The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment
Abstract
1. Introduction
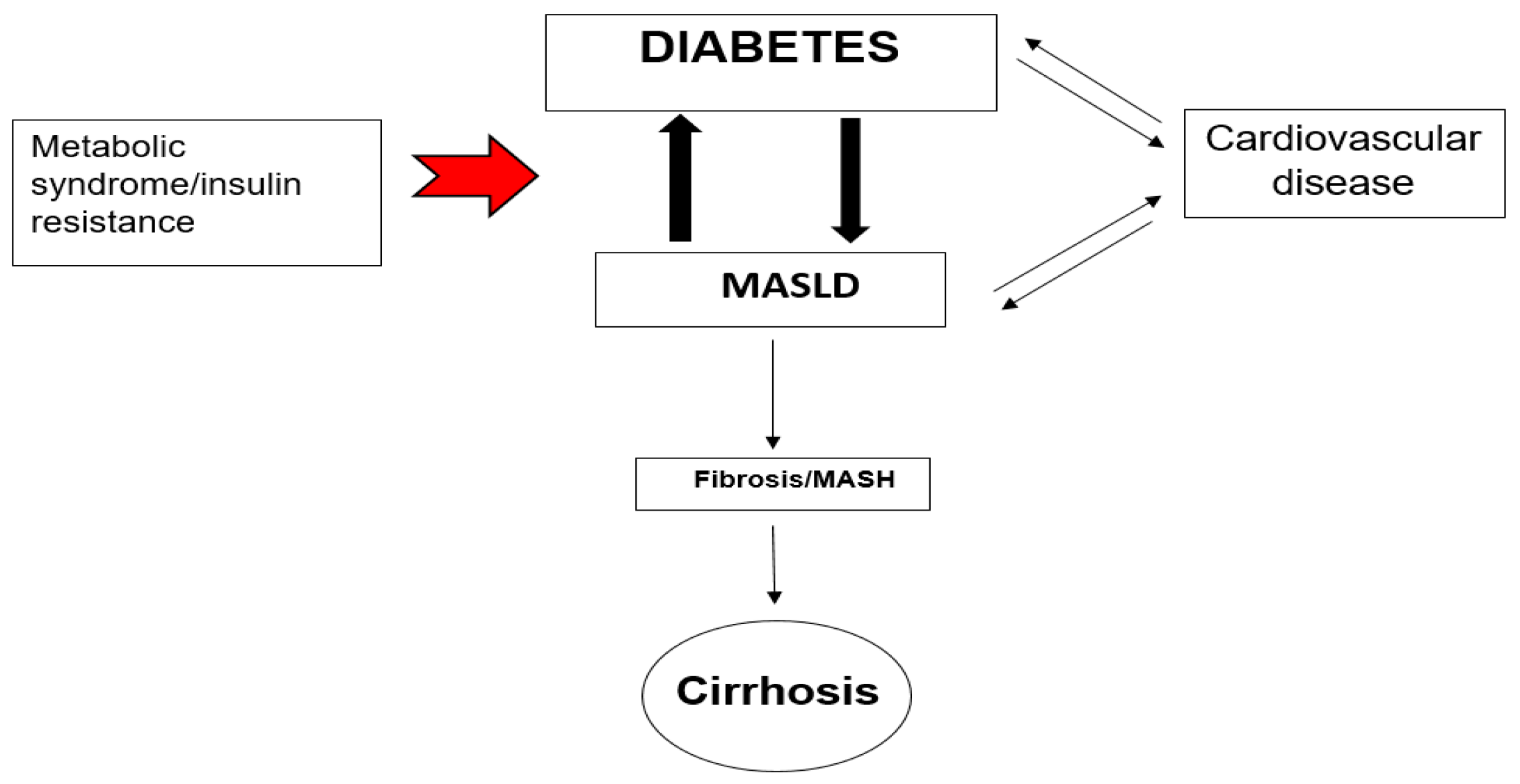
2. Pathophysiological Link Between MASLD, T2DM and CVD
2.1. Genetic Factors
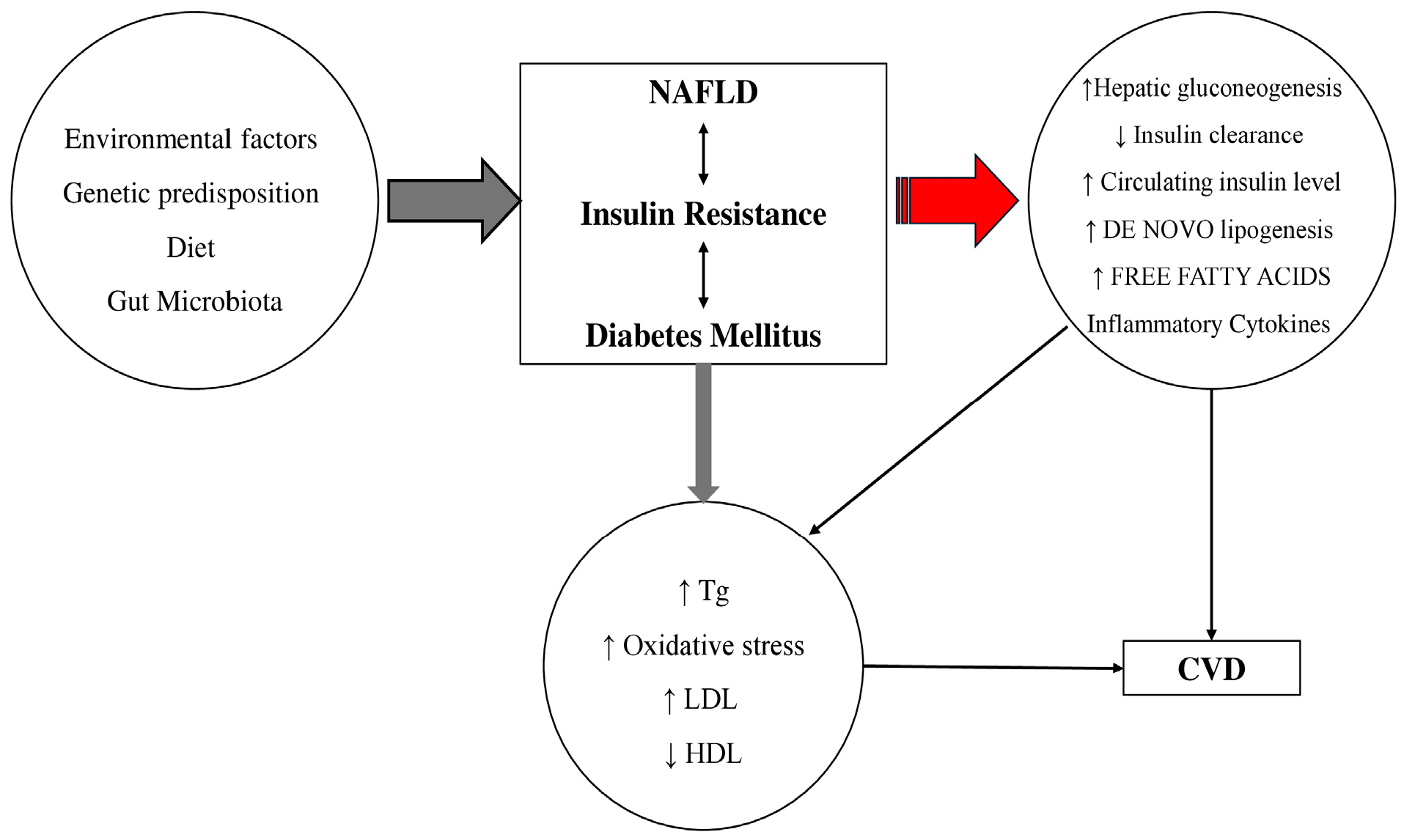
2.2. Insulin Resistance, Lipotoxicity
2.3. Inflammation, Cytokines, Oxidative Stress
2.4. ΜAFLD and CVD
2.5. The Gut Microbiota
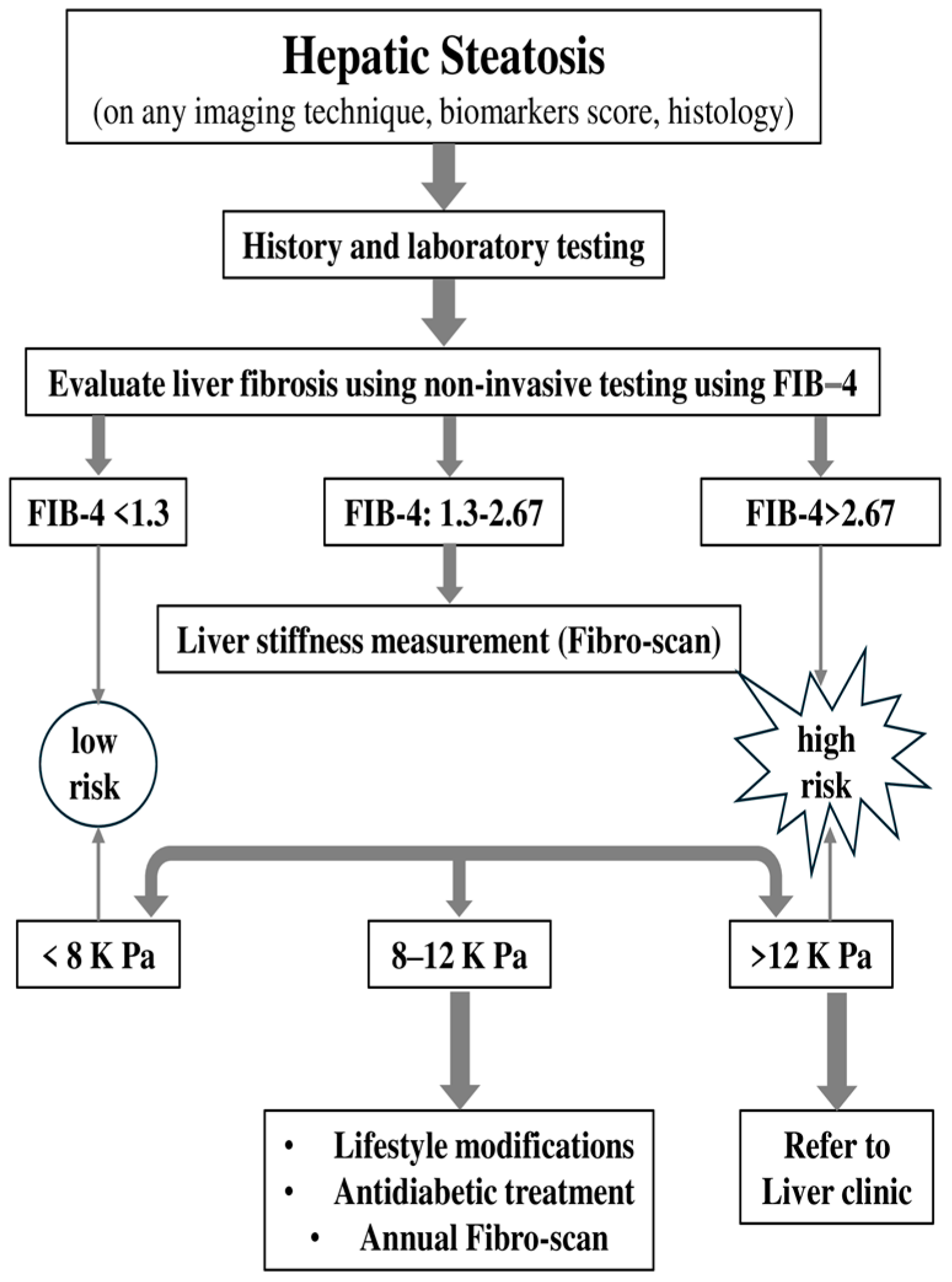
3. Monitoring Patients with ΜASLD
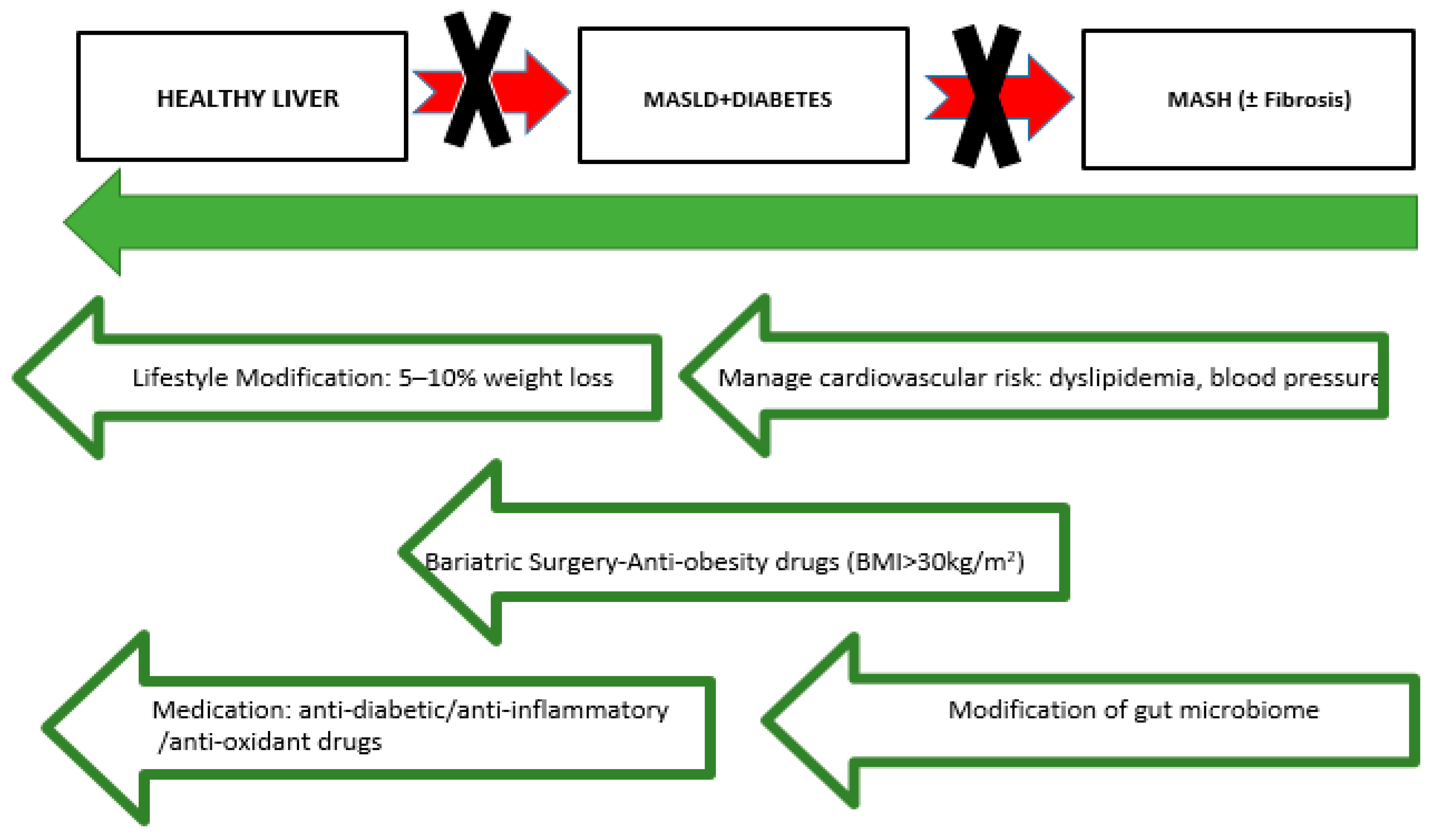
4. Current Therapeutic Approaches for Managing NAFLD-MASLD, T2DM, and CVD Risk Factors
4.1. Lifestyle Modifications
4.2. Vitamin E
4.3. Lipid-Lowering Therapies
4.4. Blood Pressure Control
4.5. Metformin: The First-Line Drug
4.6. GLP-1 Receptor Agonists
4.7. SGLT2 Inhibitors
4.8. Combination Therapy GLP-1 Receptor Agonists/SGLT2 Inhibitors
4.9. Pioglitazone
4.10. DPP-4 Inhibitors
4.11. Bariatric Surgery
4.12. Other Therapeutic Targets
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Gaal, L.F.; Mertens, J.; Francque, S.; De Block, C. Therapeutic approaches for non-alcoholic steatohepatitis. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211034300. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef]
- Wong, V.W.; Ekstedt, M.; Wong, G.L.; Hagstrom, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Leyh, C.; Coombes, J.D.; Schmidt, H.H.; Canbay, A.; Manka, P.P.; Best, J. MASLD-Related HCC-Update on Pathogenesis and Current Treatment Options. J. Pers. Med. 2024, 14, 370. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Cariou, B.; Byrne, C.D.; Loomba, R.; Sanyal, A.J. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes. Metab. 2021, 23, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- McGlone, E.R.; Tan, T.M. Glucagon-based therapy for people with diabetes and obesity: What is the sweet spot? Peptides 2024, 176, 171219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fleishman, J.S.; Li, T.; Li, Y.; Ren, Z.; Chen, J.; Ding, M. Pharmacological therapy of metabolic dysfunction-associated steatotic liver disease-driven hepatocellular carcinoma. Front. Pharmacol. 2023, 14, 1336216. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Caussy, C.; Aubin, A.; Loomba, R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr. Diab. Rep. 2021, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Chalasani, N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology 2009, 49, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, S.I.; Tamaki, N.; Kimura, T.; Umemura, T.; Kurosaki, M.; Izumi, N. Natural history of lean and non-lean metabolic dysfunction-associated steatotic liver disease. J. Gastroenterol. 2024, 59, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.; Dhami-Shah, H.; Shah, S.R. Antidiabetic Drugs and Statins in Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mi, Z.; Peng, J.; Yang, T.; Han, Y.; Zhai, Y.; Song, C.; Teng, X.; Sun, W.; Guo, J.; et al. Nonalcoholic Fatty Liver Disease as an Emerging Risk Factor and Potential Intervention Target for Atherosclerotic Cardiovascular Diseases. J. Cardiovasc. Pharmacol. 2023, 81, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego-Duran, R.; Gallego, P.; Grande, L. Genetic and Epigenetic Regulation in Nonalcoholic Fatty Liver Disease (NAFLD). Int. J. Mol. Sci. 2018, 19, 911. [Google Scholar] [CrossRef] [PubMed]
- Kahali, B.; Liu, Y.L.; Daly, A.K.; Day, C.P.; Anstee, Q.M.; Speliotes, E.K. TM6SF2: Catch-22 in the fight against nonalcoholic fatty liver disease and cardiovascular disease? Gastroenterology 2015, 148, 679–684. [Google Scholar] [CrossRef]
- Lee, B.W.; Lee, Y.H.; Park, C.Y.; Rhee, E.J.; Lee, W.Y.; Kim, N.H.; Choi, K.M.; Park, K.G.; Choi, Y.K.; Cha, B.S.; et al. Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Position Statement of the Fatty Liver Research Group of the Korean Diabetes Association. Diabetes Metab. J. 2020, 44, 382–401. [Google Scholar] [CrossRef]
- Wu, B.; Lan, X.; Gao, M.; Wei, W.; Wang, Y.; Yang, Y.; Yu, Z.; Huang, M.; Wu, Q. Elucidation of the molecular mechanism of type 2 diabetes mellitus affecting the progression of nonalcoholic steatohepatitis using bioinformatics and network pharmacology: A review. Medicine 2024, 103, e39731. [Google Scholar] [CrossRef]
- Zachou, M.; Flevari, P.; Nasiri-Ansari, N.; Varytimiadis, C.; Kalaitzakis, E.; Kassi, E.; Androutsakos, T. The role of anti-diabetic drugs in NAFLD. Have we found the Holy Grail? A narrative review. Eur. J. Clin. Pharmacol. 2024, 80, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab. Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Jelenik, T.; Kaul, K.; Sequaris, G.; Flogel, U.; Phielix, E.; Kotzka, J.; Knebel, B.; Fahlbusch, P.; Horbelt, T.; Lehr, S.; et al. Mechanisms of Insulin Resistance in Primary and Secondary Nonalcoholic Fatty Liver. Diabetes 2017, 66, 2241–2253. [Google Scholar] [CrossRef]
- Finck, B.N. Targeting Metabolism, Insulin Resistance, and Diabetes to Treat Nonalcoholic Steatohepatitis. Diabetes 2018, 67, 2485–2493. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef]
- Manka, P.P.; Kaya, E.; Canbay, A.; Syn, W.K. A Review of the Epidemiology, Pathophysiology, and Efficacy of Anti-diabetic Drugs Used in the Treatment of Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2021, 66, 3676–3688. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Ziamanesh, F.; Mohammadi, M.; Ebrahimpour, S.; Tabatabaei-Malazy, O.; Mosallanejad, A.; Larijani, B. Unraveling the link between insulin resistance and Non-alcoholic fatty liver disease (or metabolic dysfunction-associated steatotic liver disease): A Narrative Review. J. Diabetes Metab. Disord. 2023, 22, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Deng, H.; Ma, R.; Liao, J.Y.; Liang, H.; Hu, J.; Li, J.; Guo, Z.; Cai, J.; et al. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell 2020, 183, 76–93.e22. [Google Scholar] [CrossRef]
