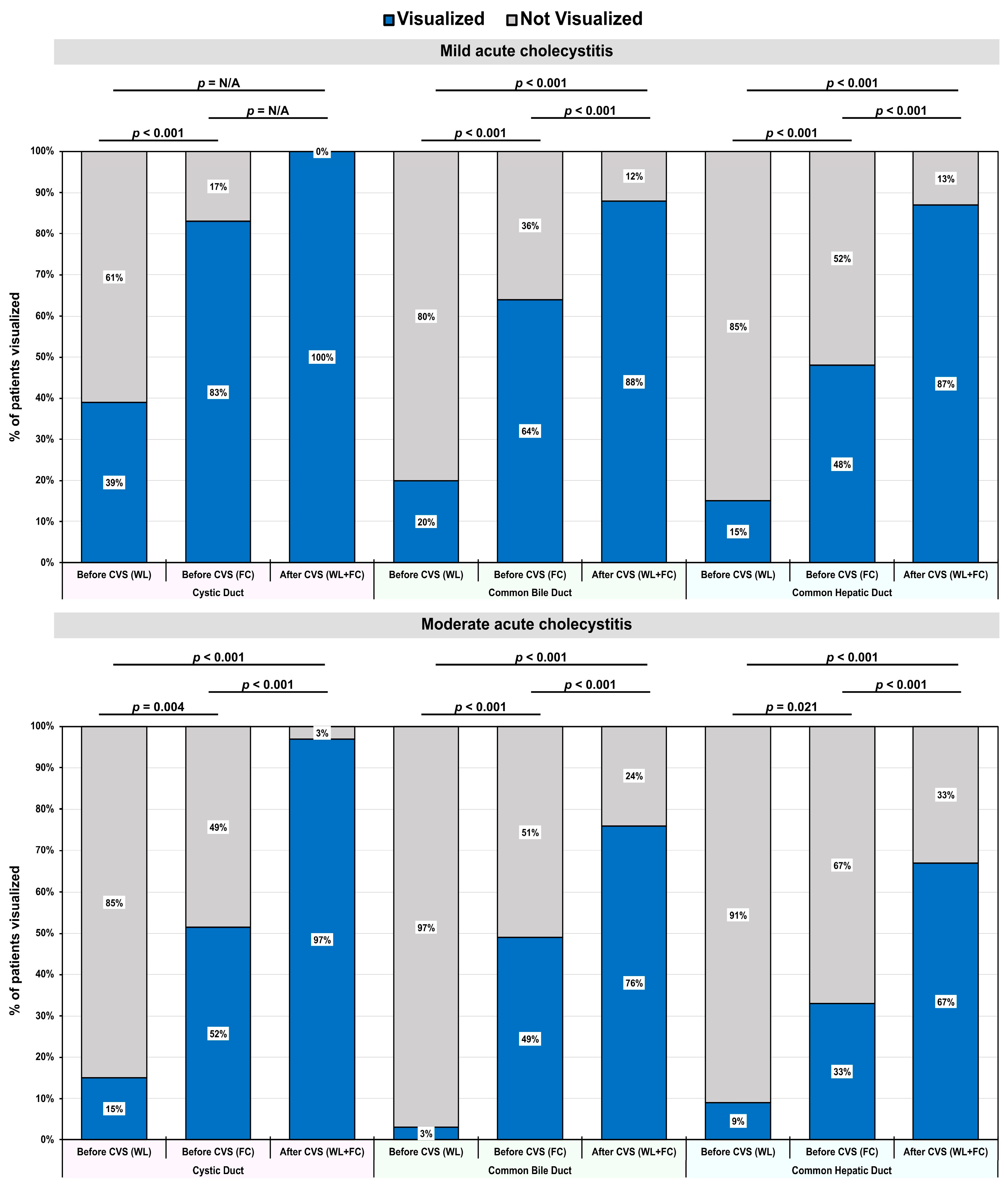
3.3. Primary Outcomes: Visualization of Bile Ducts Using FC
3.4. Usefulness of ICG for Visualization of Bile Ducts
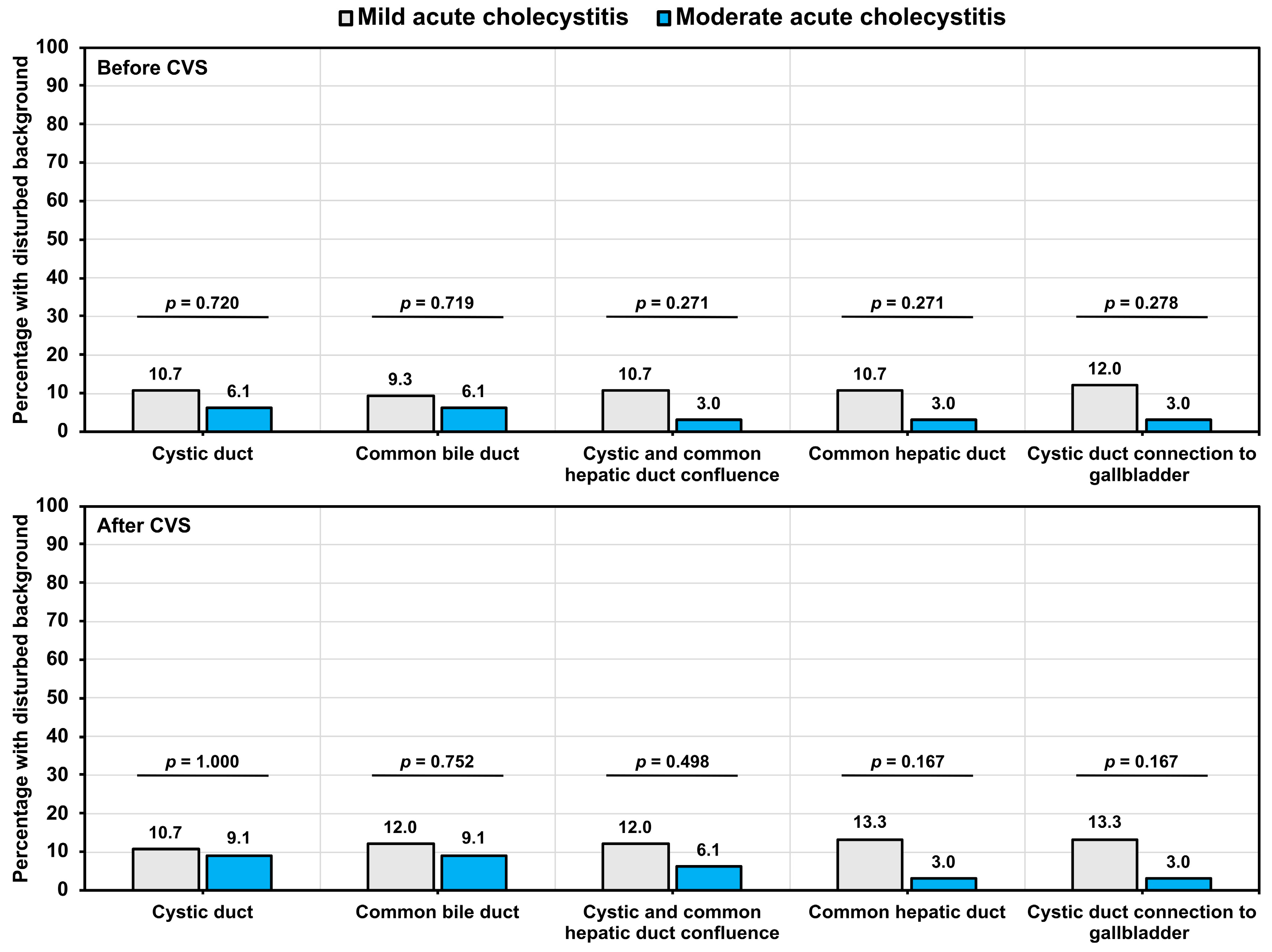
3.5. Assessment of Background Liver Fluorescence
3.6. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tazuma, S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract. Res. Clin. Gastroenterol. 2006, 20, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.E.; Khare, M.; Hill, M.; Maurer, K.R. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999, 117, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.F.; Méndez-Sánchez, N.; Portincasa, P.; van Erpecum, K.J. Gallstones. Nat. Rev. Dis. Primers 2016, 2, 16024. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W.; Ferguson, T. Acalculous Cholecystitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Hassler, K.R.; Collins, J.T.; Philip, K.; Jones, M.W. Laparoscopic Cholecystectomy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Huntington, C.R.; Cox, T.C.; Blair, L.J.; Prasad, T.; Lincourt, A.E.; Heniford, B.T.; Augenstein, V.A. Nationwide variation in outcomes and cost of laparoscopic procedures. Surg. Endosc. 2016, 30, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.A.; Junior, C.S.; Di Saveiro, S.; Sartelli, M.; Kelly, M.D.; Gomes, C.C.; Gomes, F.C.; Corrêa, L.D.; Alves, C.B.; de Fádel Guimarães, S. Acute calculous cholecystitis: Review of current best practices. World J. Gastrointest. Surg. 2017, 9, 118–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ismaeil, D.A. Avoidance of bile duct injury in laparoscopic cholecystectomy with feasible intraoperative resources: A cohort study. Biomed. Rep. 2024, 21, 110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seeras, K.; Qasawa, R.N.; Kashyap, S.; Kalani, A.D. Bile Duct Repair. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Liu, Y.; Peng, Y.; Su, S.; Fang, C.; Qin, S.; Wang, X.; Xia, X.; Li, B.; He, P. A meta-analysis of indocyanine green fluorescence image-guided laparoscopic cholecystectomy for benign gallbladder disease. Photofiagn. Photodyn. Ther. 2020, 32, 101948. [Google Scholar] [CrossRef] [PubMed]
