A Unique Case of Extramedullary Relapse in Acute Lymphoblastic Leukemia: Testicular to Ocular, Cardiac, and Colonic Involvement and the Role of Sperm Phenotyping in Diagnosis—Case Report and Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Ashraf, D.C.; Kinde, B.; Ohgami, R.S.; Kumar, J.; Kersten, R.C. Hypodiploid B-lymphoblastic leukemia presenting as an isolated orbital mass prior to systemic involvement: A case report and review of the literature. Diagnostics 2021, 11, 25. [Google Scholar] [CrossRef]
- Pui, C.-H.; Robison, L.L.; Look, A.T. Acute lymphoblastic leukaemia. Lancet 2008, 371, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.-H. Is testicular irradiation necessary for patients with acute lymphoblastic leukemia and testicular relapse? Pediatr. Blood Cancer 2018, 65, e26977. [Google Scholar] [CrossRef]
- Shahriari, M.; Shakibazad, N.; Haghpanah, S.; Ghasemi, K. Extramedullary manifestations in acute lymphoblastic leukemia in children: A systematic review and guideline-based approach of treatment. Am. J. Blood Res. 2020, 10, 360–374. [Google Scholar]
- Abbasi, S.; Maleha, F.; Shobaki, M. Acute lymphoblastic leukemia experience: Epidemiology and outcome of two different regimens. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013024. [Google Scholar] [CrossRef] [PubMed]
- Gökbuget, N.; Stanze, D.; Beck, J.; Diedrich, H.; Horst, H.-A.; Hüttmann, A.; Kobbe, G.; Kreuzer, K.-A.; Leimer, L.; Reichle, A.; et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012, 120, 2032–2041. [Google Scholar] [CrossRef]
- Skeith, L.; Lazo-Langner, A.; Mangel, J. Kidney and pancreatic extramedullary relapse in adult acute lymphoblastic leukemia: A case report and review of the literature. Case Rep. Hematol. 2013, 2013, 637264. [Google Scholar] [CrossRef]
- Shem-Tov, N.; Saraceni, F.; Danylesko, I.; Shouval, R.; Yerushalmi, R.; Nagler, A.; Shimoni, A. Isolated extramedullary relapse of acute leukemia after allogeneic stem cell transplantation: Different kinetics and better prognosis than systemic relapse. Biol. Blood Marrow Transplant. 2017, 23, 1087–1094. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Harald, S.; Thiele, J.; Vardiman, J.W. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2008. [Google Scholar]
- Shimizu, H.; Ishizaki, T.; Hatsumi, N.; Takada, S.; Yokohama, A.; Saitoh, T.; Sakura, T.; Handa, H. Extramedullary relapses after allogeneic stem cell transplant (allo-SCT) in adult patients with acute lymphoblastic leukemia (ALL). Blood 2019, 134 (Suppl. S1), 5690. [Google Scholar] [CrossRef]
- Ding, L.-W.; Sun, Q.-Y.; Mayakonda, A.; Tan, K.-T.; Chien, W.; Lin, D.-C.; Jiang, Y.-Y.; Xu, L.; Garg, M.; Lao, Z.-T.; et al. Mutational profiling of acute lymphoblastic leukemia with testicular relapse. J. Hematol. Oncol. 2017, 10, 65. [Google Scholar] [CrossRef]
- Nguyen, H.T.K.; Terao, M.A.; Green, D.M.; Pui, C.-H.; Inaba, H. Testicular involvement of acute lymphoblastic leukemia in children and adolescents: Diagnosis, biology, and management. Cancer 2021, 127, 3067–3081. [Google Scholar] [CrossRef] [PubMed]
- Skroblyn, T.; Joedicke, J.J.; Pfau, M.; Krüger, K.; Bourquin, J.-P.; Izraeli, S.; Eckert, C.; Höpken, U.E. P319: The testicular niche of acute lymphoblastic leukemia—Molecular and cellular factors for the preferential migration and survival. HemaSphere 2022, 6, 219–220. [Google Scholar] [CrossRef]
- Chu, J.-Y.; O’Connor, D.M.; Cradock, T.V.; Gale, G.B. Isolated testicular relapse five years after cessation of chemotherapy for acute lymphoblastic leukemia. Clin. Pediatr. 1982, 21, 376–377. [Google Scholar] [CrossRef]