- Che, W.; Zhao, M.; Li, X.; Li, C.; Cho, W.C.; Yu, S. Current insights in molecular characterization of non-alcoholic fatty liver disease and treatment. Front. Endocrinol. 2022, 13, 1002916. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.S.; Curzen, N.P.; Calder, P.C.; Byrne, C.D. Non-alcoholic fatty liver disease: A new and important cardiovascular risk factor? Eur. Heart J. 2012, 33, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef]
- Cakir, E.; Ozbek, M.; Colak, N.; Cakal, E.; Delibasi, T. Is NAFLD an independent risk factor for increased IMT in T2DM? Minerva Endocrinol. 2012, 37, 187–193. [Google Scholar]
- Ismaiel, A.; Dumitrascu, D.L. Cardiovascular Risk in Fatty Liver Disease: The Liver-Heart Axis-Literature Review. Front. Med. 2019, 6, 202. [Google Scholar] [CrossRef]
- Bhatia, L.S.; Curzen, N.P.; Byrne, C.D. Nonalcoholic fatty liver disease and vascular risk. Curr. Opin. Cardiol. 2012, 27, 420–428. [Google Scholar] [CrossRef]
- Hassen, G.; Singh, A.; Belete, G.; Jain, N.; De la Hoz, I.; Camacho-Leon, G.P.; Dargie, N.K.; Carrera, K.G.; Alemu, T.; Jhaveri, S.; et al. Nonalcoholic Fatty Liver Disease: An Emerging Modern-Day Risk Factor for Cardiovascular Disease. Cureus 2022, 14, e25495. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. Metabolic-Dysfunction-Associated Steatotic Liver Disease-Its Pathophysiology, Association with Atherosclerosis and Cardiovascular Disease, and Treatments. Int. J. Mol. Sci. 2023, 24, 5473. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.J.; Ji, Y.X.; Zhang, P.; She, Z.G.; Li, H. Nonalcoholic Fatty Liver Disease Pandemic Fuels the Upsurge in Cardiovascular Diseases. Circ. Res. 2020, 126, 679–704. [Google Scholar] [CrossRef]
- Al Hashmi, K.; Giglio, R.V.; Pantea Stoian, A.; Patti, A.M.; Al Waili, K.; Al Rasadi, K.; Ciaccio, M.; Rizzo, M. Metabolic dysfunction-associated fatty liver disease: Current therapeutic strategies. Front. Nutr. 2024, 11, 1355732. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: A systematic review. J. Hepatol. 2008, 49, 600–607. [Google Scholar] [CrossRef]
- Galatou, E.; Mourelatou, E.; Hatziantoniou, S.; Vizirianakis, I.S. Nonalcoholic Steatohepatitis (NASH) and Atherosclerosis: Explaining Their Pathophysiology, Association and the Role of Incretin-Based Drugs. Antioxidants 2022, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Tessari, R.; Zenari, L.; Day, C.; Arcaro, G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007, 30, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Danzeng, A.; Liu, Q.; Zeng, C.; Xu, L.; Mo, J.; Pingcuo, C.; Wang, X.; Wang, C.; Zhang, B.; et al. The Role of Nuclear Receptors in the Pathogenesis and Treatment of Non-alcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2024, 20, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Senesi, P.; Ferrulli, A.; Luzi, L.; Terruzzi, I. Diabetes Mellitus and Cardiovascular Diseases: Nutraceutical Interventions Related to Caloric Restriction. Int. J. Mol. Sci. 2021, 22, 7772. [Google Scholar] [CrossRef]
- Boeckmans, J.; Natale, A.; Rombaut, M.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, M.R. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells 2019, 9, 37. [Google Scholar] [CrossRef]
- Athyros, V.G.; Polyzos, S.A.; Kountouras, J.; Katsiki, N.; Anagnostis, P.; Doumas, M.; Mantzoros, C.S. Non-Alcoholic Fatty Liver Disease Treatment in Patients with Type 2 Diabetes Mellitus; New Kids on the Block. Curr. Vasc. Pharmacol. 2020, 18, 172–181. [Google Scholar] [CrossRef]
- Khaznadar, F.; Petrovic, A.; Khaznadar, O.; Roguljic, H.; Bojanic, K.; Kuna Roguljic, L.; Siber, S.; Smolic, R.; Bilic-Curcic, I.; Wu, G.Y.; et al. Biomarkers for Assessing Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus on Sodium-Glucose Cotransporter 2 Inhibitor Therapy. J. Clin. Med. 2023, 12, 6561. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: Current approaches and future directions. Diabetologia 2016, 59, 1112–1120. [Google Scholar] [CrossRef]
- Marusic, M.; Paic, M.; Knobloch, M.; Liberati Prso, A.M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome. Diabetes Metab. J. 2024, 48, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Noureddin, M.; Clark, J.M. Nonalcoholic Fatty Liver Disease: Review of Management for Primary Care Providers. Mayo Clin. Proc. 2022, 97, 1700–1716. [Google Scholar] [CrossRef]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Flavin, B. Nonalcoholic steatohepatitis/metabolic dysfunction-associated steatohepatitis emerging market: Preparing managed care for early intervention, equitable access, and integrating the patient perspective. J. Manag. Care Spec. Pharm. 2024, 30, S1–S13. [Google Scholar] [CrossRef]
- Grander, C.; Grabherr, F.; Tilg, H. Non-alcoholic fatty liver disease: Pathophysiological concepts and treatment options. Cardiovasc. Res. 2023, 119, 1787–1798. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, S.S.; Chan, S.P.; Lee, Y.Y.; Tee, H.P.; Mahadeva, S.; Goh, K.L.; Ramli, A.S.; Mustapha, F.; Kosai, N.R.; et al. Malaysian Society of Gastroenterology and Hepatology consensus statement on metabolic dysfunction-associated fatty liver disease. J. Gastroenterol. Hepatol. 2022, 37, 795–811. [Google Scholar] [CrossRef]
- de Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Tsigkou, V.; Oikonomou, E.; Anastasiou, A.; Lampsas, S.; Zakynthinos, G.E.; Kalogeras, K.; Katsioupa, M.; Kapsali, M.; Kourampi, I.; Pesiridis, T.; et al. Molecular Mechanisms and Therapeutic Implications of Endothelial Dysfunction in Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 4321. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.; Dirinck, E. Pharmacological Approaches in the Treatment and Maintenance of Weight Loss. Diabetes Care 2016, 39 (Suppl. S2), S260–S267. [Google Scholar] [CrossRef]