- Sherwinter, D.A. Identification of anomolous biliary anatomy using near-infrared cholangiography. J. Gastrointest. Surg. 2012, 16, 1814–1815. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.; Badiu, D.C.; Davitoiu, D.; Tanasescu, C.; Tudosie, M.S.; Sabau, A.D.; Dascalu, A.M.; Tudor, C.; Balasescu, S.A.; Socea, B.; et al. Systematic review of the role of indocyanine green near-infrared fluorescence in safe laparoscopic cholecystectomy (Review). Exp. Ther. Med. 2022, 23, 187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meira, J.; Marques, M.L.; Falcão-Reis, F.; Rebelo Gomes, E.; Carneiro, Â. Immediate Reactions to Fluorescein and Indocyanine Green in Retinal Angiography: Review of Literature and Proposal for Patient’s Evaluation. Clin. Ophthalmol. 2020, 14, 171–178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Peng, W.; Yang, J.; Li, Y.; Yang, J.; Hu, X.; Xia, L.; Zhang, L.; Zhong, Y.; Qiao, L.; et al. Application of near-infrared fluorescent cholangiography using indocyanine green in laparoscopic cholecystectomy. J. Int. Med. Res. 2020, 48, 300060520979224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yokoe, M.; Hata, J.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Wakabayashi, G.; Kozaka, K.; Endo, I.; Deziel, D.J.; Miura, F.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018, 25, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Vettoretto, N.; Saronni, C.; Harbi, A.; Balestra, L.; Taglietti, L.; Giovanetti, M. Critical view of safety during laparoscopic cholecystectomy. JSLS J. Soc. Laparoendosc. Surg. 2011, 15, 322–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agnus, V.; Pesce, A.; Boni, L.; Van Den Bos, J.; Morales-Conde, S.; Paganini, A.M.; Quaresima, S.; Balla, A.; La Greca, G.; Plaudis, H.; et al. Fluorescence-based cholangiography: Preliminary results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg. Endosc. 2020, 34, 3888–3896. [Google Scholar] [CrossRef] [PubMed]
- Dip, F.; LoMenzo, E.; Sarotto, L.; Phillips, E.; Todeschini, H.; Nahmod, M.; Alle, L.; Schneider, S.; Kaja, L.; Boni, L.; et al. Randomized Trial of Near-infrared Incisionless Fluorescent Cholangiography. Ann. Surg. 2019, 270, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Fassari, A.; Bianucci, A.; Lucchese, S.; Santoro, E.; Lirici, M.M. Fluorescence cholangiography for laparoscopic cholecystectomy: How, when, and why? A single-center preliminary study. Minim. Invasive Ther. Allied Technol. 