Elevated Interleukin-6 Is Associated with an Increased Risk of Long-Term Arteriovenous Fistula Failure for Dialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Pre-Operative Vascular Mapping
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 18 August 2024).
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ashby, D.; Borman, N.; Burton, J.; Corbett, R.; Davenport, A.; Farrington, K.; Flowers, K.; Fotheringham, J.; Andrea Fox, R.N.; Franklin, G.; et al. Renal Association Clinical Practice Guideline on Haemodialysis. BMC Nephrol. 2019, 20, 379. [Google Scholar] [CrossRef]
- Schmidli, J.; Widmer, M.K.; Basile, C.; de Donato, G.; Gallieni, M.; Gibbons, C.P.; Haage, P.; Hamilton, G.; Hedin, U.; Kamper, L.; et al. Editor’s Choice—Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2018, 55, 757–818. [Google Scholar] [CrossRef]
- Brown, R.S. Barriers to Optimal Vascular Access for Hemodialysis. Semin. Dial. 2020, 33, 457–463. [Google Scholar] [CrossRef]
- Lawson, J.H.; Niklason, L.E.; Roy-Chaudhury, P. Challenges and Novel Therapies for Vascular Access in Haemodialysis. Nat. Rev. Nephrol. 2020, 16, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.E.; Huber, T.S.; Orchanian-Cheff, A.; Rajan, D.K. Arteriovenous Access for Hemodialysis: A Review. JAMA 2024, 331, 1307–1317. [Google Scholar] [CrossRef]
- Brahmbhatt, A.; Remuzzi, A.; Franzoni, M.; Misra, S. The Molecular Mechanisms of Hemodialysis Vascular Access Failure. Kidney Int. 2016, 89, 303–316. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Florea, E.; Arbănași, E.-M.; Bartus, R.; Arbănași, E.-M.; Ion, A.P.; Cordoș, B.A.; Halatiu, V.B.; Niculescu, R.; Stoian, A.; et al. Elevated Leukocyte Glucose Index Is Associated with Long-Term Arteriovenous Fistula Failure in Dialysis Patients. J. Clin. Med. 2024, 13, 2037. [Google Scholar] [CrossRef] [PubMed]
- Kaller, R.; Russu, E.; Arbănași, E.M.; Mureșan, A.V.; Jakab, M.; Ciucanu, C.C.; Arbănași, E.M.; Suciu, B.A.; Hosu, I.; Demian, L.; et al. Intimal CD31-Positive Relative Surfaces Are Associated with Systemic Inflammatory Markers and Maturation of Arteriovenous Fistula in Dialysis Patients. J. Clin. Med. 2023, 12, 4419. [Google Scholar] [CrossRef] [PubMed]