- Fappi, A.; Mittendorfer, B. Dietary protein intake and obesity-associated cardiometabolic function. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, E.; Negri, C.; Targher, G.; Faccioli, N.; Lanza, M.; Zoppini, G.; Zanolin, E.; Schena, F.; Bonora, E.; Moghetti, P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013, 58, 1287–1295. [Google Scholar] [CrossRef]
- Wu, T.; Gao, X.; Chen, M.; van Dam, R.M. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: A meta-analysis. Obes. Rev. 2009, 10, 313–323. [Google Scholar] [CrossRef]
- Lampsas, S.; Marinos, G.; Lambrinos, D.; Theofilis, P.; Gialamas, I.; Pantelidis, P.; Zakynthinos, G.E.; Kalogera, V.; Pililis, S.; Korakas, E.; et al. Physical Activity Habits Among Physicians: Data From the Athens Medical Association. Am. J. Lifestyle Med. 2024, 15598276241267213. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Solga, S.F.; Horska, A.; Bonekamp, S.; Diehl, A.M.; Brancati, F.L.; Wagenknecht, L.E.; Pi-Sunyer, F.X.; Kahn, S.E.; Clark, J.M.; et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010, 33, 2156–2163. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, B.W.; Kim, Y.J.; Lee, D.H.; Cha, B.S.; Park, C.Y. Nonalcoholic Fatty Liver Disease and Diabetes: Part II: Treatment. Diabetes Metab. J. 2019, 43, 127–143. [Google Scholar] [CrossRef]
- Lee, I.M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Biernacki, D.M.; Kalavalapalli, S.; Lomonaco, R.; Subbarayan, S.K.; Lai, J.; Tio, F.; Suman, A.; Orsak, B.K.; Hecht, J.; et al. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2019, 42, 1481–1488. [Google Scholar] [CrossRef]
- Hochberg, I.; Berinstein, E.M.; Milman, U.; Shapira, C.; Levy, A.P. Interaction Between the Haptoglobin Genotype and Vitamin E on Cardiovascular Disease in Diabetes. Curr. Diab. Rep. 2017, 17, 42. [Google Scholar] [CrossRef]
- Connelly, M.A.; Velez Rivera, J.; Guyton, J.R.; Siddiqui, M.S.; Sanyal, A.J. Review article: The impact of liver-directed therapies on the atherogenic risk profile in non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2020, 52, 619–636. [Google Scholar] [CrossRef]
- Barb, D.; Portillo-Sanchez, P.; Cusi, K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism 2016, 65, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.S.; Chua, D.; Lim, C.O.; Ho, W.X.; Tan, N.S. Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials. Int. J. Mol. Sci. 2022, 24, 158. [Google Scholar] [CrossRef]
- Lampsas, S.; Xenou, M.; Oikonomou, E.; Pantelidis, P.; Lysandrou, A.; Sarantos, S.; Goliopoulou, A.; Kalogeras, K.; Tsigkou, V.; Kalpis, A.; et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules 2023, 28, 969. [Google Scholar] [CrossRef]
- Athyros, V.G.; Papageorgiou, A.A.; Mercouris, B.R.; Athyrou, V.V.; Symeonidis, A.N.; Basayannis, E.O.; Demitriadis, D.S.; Kontopoulos, A.G. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ’usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr. Med. Res. Opin. 2002, 18, 220–228. [Google Scholar] [CrossRef]
- Prentza, V.; Pavlidis, G.; Ikonomidis, I.; Pililis, S.; Lampsas, S.; Kountouri, A.; Pliouta, L.; Korakas, E.; Thymis, J.; Palaiodimou, L.; et al. Antidiabetic Treatment and Prevention of Ischemic Stroke: A Systematic Review. J. Clin. Med. 2024, 13, 5786. [Google Scholar] [CrossRef]
- Arad, Y.; Spadaro, L.A.; Roth, M.; Newstein, D.; Guerci, A.D. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: The St. Francis Heart Study randomized clinical trial. J. Am. Coll. Cardiol. 2005, 46, 166–172. [Google Scholar] [CrossRef]
- Hardy, T.; Anstee, Q.M.; Day, C.P. Nonalcoholic fatty liver disease: New treatments. Curr. Opin. Gastroenterol. 2015, 31, 175–183. [Google Scholar] [CrossRef]
- Bril, F.; Portillo Sanchez, P.; Lomonaco, R.; Orsak, B.; Hecht, J.; Tio, F.; Cusi, K. Liver Safety of Statins in Prediabetes or T2DM and Nonalcoholic Steatohepatitis: Post Hoc Analysis of a Randomized Trial. J. Clin. Endocrinol. Metab. 2017, 102, 2950–2961. [Google Scholar] [CrossRef]
- Pastori, D.; Polimeni, L.; Baratta, F.; Pani, A.; Del Ben, M.; Angelico, F. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig. Liver Dis. 2015, 47, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Boutari, C.; Stavropoulos, K.; Anagnostis, P.; Imprialos, K.P.; Doumas, M.; Karagiannis, A. Statins: An Under-Appreciated Asset for the Prevention and the Treatment of NAFLD or NASH and the Related Cardiovascular Risk. Curr. Vasc. Pharmacol. 2018, 16, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Elam, M.; Lovato, L.; Ginsberg, H. The ACCORD-Lipid study: Implications for treatment of dyslipidemia in Type 2 diabetes mellitus. Clin. Lipidol. 2011, 6, 9–20. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Perdomo, C.; D’Ingianna, P.; Escalada, J.; Petta, S.; Romero Gomez, M.; Ampuero, J. Nonalcoholic fatty liver disease and the risk of metabolic comorbidities: How to manage in clinical practice. Pol. Arch. Intern. Med. 2020, 130, 975–985. [Google Scholar] [CrossRef]
- Wilson, P.W.; Abbott, R.D.; Castelli, W.P. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 1988, 8, 737–741. [Google Scholar] [CrossRef]
- Pantelidis, P.; Oikonomou, E.; Lampsas, S.; Zakynthinos, G.E.; Lysandrou, A.; Kalogeras, K.; Katsianos, E.; Theofilis, P.; Siasos, G.; Vavuranakis, M.A.; et al. Lipoprotein(a) and calcific aortic valve disease initiation and progression: A systematic review and meta-analysis. Cardiovasc. Res. 2023, 119, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Oakley, F.; Teoh, V.; Ching, A.S.G.; Bataller, R.; Colmenero, J.; Jonsson, J.R.; Eliopoulos, A.G.; Watson, M.R.; Manas, D.; Mann, D.A. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology 2009, 136, 2334–2344.e1. [Google Scholar] [CrossRef]