2023, 32, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Bleszynski, M.S.; DeGirolamo, K.M.; Meneghetti, A.T.; Chiu, C.J.; Panton, O.N. Fluorescent Cholangiography in Laparoscopic Cholecystectomy: An Updated Canadian Experience. Surg. Innov. 2020, 27, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Broderick, R.C.; Li, J.Z.; Huang, E.Y.; Blitzer, R.R.; Lee, A.M.; Serra, J.L.; Bouvet, M.; Sandler, B.J.; Jacobsen, G.R.; Horgan, S. Lighting the Way with Fluorescent Cholangiography in Laparoscopic Cholecystectomy: Reviewing 7 Years of Experience. J. Am. Coll. Surg. 2022, 235, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekara, C.S.; Shrestha, K.; Grossman, H.; Garcia, L.M.; Maqbool, B.; Luppens, C.; Dumas, R.P.; Morales, L.R.T.; Brahmbhatt, T.S.; Haqqani, M.; et al. A comparison of outcomes including bile duct injury of subtotal cholecystectomy versus open total cholecystectomy as bailout procedures for severe cholecystitis: A multicenter real-world study. Surgery 2024, 176, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Dip, F.; Aleman, J.; DeBoer, E.; Boni, L.; Bouvet, M.; Buchs, N.; Carus, T.; Diana, M.; Elli, E.F.; Hutteman, M.; et al. Use of fluorescence imaging and indocyanine green during laparoscopic cholecystectomy: Results of an international Delphi survey. Surgery 2022, 172, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, A.M.; Busch, O.R.; Besselink, M.G.; Ignatavicius, P.; Gulbinas, A.; Barauskas, G.; Gouma, D.J.; van Gulik, T.M. Long-Term Impact of Iatrogenic Bile Duct Injury. Dig. Surg. 2020, 37, 10–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiche, L.; Guieu, M.; Bachellier, P.; Suc, B.; Soubrane, O.; Boudjema, K.; Navarro, F.; Adam, R.; Vaillant, J.C.; Salame, E.; et al. Liver transplantation for iatrogenic bile duct injury during cholecystectomy: A French retrospective multicenter study. HPB 2022, 24, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kwon, J. Delayed laparoscopic cholecystectomy after more than 6 weeks on easily controlled cholecystitis patients. Korean J. Hepatobiliary Pancreat. Surg. 2013, 17, 60–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Liu, Y.Q.; Wang, C.; Cai, X.; Zheng, Z.X.; Bi, J.T. Can the parkland grading scale