- Kaller, R.; Arbănași, E.M.; Mureșan, A.V.; Voidăzan, S.; Arbănași, E.M.; Horváth, E.; Suciu, B.A.; Hosu, I.; Halmaciu, I.; Brinzaniuc, K.; et al. The Predictive Value of Systemic Inflammatory Markers, the Prognostic Nutritional Index, and Measured Vessels’ Diameters in Arteriovenous Fistula Maturation Failure. Life 2022, 12, 1447. [Google Scholar] [CrossRef] [PubMed]
- Fattori, E.; Cappelletti, M.; Costa, P.; Sellitto, C.; Cantoni, L.; Carelli, M.; Faggioni, R.; Fantuzzi, G.; Ghezzi, P.; Poli, V. Defective Inflammatory Response in Interleukin 6-Deficient Mice. J. Exp. Med. 1994, 180, 1243–1250. [Google Scholar] [CrossRef]
- Baek, J.; Lee, H.; Yang, T.; Lee, S.-Y.; Kim, Y.G.; Kim, J.S.; Ahn, S.; Kim, K.; Kang, S.H.; Lee, M.-J.; et al. Plasma Interleukin-6 Level Predicts the Risk of Arteriovenous Fistula Dysfunction in Patients Undergoing Maintenance Hemodialysis. J. Pers. Med. 2023, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Ko, Y.-S.; Ko, P.-J.; Hsu, L.-A.; Chen, C.-F.; Yang, C.-W.; Hsu, T.-S.; Pang, J.-H.S. Thrombosed Arteriovenous Fistula for Hemodialysis Access Is Characterized by a Marked Inflammatory Activity. Kidney Int. 2005, 68, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Dukkipati, R.; Molnar, M.Z.; Park, J.; Jing, J.; Kovesdy, C.P.; Kajani, R.; Kalantar-Zadeh, K. Association of Vascular Access Type with Inflammatory Marker Levels in Maintenance Hemodialysis Patients. Semin. Dial. 2014, 27, 415–423. [Google Scholar] [CrossRef]
- Kaygin, M.A.; Halici, U.; Aydin, A.; Dag, O.; Binici, D.N.; Limandal, H.K.; Arslan, Ü.; Kiymaz, A.; Kahraman, N.; Calik, E.S.; et al. The Relationship between Arteriovenous Fistula Success and Inflammation. Ren. Fail. 2013, 35, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, L.; Gao, M.; Wang, Y.; Yu, J. Microinflammation Is Involved in the Dysfunction of Arteriovenous Fistula in Patients with Maintenance Hemodialysis. Chin. Med. J. 2008, 121, 2157–2161. [Google Scholar] [CrossRef]
- Hu, S.; Wang, D.; Ma, T.; Yuan, F.; Zhang, Y.; Gao, X.; Lei, Q.; Cheng, J. Association between Preoperative Monocyte-to-Lymphocyte Ratio and Late Arteriovenous Fistula Dysfunction in Hemodialysis Patients: A Cohort Study. Am. J. Nephrol. 2021, 52, 854–860. [Google Scholar] [CrossRef]
- Sarioglu, O.; Capar, A.E.; Belet, U. Relationship of Arteriovenous Fistula Stenosis and Thrombosis with the Platelet–Lymphocyte Ratio in Hemodialysis Patients. J. Vasc. Access 2020, 21, 630–635. [Google Scholar] [CrossRef]