- Yokohama, S.; Yoneda, M.; Haneda, M.; Okamoto, S.; Okada, M.; Aso, K.; Hasegawa, T.; Tokusashi, Y.; Miyokawa, N.; Nakamura, K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 2004, 40, 1222–1225. [Google Scholar] [CrossRef]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef]
- Bataller, R.; Sancho-Bru, P.; Gines, P.; Lora, J.M.; Al-Garawi, A.; Sole, M.; Colmenero, J.; Nicolas, J.M.; Jimenez, W.; Weich, N.; et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology 2003, 125, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef]
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Averet, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mazza, A.; Fruci, B.; Garinis, G.A.; Giuliano, S.; Malaguarnera, R.; Belfiore, A. The role of metformin in the management of NAFLD. Exp. Diabetes Res. 2012, 2012, 716404. [Google Scholar] [CrossRef] [PubMed]
- Fruci, B.; Giuliano, S.; Mazza, A.; Malaguarnera, R.; Belfiore, A. Nonalcoholic Fatty liver: A possible new target for type 2 diabetes prevention and treatment. Int. J. Mol. Sci. 2013, 14, 22933–22966. [Google Scholar] [CrossRef]
- Lavine, J.E.; Schwimmer, J.B.; Molleston, J.P.; Scheimann, A.O.; Murray, K.F.; Abrams, S.H.; Rosenthal, P.; Sanyal, A.J.; Robuck, P.R.; Brunt, E.M.; et al. Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp. Clin. Trials 2010, 31, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, X.; Yan, C.; Li, C.; Zhang, L.; Zhang, L.; Liang, E.; Liu, T.; Mao, J. Effect of metformin on nonalcoholic fatty liver based on meta-analysis and network pharmacology. Medicine 2022, 101, e31437. [Google Scholar] [CrossRef]
- King, P.; Peacock, I.; Donnelly, R. The UK prospective diabetes study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 1999, 48, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Roumane, A.; McIlroy, G.D.; Sommer, N.; Han, W.; Heisler, L.K.; Rochford, J.J. GLP-1 receptor agonist improves metabolic disease in a pre-clinical model of lipodystrophy. Front. Endocrinol. 2024, 15, 1379228. [Google Scholar] [CrossRef]
- Snyder, H.S.; Sakaan, S.A.; March, K.L.; Siddique, O.; Cholankeril, R.; Cummings, C.D.; Gadiparthi, C.; Satapathy, S.K.; Ahmed, A.; Cholankeril, G. Non-alcoholic Fatty Liver Disease: A Review of Anti-diabetic Pharmacologic Therapies. J. Clin. Transl. Hepatol. 2018, 6, 168–174. [Google Scholar] [CrossRef]
- Binet, Q.; Loumaye, A.; Preumont, V.; Thissen, J.P.; Hermans, M.P.; Lanthier, N. Non-invasive screening, staging and management of metabolic dysfunction-associated fatty liver disease (MAFLD) in type 2 diabetes mellitus patients: What do we know so far ? Acta Gastroenterol. Belg. 2022, 85, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; DeMarsilis, A.; Mantzoros, C.S. Obesity and diabetes. Diabetes Res. Clin. Pract. 2023, 202, 110773. [Google Scholar] [CrossRef] [PubMed]
- Liarakos, A.L.; Koliaki, C. Novel Dual Incretin Receptor Agonists in the Spectrum of Metabolic Diseases with a Focus on Tirzepatide: Real Game-Changers or Great Expectations? A Narrative Review. Biomedicines 2023, 11, 1875. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, B.W. Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2020, 26, 430–443. [Google Scholar] [CrossRef]
- Brouwers, B.; Rao, G.; Tang, Y.; Rodriguez, A.; Glass, L.C.; Hartman, M.L. Incretin-based investigational therapies for the treatment of MASLD/MASH. Diabetes Res. Clin. Pract. 2024, 211, 111675. [Google Scholar] [CrossRef] [PubMed]
- Yabiku, K. Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Patients With Concurrent Type 2 Diabetes Mellitus and Non-Alcoholic Steatohepatitis: A Review of the Evidence. Front. Endocrinol. 2021, 12, 768850. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, H.Y. Therapeutic Mechanisms and Clinical Effects of Glucagon-like Peptide 1 Receptor Agonists in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 9324. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Gusdon, A.M.; Liu, H.; Qu, S. Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J. Gastroenterol. 2014, 20, 14821–14830. [Google Scholar] [CrossRef]
- Newsome, P.; Francque, S.; Harrison, S.; Ratziu, V.; Van Gaal, L.; Calanna, S.; Hansen, M.; Linder, M.; Sanyal, A. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment. Pharmacol. Ther. 2019, 50, 193–203. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; Investigators, N.N. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Korakas, E.; Kountouri, A.; Pavlidis, G.; Oikonomou, E.; Vrentzos, E.; Michalopoulou, E.; Tsigkou, V.; Katogiannis, K.; Pliouta, L.; Balampanis, K.; et al. Semaglutide Concurrently Improves Vascular and Liver Indices in Patients With Type 2 Diabetes and Fatty Liver Disease. J. Endocr. Soc. 2024, 8, bvae122. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Xenou, M.; Zakynthinos, G.E.; Tsaplaris, P.; Lampsas, S.; Bletsa, E.; Gialamas, I.; Kalogeras, K.; Goliopoulou, A.; Gounaridi, M.I.; et al. Novel Approaches to the Management of Diabetes Mellitus in Patients with Coronary Artery Disease. Curr. Pharm. Des. 2023, 29, 1844–1862. [Google Scholar] [CrossRef]
- Cigrovski Berkovic, M.; Virovic-Jukic, L.; Bilic-Curcic, I.; Mrzljak, A. Post-transplant diabetes mellitus and preexisting liver disease—A bidirectional relationship affecting treatment and management. World J. Gastroenterol. 2020, 26, 2740–2757. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN trial team ; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.M.; Cercueil, J.P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Verges, B. Effect of Liraglutide Therapy on Liver Fat Content in Patients With Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J. Clin. Endocrinol. Metab. 2017, 102, 407–415. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Buse, J.B.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Wintle, M.E.; Maggs, D.G. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 2008, 24, 275–286. [Google Scholar] [CrossRef]