predict the difficulty of laparoscopic cholecystectomy? A new approach to validation. BMC Surg. 2023, 23, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pesce, A.; Piccolo, G.; Lecchi, F.; Fabbri, N.; Diana, M.; Feo, C.V. Fluorescent cholangiography: An up-to-date overview twelve years after the first clinical application. World J. Gastroenterol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pesce, A.; Piccolo, G.; La Greca, G.; Puleo, S. Utility of fluorescent cholangiography during laparoscopic cholecystectomy: A systematic review. World J. Gastroenterol. 2015, 21, 7877–7883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koong, J.K.; Ng, G.H.; Ramayah, K.; Koh, P.S.; Yoong, B.K. Early identification of the critical view of safety in laparoscopic cholecystectomy using indocyanine green fluorescence cholangiography: A randomised controlled study. Asian J. Surg. 2021, 44, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, G.; Wu, Y.; Cai, H. Assessment of liver injury using indocyanine green fluorescence imaging. Ann. Transl. Med. 2021, 9, 1167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakka, S.G. Assessment of liver perfusion and function by indocyanine green in the perioperative setting and in critically ill patients. J. Clin. Monit. Comput. 2018, 32, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Luo, H.; Zhu, W.; Yang, J.; Zeng, N.; Fan, Y.; Wen, S.; Xiang, N.; Jia, F.; Fang, C. Real-time navigation for laparoscopic hepatectomy using image fusion of preoperative 3D surgical plan and intraoperative indocyanine green fluorescence imaging. Surg. Endosc. 2020, 34, 3449–3459. [Google Scholar] [CrossRef] [PubMed]
- Aranda, F.P.; Škrabec, C.G.; López-Sánchez, J.; Pinedo, A.Z.; Álvarez, F.E.; Pérez, M.C.; López, J.N.; Vicente, C.H.; Piñeiro, L.V.; Andorrà, E.C. Indocyanine green (ICG) fluorescent cholangiography in laparoscopic cholecystectomy: Simplifying time and dose. Dig. Liver Dis. 2023, 55, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liao, C.H.; Diana, M.; Wang, S.Y.; Kong, S.H.; Yeh, C.N.; Dallemagne, B.; Marescaux, J.; Yeh, T.S. Near-infrared cholecystocholangiography with direct intragallbladder indocyanine green injection: Preliminary clinical results. Surg. Endosc. 