- Pasqui, E.; de Donato, G.; Lazzeri, E.; Molino, C.; Galzerano, G.; Giubbolini, M.; Palasciano, G. High Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios Are Associated with a Higher Risk of Hemodialysis Vascular Access Failure. Biomedicines 2022, 10, 2218. [Google Scholar] [CrossRef] [PubMed]
- Wongmahisorn, Y. Role of Neutrophil-to-Lymphocyte Ratio as a Prognostic Indicator for Hemodialysis Arteriovenous Fistula Failure. J. Vasc. Access 2019, 20, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Usman, R.; Jamil, M.; Naveed, M. High Preoperative Neutrophil-Lymphocyte Ratio (NLR) and Red Blood Cell Distribution Width (RDW) as Independent Predictors of Native Arteriovenous Fistula Failure. Ann. Vasc. Dis. 2017, 10, 205–210. [Google Scholar] [CrossRef][Green Version]
- Yilmaz, H.; Bozkurt, A.; Cakmak, M.; Celik, H.T.; Bilgic, M.A.; Bavbek, N.; Akcay, A. Relationship between Late Arteriovenous Fistula (AVF) Stenosis and Neutrophil-Lymphocyte Ratio (NLR) in Chronic Hemodialysis Patients. Ren. Fail. 2014, 36, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Bashar, K.; Zafar, A.; Ahmed, K.; Kheirelseid, E.A.H.; Healy, D.; Clarke-Moloney, M.; Burke, P.E.; Kavanagh, E.G.; Walsh, S.R. Can a Neutrophil-Lymphocyte Ratio Derived from Preoperative Blood Tests Predict Arteriovenous Fistula Maturation? Ann. Vasc. Surg. 2016, 35, 60–67. [Google Scholar] [CrossRef]
- Martinez, L.; Perla, M.; Tabbara, M.; Duque, J.C.; Rojas, M.G.; Falcon, N.S.; Pereira-Simon, S.; Salman, L.H.; Vazquez-Padron, R.I. Systemic Profile of Cytokines in Arteriovenous Fistula Patients and Their Associations with Maturation Failure. Kidney360 2022, 3, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Maggiore, U.; Taccola, D.; Migliori, M.; Rizza, G.M.; Consani, C.; Bertini, A.; Sposini, S.; Perez-Garcia, R.; Rindi, P.; et al. Interleukin-6 Is a Stronger Predictor of Total and Cardiovascular Mortality than C-Reactive Protein in Haemodialysis Patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2004, 19, 1154–1160. [Google Scholar] [CrossRef]

| Variables | All Patients n = 91 | Functional AVF n = 74 | AVF Failure n = 17 | p Value |
|---|---|---|---|---|
| Age mean ± SD | 62.93 ± 13.28 | 62.97 ± 13.28 | 62.76 ± 13.68 | 0.955 |
| Male gender no. (%) | 49 (53.85%) | 42 (56.76%) | 7 (41.18%) | 0.245 |