- Dini, G.; Capolsini, I.; Cerri, C.; Massei, M.S.; Mastrodicasa, E.; Perruccio, K.; Gorello, P.; Caniglia, M.; Verrotti, A.; Arcioni, F. Acute lymphoblastic leukemia relapse presenting with optic nerve infiltration. SAGE Open Med. Case Rep. 2023, 11, 2050313X231175020. [Google Scholar] [CrossRef]
- Ge, L.; Ye, F.; Mao, X.; Chen, J.; Sun, A.; Zhu, X.; Qiu, H.; Jin, Z.; Miao, M.; Fu, C.; et al. Extramedullary relapse of acute leukemia after allogeneic hematopoietic stem cell transplantation: Different characteristics between acute myelogenous leukemia and acute lymphoblastic leukemia. Biol. Blood Marrow Transplant. 2014, 20, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, I.; Pierce, E.; Scheuing, L.; Morris, A.; El Chaer, F.; Keng, M. Post-hematopoietic cell transplantation relapsed acute lymphoblastic leukemia: Current challenges and future directions. OncoTargets Ther. 2023, 16, 1–16. [Google Scholar] [CrossRef]
- Le Louet, S.; Icart, V.; Strullu, M.; Petit, A.; Freycon, C.; Blouin, P.; Serre, J.; Rama, N.; Reguerre, Y.; Piguet, C.; et al. Novel Insights into Pediatric Acute Lymphoblastic Leukemia Ophthalmic Relapses from a Nationwide Cohort Study. J. Cancer 2022, 13, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Ninane, J.; Taylor, D.; Day, S. The Eye as a Sanctuary in Acute Lymphoblastic Leukæmia. Lancet 1980, 315, 452–453. [Google Scholar] [CrossRef]
- Novakovic, P.; Kellie, S.J.; Taylor, D. Childhood leukaemia: Relapse in the anterior segment of the eye. Br. J. Ophthalmol. 1989, 73, 354–359. [Google Scholar] [CrossRef]
- Kakefuda, Y.; Sato, A.; Hoshi, T.; Ishizu, T.; Tada, H.; Satomi, K.; Morishita, Y.; Okoshi, Y.; Tokunaga, C.; Sakakibara, Y.; et al. Isolated cardiac involvement of B-cell acute lymphoblastic leukemia mimicking acute myocardial infarction with persistent broad ST-segment elevation. Circulation 2012, 125, e979–e982. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.N.; Ragoonanan, D.; Franklin, A.; Srinivasan, C.; Zhao, B.; Petropoulos, D.; Mahadeo, K.M.; Tewari, P.; Khazal, S.J. Cardiac relapse of acute lymphoblastic leukemia following hematopoietic stem cell transplantation: A case report and review of literature. Cancers 2021, 13, 5814. [Google Scholar] [CrossRef]
- Vukelja, S.J.; Swanson, S.J.; Knight, R.D.; Weiss, R.B. Testicular relapse in adult acute lymphocytic leukemia: A case report and literature review. Med. Pediatr. Oncol. 1989, 17, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shing, M.; Chik, K.; Kwan, W.; Lai, D.; Leung, T.; Yuen, P. Isolated testicular relapse after bone marrow transplant with total body irradiation and testicular boost in acute lymphoblastic leukemia. Bone Marrow Transplant. 1998, 22, 397–399. [Google Scholar] [CrossRef]
- Uderzo, C.; Zurlo, M.G.; Adamoli, L.; Zanesco, L.; Aricò, M.; Calculli, G.; Comelli, A.; di Montezemolo, L.C.; Di Tullio, M.T.; Guazzelli, C.; et al. Treatment of isolated testicular relapse in childhood acute lymphoblastic leukemia: An Italian multicenter study. Associazione Italiana Ematologia ED Oncologia Pediatrica. J. Clin. Oncol. 1990, 8, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Ritzén, E.M. Testicular relapse of acute lymphoblastic leukemia (ALL). J. Reprod. Immunol. 1990, 18, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Jahnukainen, K.; Morris, I.; Roe, S.; Salmi, T.; Mäkipernaa, A.; Pöllänen, P. A rodent model for testicular involvement in acute lymphoblastic leukaemia. Br. J. Cancer 1993, 67, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, R.; Kulkarni, K.; Trehan, A.; Bansal, D. Testicular relapse in childhood acute lymphoblastic leukemia: The challenges and lessons. Indian J. Cancer 2010, 47, 134–138. [Google Scholar] [CrossRef]