2018, 32, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Buchs, N.C.; Hagen, M.E.; Pugin, F.; Volonte, F.; Bucher, P.; Schiffer, E.; Morel, P. Intra-operative fluorescent cholangiography using indocyanin green during robotic single site cholecystectomy. Int. J. Med. Robot. 2012, 8, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Spinoglio, G.; Priora, F.; Bianchi, P.P.; Lucido, F.S.; Licciardello, A.; Maglione, V.; Grosso, F.; Quarati, R.; Ravazzoni, F.; Lenti, L.M. Real-time near-infrared (NIR) fluorescent cholangiography in single-site robotic cholecystectomy (SSRC): A single-institutional prospective study. Surg. Endosc. 2013, 27, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, D.; Fernandes, E.; Wang, X.; Bianco, F.M.; Elli, E.F.; Ayloo, S.; Masrur, M.; Milone, L.; Giulianotti, P.C. Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: Results of 184 consecutive cases in a single institution. Surg. Innov. 2014, 21, 615–621. [Google Scholar] [CrossRef] [PubMed]
- van den Bos, J.; Schols, R.M.; Boni, L.; Cassinotti, E.; Carus, T.; Luyer, M.D.; Vahrmeijer, A.L.; Mieog, J.S.D.; Warnaar, N.; Berrevoet, F.; et al. Near-infrared fluorescence cholangiography assisted laparoscopic cholecystectomy (FALCON): An international multicentre randomized controlled trial. Surg. Endosc. 2023, 37, 4574–4584. [Google Scholar] [CrossRef] [PubMed]
- Manasseh, M.; Davis, H.; Bowling, K. Evaluating the Role of Indocyanine Green Fluorescence Imaging in Enhancing Safety and Efficacy During Laparoscopic Cholecystectomy: A Systematic Review. Cureus 2024, 16, e73388. [Google Scholar] [CrossRef]
- Onoe, S.; Maeda, A.; Takayama, Y.; Fukami, Y.; Kaneoka, Y. A preoperative predictive scoring system to predict the ability to achieve the critical view of safety during laparoscopic cholecystectomy for acute cholecystitis. HPB 2017, 19, 406–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dip, F.; Aleman, R.; Frieder, J.S.; Gomez, C.O.; Menzo, E.L.; Szomstein, S.; Rosenthal, R.J. Understanding intraoperative fluorescent cholangiography: Ten steps for an effective and successful procedure. Surg. Endosc. 2021, 35, 7042–7048. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.H.M.; Ng, H.J.; Wysocki, A.P.; Khan, K.S.; Gil, I.C. Achieving the critical view of safety in the difficult laparoscopic cholecystectomy: A prospective study of predictors of failure. Surg. Endosc. 2021, 35, 6039–6047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