- Yazici, D.; Yapici Eser, H.; Kiyici, S.; Sancak, S.; Sezer, H.; Uygur, M.; Yumuk, V. Clinical Impact of Glucagon-Like Peptide-1 Receptor Analogs on the Complications of Obesity. Obes. Facts. 2023, 16, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Abushamat, L.A.; Shah, P.A.; Eckel, R.H.; Harrison, S.A.; Barb, D. The Emerging Role of Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Metabolic Dysfunction-Associated Steatohepatitis. Clin. Gastroenterol. Hepatol. 2024, 22, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Barritt, A.S., 4th; Marshman, E.; Noureddin, M. Review article: Role of glucagon-like peptide-1 receptor agonists in non-alcoholic steatohepatitis, obesity and diabetes-what hepatologists need to know. Aliment. Pharmacol. Ther. 2022, 55, 944–959. [Google Scholar] [CrossRef]
- Barritt, A.S.; Watkins, S.; Gitlin, N.; Klein, S.; Lok, A.S.; Loomba, R.; Schoen, C.; Reddy, K.R.; Trinh, H.N.; Mospan, A.R.; et al. Patient Determinants for Histologic Diagnosis of NAFLD in the Real World: A TARGET-NASH Study. Hepatol. Commun. 2021, 5, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Umemura, A.; Kataoka, S.; Okuda, K.; Seko, Y.; Yamaguchi, K.; Moriguchi, M.; Okanoue, T.; Itoh, Y. Potential Therapeutic Targets and Promising Agents for Combating NAFLD. Biomedicines 2022, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Ye, Z.; Yang, H.; Cui, X.; Wang, Z.; Liu, L. Effects of Canagliflozin on Fatty Liver Indexes in Patients with Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. J. Pharm. Pharm. Sci. 2018, 21, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Huang, Z.; Liang, Y.; Liao, Y.; Xia, N. The safety and efficacy evaluation of sodium-glucose co-transporter 2 inhibitors for patients with non-alcoholic fatty liver disease: An updated meta-analysis. Dig. Liver Dis. 2022, 54, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Chino, Y.; Samukawa, Y.; Sakai, S.; Nakai, Y.; Yamaguchi, J.; Nakanishi, T.; Tamai, I. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm. Drug Dispos. 2014, 35, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Androutsakos, T.; Nasiri-Ansari, N.; Bakasis, A.D.; Kyrou, I.; Efstathopoulos, E.; Randeva, H.S.; Kassi, E. SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. Int. J. Mol. Sci. 2022, 23, 3107. [Google Scholar] [CrossRef]
- Amjad, W.; Malik, A.; Qureshi, W.; Dennis, B.; Mumtaz, M.; Haider, R.; Jamal, S.; Jaura, F.; Ahmed, A. Sodium-glucose cotransporter-2 inhibitors improve liver enzymes in patients with co-existing non-alcoholic fatty liver disease: A systematic review and meta-analysis. Prz. Gastroenterol. 2022, 17, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Jung, C.H.; Mok, J.O.; Kim, C.H.; Kang, S.K.; Kim, B.Y. Effect of Dapagliflozin on Alanine Aminotransferase Improvement in Type 2 Diabetes Mellitus with Non-alcoholic Fatty Liver Disease. Endocrinol. Metab. 2018, 33, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kurinami, N.; Sugiyama, S.; Yoshida, A.; Hieshima, K.; Miyamoto, F.; Kajiwara, K.; Jinnouch, K.; Jinnouchi, T.; Jinnouchi, H. Dapagliflozin significantly reduced liver fat accumulation associated with a decrease in abdominal subcutaneous fat in patients with inadequately controlled type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 142, 254–263. [Google Scholar] [CrossRef]
- Tobita, H.; Sato, S.; Miyake, T.; Ishihara, S.; Kinoshita, Y. Effects of Dapagliflozin on Body Composition and Liver Tests in Patients with Nonalcoholic Steatohepatitis Associated with Type 2 Diabetes Mellitus: A Prospective, Open-label, Uncontrolled Study. Curr. Ther. Res. Clin. Exp. 2017, 87, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.W.; Lundkvist, P.; Jansson, P.A.; Johansson, L.; Kvarnstrom, M.; Moris, L.; Miliotis, T.; Forsberg, G.B.; Riserus, U.; Lind, L.; et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: A double-blind randomised placebo-controlled study. Diabetologia 2018, 61, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Bril, F.; Barb, D.; Polidori, D.; Sha, S.; Ghosh, A.; Farrell, K.; Sunny, N.E.; Kalavalapalli, S.; Pettus, J.; et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes. Metab. 2019, 21, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hayashi, A.; Taguchi, T.; Arai, R.; Sasaki, S.; Takano, K.; Inoue, Y.; Shichiri, M. Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease. J. Diabetes Investig. 2019, 10, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Kahl, S.; Gancheva, S.; Strassburger, K.; Herder, C.; Machann, J.; Katsuyama, H.; Kabisch, S.; Henkel, E.; Kopf, S.; Lagerpusch, M.; et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care 2020, 43, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, A.; Maegawa, H. Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J. Diabetes Investig. 2017, 8, 416–427. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Aoun, M.; Jadoul, M.; Anders, H.J. Erythrocytosis and CKD: A Review. Am. J. Kidney Dis. 2024, 84, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Lian, D.; Xie, Y.; Chen, Z.; Wang, Y.; Mu, L.; Wang, Y.; Zhang, B. SGLT-2 Inhibitors for Non-Alcoholic Fatty Liver Disease: A Review. Front. Biosci. 2023, 28, 134. [Google Scholar] [CrossRef] [PubMed]
- Townsend, S.A.; Newsome, P.N. Review article: New treatments in non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 46, 494–507. [Google Scholar] [CrossRef]