- Dördelmann, M.; Reiter, A.; Zimmermann, M.; Fengler, R.; Henze, G.; Riehm, H.; Schrappe, M. Intermediate dose methotrexate is as effective as high dose methotrexate in preventing isolated testicular relapse in childhood acute lymphoblastic leukemia. J. Pediatr. Hematol./Oncol. 1998, 20, 444–450. [Google Scholar] [CrossRef]
- Banavali, S.D.; Shama, G.; Bhagwat, R.; Pai, S.K.; Kurkure, P.; Nair, C.N.; Parikh, P.M.; Mukaden, M. Isolated testicular relapse in acute lymphoblastic leukemia—Effective treatment with the modified CCG-112 protocol. Indian J. Cancer 2005, 42, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Okamoto, Y.; Ijichi, O.; Shinkoda, Y.; Nishikawa, T.; Tanabe, T.; Yoshioka, T.; Tashiro, Y.; Mougi, H.; Kawano, Y. Continued complete remission without systemic therapy for isolated testicular relapse after bone marrow transplantation in a boy with acute lymphoblastic leukemia. Pediatr. Transplant. 2009, 13, 769–772. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Y.; Pu, C.; Liang, Z.; Cui, Q.; Zhang, H.; Luo, Y.; Shi, J.; Jin, A.; Xiao, L.; et al. Successful chimeric Ag receptor modified T cell therapy for isolated testicular relapse after hematopoietic cell transplantation in an acute lymphoblastic leukemia patient. Bone Marrow Transplant. 2017, 52, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Thacker, L.R.; McCune, T.R.; Harland, R.C. Kidney sharing by centers of the south-eastern organ procurement Foundation. Clin. Transplant. 1996, 129–137. [Google Scholar] [PubMed]
- Wofford, M.M.; Smith, S.D.; Shuster, J.J.; Johnson, W.; Buchanan, G.R.; Wharam, M.D.; Ritchey, A.K.; Rosen, D.; Haggard, M.E.; Golembe, B.L.; et al. Treatment of occult or late overt testicular relapse in children with acute lymphoblastic leukemia: A pediatric Oncology Group study. J. Clin. Oncol. 1992, 10, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Gomel, N.; Levinger, E.; Ram, R.; Limon, D.; Habot-Wilner, Z. Acute lymphoblastic leukemia relapse limited to the anterior chamber of the eye and treated with novel CAR T-cell therapy. Case Rep. Ophthalmol. 2021, 12, 994–1001. [Google Scholar] [CrossRef]
- Jayakrishnan, T.; Shaikh, H.; Samhouri, Y.; Sandhu, A. Isolated testicular recurrence of B cell acute lymphoblastic leukaemia in an adult: Rare case. BMJ Case Rep. 2019, 12, e232286. [Google Scholar] [CrossRef]
- Veys, D.; Norton, A.; Ainsworth, J.R.; Amrolia, P.; Lucchini, G. Isolated Intraocular Relapse of Pediatric B-cell Precursor Acute Lymphoblastic Leukaemia Following Chimeric Antigen Receptor T-lymphocyte Therapy. Cureus 2020, 12, e10937. [Google Scholar] [CrossRef] [PubMed]
- Willier, S.; Raedler, J.; Blaeschke, F.; Stenger, D.; Escudero, M.P.; Jurgeleit, F.; Grünewald, T.G.P.; Binder, V.; Schmid, I.; Albert, M.H.; et al. Leukemia escape in immune desert: Intraocular relapse of pediatric pro-BALL during systemic control by CD19-CAR T cells. J. Immunother. Cancer 2020, 8, e001052. [Google Scholar] [CrossRef]
- Luo, Z.; Cheng, J.; Wang, Y. Cardiac Infiltration as the First Manifestation of Acute Lymphoblastic Leukemia: A Systematic Review. Front. Oncol. 2022, 12, 805981. [Google Scholar] [CrossRef]