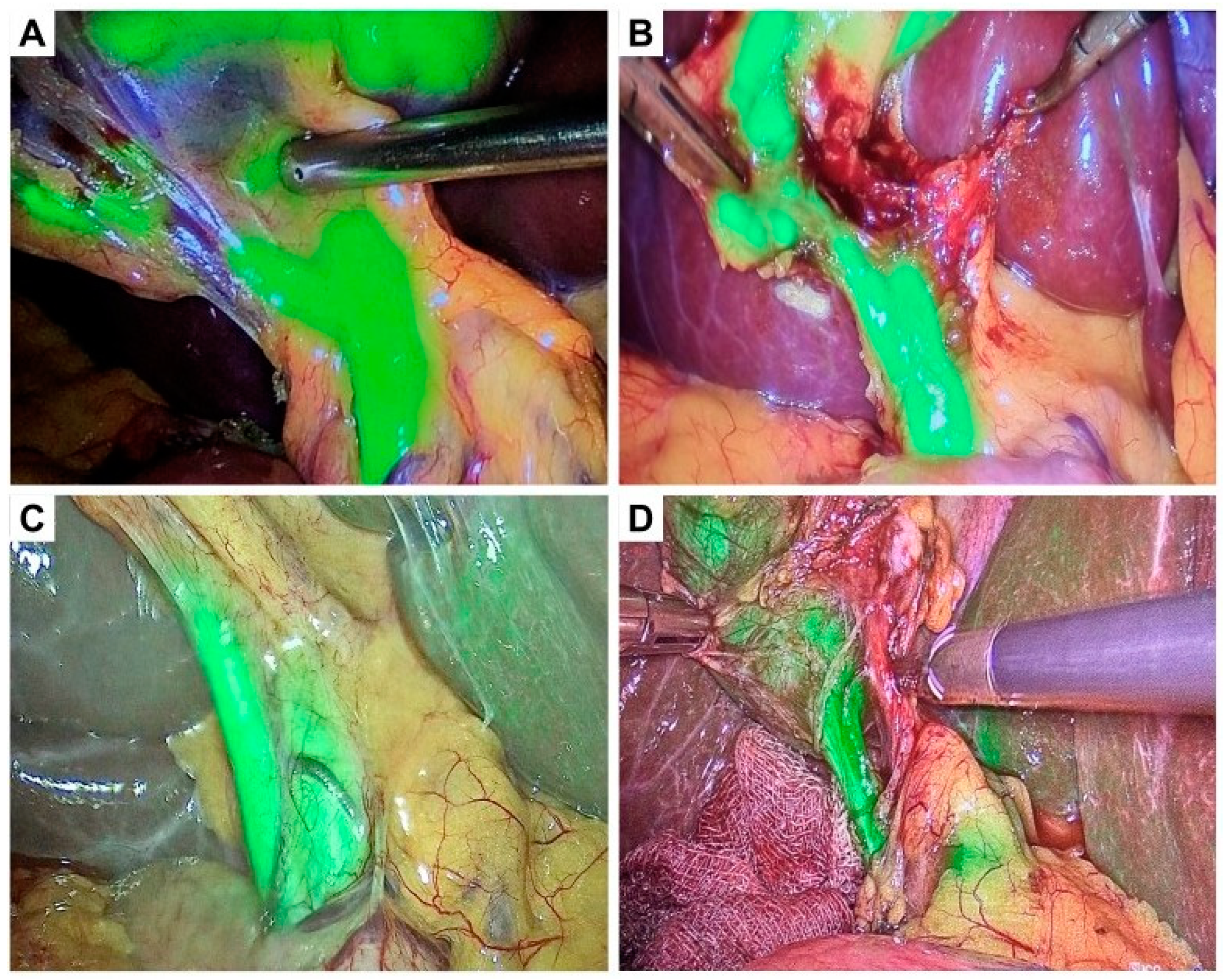
| Severity Grade | Diagnostic Criteria |
|---|---|
| Acute Cholecystitis | Definite diagnosis: one item in A + one item in B + C. Suspected diagnosis: one item in A + one item in B. A. Local signs of inflammation—(1) Murphy’s sign or (2) right upper quadrant mass/pain/tenderness. B. Systemic signs of inflammation—(1) Fever, (2) elevated CRP, or (3) elevated WBC count. C. Imaging findings—Imaging findings characteristic of acute cholecystitis. |
| Mild (Grade I) | Acute cholecystitis patient meeting any of the following criteria:
|
| Moderate (Grade II) | Acute cholecystitis patient presenting with any of the following symptoms:
|
| Severe (Grade III) | Acute cholecystitis patient presenting with dysfunction of any of the following organs/organ systems:
|
| CVS Step | CVS Step Description |
|---|---|
| I | Cranial retraction of the fundus part of the gallbladder. |
| II | Lateral retraction of the infundibulum part of the gallbladder. |
| III | Dissection of the visceral peritoneum with electrocoagulation, either laterally or medially, from the infundibulum part, ascending to the fundus part of the gallbladder. |
| IV | Dissection of the medial adipose tissue of the gallbladder with electrocoagulation, visualization and release of the cystic duct, and visualization of its entry into the gallbladder. |
| V | Total dissection of adipose tissue and formation of a “critical safety triangle” by separating cystic duct and cystic artery. |
| VI | Dissection of the infundibulum part of the gallbladder from adipose tissue and mobilization in the anterior/posterior parts, creating the “Calot’s triangle”. Visualization of the margin of the liver. |
| VII | Clipping of the cystic duct (distal/proximal) from the gallbladder and its resection. Clipping of cystic artery and its resection. |
| VIII | Dissection of the gallbladder from the liver bed. |
| Characteristics † | Mild Acute Cholecystitis (n = 75) | Moderate Acute Cholecystitis (n = 33) | p-Value |
|---|---|---|---|
| Patient gender | |||
| Male | 23 (59%) | 16 (41%) | p = 0.076 ** |
| Female | 52 (75%) | 17 (25%) | |