| Comorbidities and Risk factors, no. (%) | ||||
| Hypertension | 83 (91.21%) | 68 (91.89%) | 15 (88.24%) | 0.631 |
| Atrial Fibrillation | 11 (12.09%) | 8 (10.81%) | 3 (17.65%) | 0.436 |
| Diabetes Mellitus | 36 (39.56%) | 25 (33.78%) | 11 (64.71%) | 0.019 |
| Ischemic Heart Disease | 58 (63.74%) | 45 (60.81%) | 13 (76.47%) | 0.226 |
| Peripheral Arterial Disease | 17 (18.68%) | 12 (16.22%) | 5 (29.41%) | 0.208 |
| History of Malignancy | 7 (7.69%) | 4 (5.41%) | 3 (17.65%) | 0.088 |
| History of Myocardial Infarction | 7 (7.69%) | 5 (6.76%) | 2 (11.76%) | 0.485 |
| History of Stroke | 6 (6.59%) | 5 (6.76%) | 1 (5.88%) | 0.895 |
| Active Smoking | 14 (15.38%) | 8 (10.81%) | 6 (35.29%) | 0.012 |
| Obesity | 23 (25.27%) | 19 (25.68%) | 4 (25.53%) | 0.854 |
| Laboratory data, median (Q1–Q3) | ||||
| WBC | 7.81 (6.34–9.51) | 7.20 (6.14–9.02) | 9.40 (8.19–10.60) | 0.008 |
| Potassium mmol/L | 5.18 (4.66–5.65) | 5.13 (4.59–5.55) | 5.23 (4.86–6.03) | 0.075 |
| Sodium mmol/L | 139 (137–141) | 140 (137–141) | 139 (138–140) | 0.674 |
| Glucose (mg/dL) | 102.50 (89–136.22) | 97.60 (88–126.90) | 128.90 (106–192.5) | 0.007 |
| BUN (mg/dL) | 134.76 (90.8–168.73) | 134.82 (93.4–167.1) | 123.90 (90.5–174) | 0.945 |
| Creatinine (mg/dL) | 6.69 (5.50–8.81) | 6.72 (5.41–9.09) | 6.65 (5.85–7.55) | 0.909 |
| Hemoglobin g/dL | 10.60 (9.21–11.4) | 10.47 (8.7–11.25) | 10.60 (10.10–11.80) | 0.158 |
| Hematocrit % | 31.72 (28.5–35.21) | 31.66 (27.57–35.07) | 32.30 (30.30–36.10) | 0.269 |
| Neutrophils ×10³/µL | 5.20 (4.17–6.54) | 4.95 (4.15–6.30) | 6.68 (4.86–8.5) | 0.023 |
| Lymphocytes ×10³/µL | 1.41 (1.11–1.93) | 1.41 (1.09–2) | 1.35 (1.22–1.81) | 0.891 |
| Monocyte ×10³/µL | 0.58 (0.445–0.77) | 0.57 (0.42–0.76) | 0.69 (0.57–0.77) | 0.132 |
| PLT ×10³/µL | 211 (171.50–281.50) | 201.5 (168.25–272) | 245 (211–304) | 0.048 |
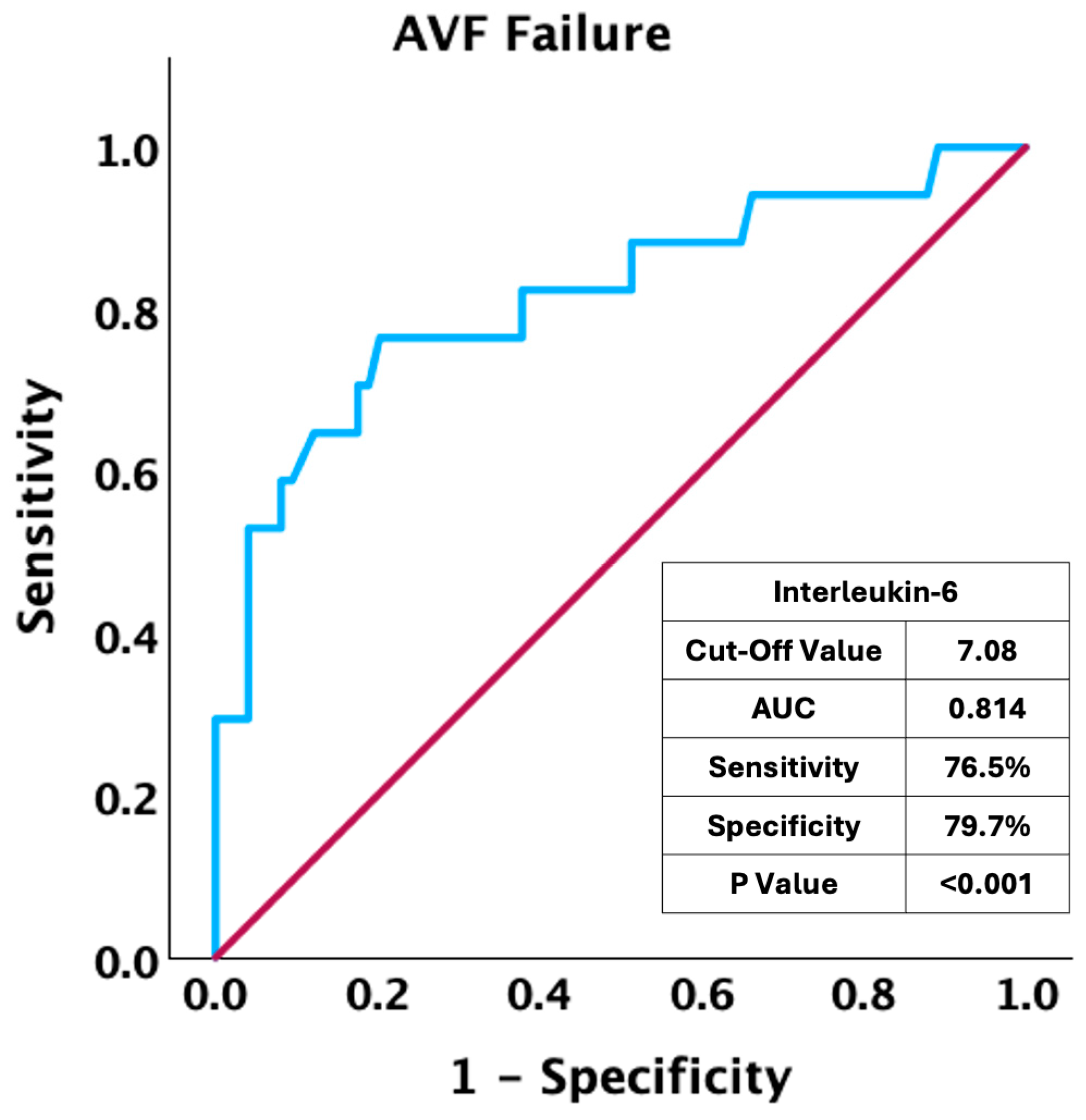
| Interleukin-6 pg/mL | 5.91 (4.71–7.61) | 5.70 (4.47–6.97) | 8.29 (6.11–11.4) | <0.001 |
| Vascular Mapping Determinations, median (Q1–Q3) | ||||
| Arterial Diameter (mm) | 2.80 (2.27–3.50) | 2.95 (2.30–3.50) | 2.35 (2–4.50) | 0.353 |
| Vein Diameter (mm) | 3 (2.40–3.50) | 3.10 (2.42–3.50) | 2.50 (2.40–3) | 0.143 |
| Vein Depth (mm) | 2.50 (1.99–3.50) | 2.50 (2–3.50) | 2.00 (1.80–3.40) | 0.430 |
| AVF Type and Placement, no. (%) | ||||
| RC-AVF | 41 (45.05%) | 31 (41.89%) | 10 (58.82%) | 0.206 |