- Ludvik, B.; Frias, J.P.; Tinahones, F.J.; Wainstein, J.; Jiang, H.; Robertson, K.E.; Garcia-Perez, L.E.; Woodward, D.B.; Milicevic, Z. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): A 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M.; Tokushige, K.; Kawanaka, M.; Fujii, H.; Yoneda, M.; Imajo, K.; Takahashi, H.; Eguchi, Y.; Ono, M.; et al. Antidiabetic Therapy in the Treatment of Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2020, 21, 1907. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Catarig, A.M.; Frias, J.P.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Lingvay, I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: A substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia 2020, 63, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, S.A.; Frias, J.P.; Ahmed, A.; Hardy, E.; Choi, J.; Sjostrom, C.D.; Guja, C. Efficacy and Safety Over 2 Years of Exenatide Plus Dapagliflozin in the DURATION-8 Study: A Multicenter, Double-Blind, Phase 3, Randomized Controlled Trial. Diabetes Care 2020, 43, 2528–2536. [Google Scholar] [CrossRef]
- Lingvay, I.; Catarig, A.M.; Frias, J.P.; Kumar, H.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Viljoen, A.; McCrimmon, R.J. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): A double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Bhosekar, V.; Busch, R.; Holst, I.; Ludvik, B.; Thielke, D.; Thrasher, J.; Woo, V.; Philis-Tsimikas, A. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): A randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Zahid, M.; Khan, S.P.; Ershad, S.; Izhar, S.; Warraich, R.; Asghar, A. Role of oral hypoglycaemic drugs in preventing complication in non-alcoholic fatty liver disease with type 2 diabetes mellitus. J. Pak. Med. Assoc. 2024, 74, 1654–1658. [Google Scholar] [CrossRef] [PubMed]
- Smith, U. Pioglitazone: Mechanism of action. Int. J. Clin. Pract. Suppl. 2001, 121, 13–18. [Google Scholar]
- Della Pepa, G.; Russo, M.; Vitale, M.; Carli, F.; Vetrani, C.; Masulli, M.; Riccardi, G.; Vaccaro, O.; Gastaldelli, A.; Rivellese, A.A.; et al. Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: Clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial. Diabetes Res. Clin. Pract. 2021, 178, 108984. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Sanyal, A.J.; Kowdley, K.V.; Robuck, P.R.; Hoofnagle, J.; Kleiner, D.E.; Unalp, A.; Tonascia, J.; Group, N.C.R. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp. Clin. Trials 2009, 30, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Genua, I.; Cusi, K. Pharmacological Approaches to Nonalcoholic Fatty Liver Disease: Current and Future Therapies. Diabetes Spectr. 2024, 37, 48–58. [Google Scholar] [CrossRef]
- Erdmann, E.; Dormandy, J.; Wilcox, R.; Massi-Benedetti, M.; Charbonnel, B. PROactive 07: Pioglitazone in the treatment of type 2 diabetes: Results of the PROactive study. Vasc. Health Risk Manag. 2007, 3, 355–370. [Google Scholar] [PubMed]
- Nissen, S.E.; Nicholls, S.J.; Wolski, K.; Nesto, R.; Kupfer, S.; Perez, A.; Jure, H.; De Larochelliere, R.; Staniloae, C.S.; Mavromatis, K.; et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: The PERISCOPE randomized controlled trial. JAMA 2008, 299, 1561–1573. [Google Scholar] [CrossRef]
- Lange, N.F.; Graf, V.; Caussy, C.; Dufour, J.F. PPAR-Targeted Therapies in the Treatment of Non-Alcoholic Fatty Liver Disease in Diabetic Patients. Int. J. Mol. Sci. 2022, 23, 4305. [Google Scholar] [CrossRef]
- Wang, Z.; Du, H.; Zhao, Y.; Ren, Y.; Ma, C.; Chen, H.; Li, M.; Tian, J.; Xue, C.; Long, G.; et al. Response to pioglitazone in non-alcoholic fatty liver disease patients with vs. without type 2 diabetes: A meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1111430. [Google Scholar] [CrossRef]
- Bae, J.C. DPP-4 Inhibitor in Type 2 Diabetes Mellitus Patient with Non-Alcoholic Fatty Liver Disease: Achieving Two Goals at Once? Endocrinol. Metab. 2022, 37, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Baumeier, C.; Schluter, L.; Saussenthaler, S.; Laeger, T.; Rodiger, M.; Alaze, S.A.; Fritsche, L.; Haring, H.U.; Stefan, N.; Fritsche, A.; et al. Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol. Metab. 2017, 6, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyan, N.A.; Koshy, R.M.; Jacob, K.; Pappachan, J.M. Efficacy and Cardiovascular Safety of DPP-4 Inhibitors. Curr. Drug Saf. 2021, 16, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Yoneda, M.; Inamori, M.; Shirakawa, J.; Higurashi, T.; Maeda, S.; Terauchi, Y.; Nakajima, A. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology 2011, 58, 2103–2105. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Yonal, O.; Deyneli, O.; Celikel, C.A.; Kalayci, C.; Duman, D.G. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol. Belg. 2012, 75, 240–244. [Google Scholar]
- Kato, H.; Nagai, Y.; Ohta, A.; Tenjin, A.; Nakamura, Y.; Tsukiyama, H.; Sasaki, Y.; Fukuda, H.; Ohshige, T.; Terashima, Y.; et al. Effect of sitagliptin on intrahepatic lipid content and body fat in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2015, 109, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Philo, L.; Nguyen, P.; Hofflich, H.; Hernandez, C.; Bettencourt, R.; Richards, L.; Salotti, J.; Bhatt, A.; Hooker, J.; et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J. Hepatol. 2016, 65, 369–376. [Google Scholar] [CrossRef]
- Fukuhara, T.; Hyogo, H.; Ochi, H.; Fujino, H.; Kan, H.; Naeshiro, N.; Honda, Y.; Miyaki, D.; Kawaoka, T.; Tsuge, M.; et al. Efficacy and safety of sitagliptin for the treatment of nonalcoholic fatty liver disease with type 2 diabetes mellitus. Hepatogastroenterology 2014, 61, 323–328. [Google Scholar]
- Macauley, M.; Hollingsworth, K.G.; Smith, F.E.; Thelwall, P.E.; Al-Mrabeh, A.; Schweizer, A.; Foley, J.E.; Taylor, R. Effect of vildagliptin on hepatic steatosis. J. Clin. Endocrinol. Metab. 2015, 100, 1578–1585. [Google Scholar] [CrossRef]