| Age (years) | 54 (24 to 85) | 64 (24 to 86) | p = 0.014 * |
| BMI (kg/m2) | 27.9 (7.1) | 28.0 (6.6) | p = 0.038 * |
| WBC count on admission (109 cells/L) | 12.0 (4.0) | 14.0 (5.5) | p < 0.001 * |
| CRP on admission (mg/L) | 16.5 (38.5) | 42.0 (67.5) | p < 0.001 * |
| Characteristics † | Mild Acute Cholecystitis (n = 75) | Moderate Acute Cholecystitis (n = 33) | p-Value |
|---|---|---|---|
| Intraoperative detection of peri-vesicular infiltration | |||
| Yes | 15 (20%) | 22 (67%) | p < 0.001 * |
| No | 60 (80%) | 11 (33%) | |
| Gallbladder empyema | |||
| Yes | 10 (13%) | 18 (55%) | p < 0.001 * |
| No | 65 (87%) | 15 (45%) | |
| No. of CVS Steps Performed | Mild Acute Cholecystitis | Moderate Acute Cholecystitis | p-Value * |
|---|---|---|---|
| 0 steps | 0 (0%) | 1 (3%) | 0.002 |
| 1 step | 0 (0%) | 1 (3%) | |
| 2 steps | 1 (1%) | 0 (0%) | |
| 3 steps | 0 (0%) | 0 (0%) | |
| 4 steps | 2 (3%) | 2 (6%) | |
| 5 steps | 5 (7%) | 6 (18%) | |
| 6 steps | 16 (21%) | 9 (27%) | |
| 7 steps | 8 (11%) | 5 (16%) | |
| 8 steps | 43 (57%) | 9 (27%) |
| No. of CVS Steps Performed | Operation Time (min) | Mann–Whitney p-Value | |||
|---|---|---|---|---|---|
| Mild Acute Cholecystitis | Moderate Acute Cholecystitis | ||||
| Median | IQR | Median | IQR | ||
| 0–4 steps (n = 7) | 45.0 | - | 105.0 | 47.5 | 0.057 |
| 5–8 steps (n = 101) | 55.0 | 25.0 | 75.0 | 30.0 | <0.001 |
| Mann–Whitney p-value | 0.106 | 0.009 | - | ||
| Structure (n = 108) | Severity of Acute Cholecystitis | Usefulness of ICG for CVS | Chi-Square Test p-Value | |
|---|---|---|---|---|
| Not Helpful | Helpful | |||
| Cystic duct | Mild (n = 75) | 13 (17%) | 62 (83%) | p < 0.001 |
| Moderate (n = 33) | 17 (52%) | 16 (49%) | ||
| Common bile duct | Mild (n = 75) | 27 (36%) | 48 (64%) | p = 0.072 |
| Moderate (n = 33) | 18 (55%) | 15 (45%) | ||
| Cystic and common hepatic duct confluence | Mild (n = 75) | 53 (71%) | 22 (29%) | p = 0.054 |
| Moderate (n = 33) | 29 (88%) | 4 (12%) | ||
| Common hepatic duct | Mild (n = 75) | 38 (51%) | 37 (49%) | p = 0.033 |
| Moderate (n = 33) | 24 (73%) | 9 (27%) | ||
| Cystic duct connection to gallbladder | Mild (n = 75) | 52 (69%) | 23 (31%) | p = 0.312 |
| Moderate (n = 33) | 26 (79%) | 7 (21%) | ||
| Characteristics † | Mild Acute Cholecystitis (n = 75) | Moderate Acute Cholecystitis (n = 33) | p-Value |