| BC-AVF | 39 (42.86%) | 34 (45.95%) | 5 (29.41%) | 0.214 |
| BB-AVF | 11 (12.09%) | 9 (12.16%) | 2 (11.76%) | 0.964 |
| Dominant Upper Limb | 15 (16.48%) | 12 (16.22%) | 3 (17.65%) | 0.886 |
| Dialysis on CVC at the time of AVF creation, no. (%) | 53 (58.24%) | 46 (62.16%) | 7 (41.18%) | 0.114 |
| Follow-up period (years) mean ± SD | 1.53 ± 0.94 | 1.67 ± 0.89 | 0.91 ± 0.93 | 0.002 |
| Variables | AVF Failure | |||
|---|---|---|---|---|
| HR * | 95% CI | p Value | ||
| Diabetes Mellitus | 2.69 | 0.99–7.31 | 0.051 | |
| History of Malignancy | 4.25 | 1.19–15.21 | 0.026 | |
| Active Smoking | 2.94 | 1.07–8.06 | 0.036 | |
| IL-6 | Model 1 | 2.23 | 1.57–3.18 | <0.001 |
| Model 2 | 2.18 | 1.53–3.12 | <0.001 | |
| Model 3 | 1.96 | 1.31–2.97 | 0.001 | |
| IL-6—RC-AVF | Model 1 | 4.06 | 1.73–9.52 | 0.001 |
| IL-6—BC-AVF | Model 1 | 2.33 | 1.21–4.51 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciucanu, C.C.; Mureșan, A.; Florea, E.; Réka, B.; Mureșan, A.V.; Szanto, L.-A.; Arbănași, E.-M.; Hosu, I.; Russu, E.; Arbănași, E.-M. Elevated Interleukin-6 Is Associated with an Increased Risk of Long-Term Arteriovenous Fistula Failure for Dialysis. J. Clin. Med. 2025, 14, 488. https://doi.org/10.3390/jcm14020488
Ciucanu CC, Mureșan A, Florea E, Réka B, Mureșan AV, Szanto L-A, Arbănași E-M, Hosu I, Russu E, Arbănași E-M. Elevated Interleukin-6 Is Associated with an Increased Risk of Long-Term Arteriovenous Fistula Failure for Dialysis. Journal of Clinical Medicine. 2025; 14(2):488. https://doi.org/10.3390/jcm14020488
Chicago/Turabian StyleCiucanu, Claudiu Constantin, Alexandru Mureșan, Elena Florea, Bartus Réka, Adrian Vasile Mureșan, Ludovic-Alexandru Szanto, Eliza-Mihaela Arbănași, Ioan Hosu, Eliza Russu, and Emil-Marian Arbănași. 2025. "Elevated Interleukin-6 Is Associated with an Increased Risk of Long-Term Arteriovenous Fistula Failure for Dialysis" Journal of Clinical Medicine 14, no. 2: 488. https://doi.org/10.3390/jcm14020488
APA StyleCiucanu, C. C., Mureșan, A., Florea, E., Réka, B., Mureșan, A. V., Szanto, L.-A., Arbănași, E.-M., Hosu, I., Russu, E., & Arbănași, E.-M. (2025). Elevated Interleukin-6 Is Associated with an Increased Risk of Long-Term Arteriovenous Fistula Failure for Dialysis. Journal of Clinical Medicine, 14(2), 488. https://doi.org/10.3390/jcm14020488