- Hussain, M.; Majeed Babar, M.Z.; Hussain, M.S.; Akhtar, L. Vildagliptin ameliorates biochemical, metabolic and fatty changes associated with non alcoholic fatty liver disease. Pak. J. Med. Sci. 2016, 32, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Udell, J.A.; Bhatt, D.L.; Braunwald, E.; Cavender, M.A.; Mosenzon, O.; Steg, P.G.; Davidson, J.A.; Nicolau, J.C.; Corbalan, R.; Hirshberg, B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: Observations from the SAVOR-TIMI 53 Trial. Diabetes Care 2015, 38, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Sanchez, P.; Cusi, K. Treatment of Nonalcoholic Fatty Liver Disease (NAFLD) in patients with Type 2 Diabetes Mellitus. Clin. Diabetes Endocrinol. 2016, 2, 9. [Google Scholar] [CrossRef]
- Young, E.N.; Dogan, M.; Watkins, C.; Bajwa, A.; Eason, J.D.; Kuscu, C.; Kuscu, C. A Review of Defatting Strategies for Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 11805. [Google Scholar] [CrossRef]
- Carlsson, L.M.; Peltonen, M.; Ahlin, S.; Anveden, A.; Bouchard, C.; Carlsson, B.; Jacobson, P.; Lonroth, H.; Maglio, C.; Naslund, I.; et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med. 2012, 367, 695–704. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Mathurin, P.; Gonzalez, F.; Kerdraon, O.; Leteurtre, E.; Arnalsteen, L.; Hollebecque, A.; Louvet, A.; Dharancy, S.; Cocq, P.; Jany, T.; et al. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology 2006, 130, 1617–1624. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Mellemkjaer, A.; Kjaer, M.B.; Haldrup, D.; Gronbaek, H.; Thomsen, K.L. Management of cardiovascular risk in patients with metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 2024, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fan, J. Therapeutic developments in metabolic dysfunction-associated fatty liver disease. Chin. Med. J. 2022, 135, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Jeeyavudeen, M.S.; Khan, S.K.A.; Fouda, S.; Pappachan, J.M. Management of metabolic-associated fatty liver disease: The diabetology perspective. World J. Gastroenterol. 2023, 29, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lee, H.; Kaura, S.; Yip, J.; Sun, H.; Guan, L.; Han, W.; Ding, Y. Effect of Bariatric Surgery on Metabolic Diseases and Underlying Mechanisms. Biomolecules 2021, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Singh, K.; Thuluvath, P.J. Bariatric Surgery in NAFLD. Dig. Dis. Sci. 2022, 67, 408–422. [Google Scholar] [CrossRef]
- Friedman, S.L.; Ratziu, V.; Harrison, S.A.; Abdelmalek, M.F.; Aithal, G.P.; Caballeria, J.; Francque, S.; Farrell, G.; Kowdley, K.V.; Craxi, A.; et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018, 67, 1754–1767. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Gargante, M.P.; Russo, C.; Antic, T.; Vacante, M.; Malaguarnera, M.; Avitabile, T.; Li Volti, G.; Galvano, F. L-carnitine supplementation to diet: A new tool in treatment of nonalcoholic steatohepatitis—A randomized and controlled clinical trial. Am. J. Gastroenterol. 2010, 105, 1338–1345. [Google Scholar] [CrossRef]
- Guixe-Muntet, S.; Biquard, L.; Szabo, G.; Dufour, J.F.; Tacke, F.; Francque, S.; Rautou, P.E.; Gracia-Sancho, J. Review article: Vascular effects of PPARs in the context of NASH. Aliment Pharmacol. Ther. 2022, 56, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016, 150, 1147–1159.e5. [Google Scholar] [CrossRef]
- Kim, W.; Kim, B.G.; Lee, J.S.; Lee, C.K.; Yeon, J.E.; Chang, M.S.; Kim, J.H.; Kim, H.; Yi, S.; Lee, J.; et al. Randomised clinical trial: The efficacy and safety of oltipraz, a liver X receptor alpha-inhibitory dithiolethione in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Butera, E.; Termite, F.; Esposto, G.; Galasso, L.; Mignini, I.; Borriello, R.; Ainora, M.E.; Miele, L.; Gasbarrini, A.; Zocco, M.A. Exploring the Role of Bempedoic Acid in Metabolic Dysfunction Associated Steatotic Liver Disease: Actual Evidence and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 6938. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.Y.; Targher, G.; Byrne, C.D.; Liu, W.Y.; Zheng, M.H. Resmetirom for MASH patients with diabetes: Challenges and opportunities in the real world. Metabolism 2024, 156, 155935. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Wang, P.C.; Zhao, S.; Yang, B.Y.; Wang, Q.H.; Kuang, H.X. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr. Polym. 2016, 148, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S60–S84. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Zhang, K.; Zhang, Y.; Fan, G. Managing metabolic diseases: The roles and therapeutic prospects of herb-derived polysaccharides. Biomed. Pharmacother. 2023, 161, 114538. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Baratta, F.; Pastori, D.; Loffredo, L.; Angelico, F. Modern approach to the clinical management of non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 8341–8350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Vigueira, P.A.; Chambers, K.T.; Hall, A.M.; Mitra, M.S.; Qi, N.; McDonald, W.G.; Colca, J.R.; Kletzien, R.F.; Finck, B.N. Insulin Resistance and Metabolic Derangements in Obese Mice Are Ameliorated by a Novel Peroxisome Proliferator-Activated Receptor γ-Sparing Thiazolidinedione. J. Biol. Chem. 2012, 287, 23537–23548. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, E.; Chiereghin, F.; Pacifici, F.; Donadel, G.; De Stefano, A.; Malatesta, G.; Valente, M.G.; Guadagni, F.; Infante, M.; Rovella, V.; et al. Novel therapeutic approaches based on the pathological role of gut dysbiosis on the link between nonalcoholic fatty liver disease and insulin resistance. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1921–1944. [Google Scholar] [CrossRef]