|---|---|---|---|
| Operation time (min) | 60.0 (25.0) | 85.0 (37.5) | p < 0.001 * |
| Conversion to open approach | |||
| Yes | 0 (0%) | 3 (9%) | p = 0.027 ** |
| No | 75 (100%) | 30 (91%) | |
| WBC count on discharge (109 cells/L) | 7.0 (3.0) | 10.0 (4.5) | p = 0.002 * |
| CRP on discharge (mg/L) | 30.0 (53.0) | 60.0 (105.5) | p = 0.002 * |
| Hospitalization length (days) | 6.0 (3.0) | 6.0 (4.5) | p = 0.437 * |
| Length of postoperative stay (days) | 2.0 (2.0) | 3.0 (2.0) | p < 0.001 * |
| Biliovascular injuries (No. of patients) | 0 (0) | 0 (0) | - |
| Mortality (No. of patients) | 0 (0) | 0 (0) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavulans, J.; Jain, N.; Zeiza, K.; Sondore, E.; Cerpakovska, K.B.; Opincans, J.; Atstupens, K.; Plaudis, H. Fluorescence Cholangiography for Extrahepatic Bile Duct Visualization in Urgent Mild and Moderate Acute Cholecystitis Patients Undergoing Laparoscopic Cholecystectomy: A Prospective Pilot Study. J. Clin. Med. 2025, 14, 541. https://doi.org/10.3390/jcm14020541
Pavulans J, Jain N, Zeiza K, Sondore E, Cerpakovska KB, Opincans J, Atstupens K, Plaudis H. Fluorescence Cholangiography for Extrahepatic Bile Duct Visualization in Urgent Mild and Moderate Acute Cholecystitis Patients Undergoing Laparoscopic Cholecystectomy: A Prospective Pilot Study. Journal of Clinical Medicine. 2025; 14(2):541. https://doi.org/10.3390/jcm14020541
Chicago/Turabian StylePavulans, Janis, Nityanand Jain, Kaspars Zeiza, Elza Sondore, Krista Brigita Cerpakovska, Janis Opincans, Kristaps Atstupens, and Haralds Plaudis. 2025. "Fluorescence Cholangiography for Extrahepatic Bile Duct Visualization in Urgent Mild and Moderate Acute Cholecystitis Patients Undergoing Laparoscopic Cholecystectomy: A Prospective Pilot Study" Journal of Clinical Medicine 14, no. 2: 541. https://doi.org/10.3390/jcm14020541
APA StylePavulans, J., Jain, N., Zeiza, K., Sondore, E., Cerpakovska, K. B., Opincans, J., Atstupens, K., & Plaudis, H. (2025). Fluorescence Cholangiography for Extrahepatic Bile Duct Visualization in Urgent Mild and Moderate Acute Cholecystitis Patients Undergoing Laparoscopic Cholecystectomy: A Prospective Pilot Study. Journal of Clinical Medicine, 14(2), 541. https://doi.org/10.3390/jcm14020541