- Janssen, A.W.F.; Houben, T.; Katiraei, S.; Dijk, W.; Boutens, L.; van der Bolt, N.; Wang, Z.; Brown, J.M.; Hazen, S.L.; Mandard, S.; et al. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: A potential role for bile acids. J. Lipid Res. 2017, 58, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Gangarapu, V.; Ince, A.T.; Baysal, B.; Kayar, Y.; Kilic, U.; Gok, O.; Uysal, O.; Senturk, H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 840–845. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. NAFLD and increased risk of cardiovascular disease: Clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Arians, M.; Karrasch, T.; Pons-Kuhnemann, J.; Schaffler, A.; Roderfeld, M.; Roeb, E. Improvement of Type 2 Diabetes Mellitus and Attenuation of NAFLD Are Associated with the Success of Obesity Therapy. J. Clin. Med. 2022, 11, 1756. [Google Scholar] [CrossRef] [PubMed]
- Bea, S.; Jeong, H.E.; Filion, K.B.; Yu, O.H.; Cho, Y.M.; Lee, B.H.; Chang, Y.; Byrne, C.D.; Shin, J.Y. Outcomes of SGLT-2i and GLP-1RA Therapy Among Patients With Type 2 Diabetes and Varying NAFLD Status. JAMA Netw. Open. 2023, 6, e2349856. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, S.; Kang, B.; Zhou, J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc. Diabetol. 2022, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Xu, J.; Targher, G.; Byrne, C.D.; Zheng, M.H. Old and new classes of glucose-lowering agents as treatments for non-alcoholic fatty liver disease: A narrative review. Clin. Mol. Hepatol. 2022, 28, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Francque, S.; Vonghia, L. Pharmacological Treatment for Non-alcoholic Fatty Liver Disease. Adv. Ther. 2019, 36, 1052–1074. [Google Scholar] [CrossRef]
| Steatosis | Liver Enzymes | Inflammation | CV Risk | |
|---|---|---|---|---|
| PUFAs | Decreased | Decreased | Decreased | Decreased |
| Statins | Decreased | Unknown/Increased | Decreased | Decreased |
| Fibrates | Unknown/Unchanged | Unknown/Unchanged | Decreased | Decreased |
| Weight | Steatosis | Liver Enzymes | CV Risk | Inflammation | Side Effects | ||
|---|---|---|---|---|---|---|---|
| Metformin | TONIC (173 patients) (Randomized double-blind, double-dummy, placebo-controlled trial) (1000 mg metformin/day, 800 IU Vitamin E/Day for 96 weeks) HOME (745 patients) (Randomized, placebo-controlled trial) (850 mg metformin/day, for 16 weeks) | Decreased | Decreased | Decreased /Unchanged | Decreased | Decreased | Gastrointestinal effects, lactic acidosis |
| GLP-1 RAs | AWARD (810 patients) (Open-label, randomized trial) (Dulaglutide 1.5 mg/week, dulaglutide 0.5 mg/week, insulin glarine daily for 52 weeks) LEAN (52 patients) (Double-blinded, placebo-controlled randomized trial) (Liraglutide 1.8 mg/daily for 48 weeks) SUSTAIN9 (302 patients) (Randomized, placebo-controlled trial) (Escalating to 1 mg semaglutide for 30 weeks) | Decreased | Decreased | Decreased | Decreased | Decreased | Gastrointestinal effects, pancreatitis, risks for cancer |
| SGLT2 inhibitors | EFFECT II (84 patients) (Double-blind randomized placebo-controlled) (Dapagliflozin 10 mg/day + 4 g omega-3 carboxylic acids for 12 weeks) E-LIFT (50 patients) (Randomized controlled trial) (Empagliflozin 10 mg/day > 12 weeks) EMPA-REG-OUTCOME (7020 patients) (10–25 mg empagliflozin/daily for 2.6 years) | Decreased | Decreased | Decreased | Decreased | Decreased | Glycosuria, cardiovascular concern, ketoacidosis, hypotension, bone fracture |
| DPP-4inhibitors | SAVOR-TIMI (16,492 patients) (Randomized, double-blind, placebo-controlled trial) (Saxagliptin 2.5–5 mg/day for 18 months) | Unchanged | Decrease/Unchanged | Decreased/ Unchanged | Decreased /Unchanged | Unchanged | Pancreatitis, risks for cancer, acute hepatitis and kidney impairment |
| Thiazolidinediones | Pro/active (952 patients) (Randomized study) (15–45 mg/day pioglitazone depending on tolerance) PERISCOPE (543 patients) (Randomized controlled trial) (15–45 mg pioglitazone/day for 18 months) | Increased | Decreased | Decreased/ Unchanged | Decreased | Decreased | Hepatitis, bladder cancer, water retention and weight gain |
| Bariatric Surgery | Decreased | Decreased | Decreased/ Unchanged | Decreased | Decreased /Unchanged | Depending on the type of surgery: indigestion, sagging skin, stomach ulcers, nausea, gall stones |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalopoulou, E.; Thymis, J.; Lampsas, S.; Pavlidis, G.; Katogiannis, K.; Vlachomitros, D.; Katsanaki, E.; Kostelli, G.; Pililis, S.; Pliouta, L.; et al. The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment. J. Clin. Med. 2025, 14, 428. https://doi.org/10.3390/jcm14020428
Michalopoulou E, Thymis J, Lampsas S, Pavlidis G, Katogiannis K, Vlachomitros D, Katsanaki E, Kostelli G, Pililis S, Pliouta L, et al. The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment. Journal of Clinical Medicine. 2025; 14(2):428. https://doi.org/10.3390/jcm14020428
Chicago/Turabian StyleMichalopoulou, Eleni, John Thymis, Stamatios Lampsas, George Pavlidis, Konstantinos Katogiannis, Dimitrios Vlachomitros, Eleni Katsanaki, Gavriella Kostelli, Sotirios Pililis, Loukia Pliouta, and et al. 2025. "The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment" Journal of Clinical Medicine 14, no. 2: 428. https://doi.org/10.3390/jcm14020428
APA StyleMichalopoulou, E., Thymis, J., Lampsas, S., Pavlidis, G., Katogiannis, K., Vlachomitros, D., Katsanaki, E., Kostelli, G., Pililis, S., Pliouta, L., Kountouri, A., Papanikolaou, I. S., Lambadiari, V., & Ikonomidis, I. (2025). The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment. Journal of Clinical Medicine, 14(2), 428. https://doi.org/10.3390/jcm14020428