- Lissat, A.; van Schewick, C.; Steffen, I.G.; Arakawa, A.; Bourquin, J.-P.; Burkhardt, B.; Henze, G.; Mann, G.; Peters, C.; Sramkova, L.; et al. Other (Non-CNS/Testicular) Extramedullary Localizations of Childhood Relapsed Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma—A Report from the ALL-REZ Study Group. J. Clin. Med. 2021, 10, 5292. [Google Scholar] [CrossRef]
- Colombini, A.; Barzaghi, A.; Castagneto, M.; Bovo, G.; Rossi, M.; Rovelli, A.; Uderzo, C. Retro-orbital late relapse in a child with leukaemia after allogeneic bone marrow transplantation. Acta Haematol. 1995, 94, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, B.C.A.M.; Denarié, B.J.A.; Bos, G.M.J. Massive cardiac involvement in acute lymphatic leukemia. BMJ J. 2003, 90, 354. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Suzuki, N.; Mizue, N.; Hatakeyama, N.; Takamuro, M.; Tsutsumi, H. Relapse of T-cell all after stem cell transplant presenting as hypertrophic cardiomyopathy: The value of non-invasive diagnostic imaging in detecting cardiac leukemia. Pediatr. Blood Cancer 2006, 46, 108–111. [Google Scholar] [CrossRef]
- Wright, T.L.; Bardy, P.G.; Disney, P.; Moore, S.; Horvath, N. Isolated cardiac recurrence of acute lymphoblastic leukemia characterized by t (11;19) two years after unrelated allogeneic bone marrow transplantation. Cancer Genet. Cytogenet. 2002, 137, 146–149. [Google Scholar] [CrossRef]
- Chang, K.; Kim, D.-Y.; Lee, K.-H.; Huh, J.; Kang, J.-W.; Shin, D.Y. An isolated cardiac relapse after allogeneic hematopoietic stem cell transplantation for acute lymphoblastic leukemia. Korean J. Intern. Med. 2016, 32, 753–757. [Google Scholar] [CrossRef]
- Forcade, E.; Leguay, T.; Vey, N.; Baruchel, A.; Delaunay, J.; Robin, M.; Socié, G.; Dombret, H.; de Latour, R.P.; Raffoux, E. Nelarabine for T Cell Acute Lymphoblastic Leukemia Relapsing after Allogeneic Hematopoietic Stem Cell Transplantation: An Opportunity to Improve Survival Marrow Transplantation. Blood Marrow Transpl. 2013, 19, 1124–1126. [Google Scholar] [CrossRef][Green Version]
- Baritussio, A.; Gately, A.; Pawade, J.; Marks, D.I.; Bucciarelli-Ducci, C. Extensive cardiac infiltration in acute T-cell lymphoblastic leukemia: Occult extra-medullary relapse and remission after salvage chemotherapy. Eur. Heart J. 2017, 38, 1933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nadel, J.; Meredith, T.; Anthony, C.; Sivasubramaniam, V.; Jabbour, A. Isolated myocardial relapse of Philadelphia-positive acute lymphoblastic leukaemia causing myocarditis: A case report. Eur. Heart J. Case Rep. 2018, 2, yty104. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Cardiac involvement in a patient with B-cell lymphoblastic lymphoma/acute lymphoblastic leukemia and a history of allogeneic hematopoietic stem cell transplantation and CAR T-cell therapy: A case report. Front. Immunol. 2023, 13, 1052336. [Google Scholar] [CrossRef]
- Hathorn, K.E.; Luskin, M.R.; Caton, M.T.; DeAngelo, D.J.; Saltzman, J.R. Colonic Wall Thickening as the First Indicator of Relapse of Acute Lymphoblastic Leukemia. ACG Case Rep. J. 2019, 6, e00207. [Google Scholar] [CrossRef] [PubMed]
- Issak, A.; Agrawal, S. Extramedullary involvement of the sigmoid colon with acute lymphocytic leukemia. J. Gastrointest. Cancer 2017, 48, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Ifthikar, Z.; Muthalib, H.A.; Mohammed, S.; Alfraih, F.A.; Aljohany, H.A.; Alsohaibani, F.I. Extramedullary Involvement of the Ascending Colon in Relapsing Acute Lymphocytic Leukemia: A Case Report. Saudi J. Med. Med. Sci. 2022, 10, 162–165. [Google Scholar] [CrossRef]
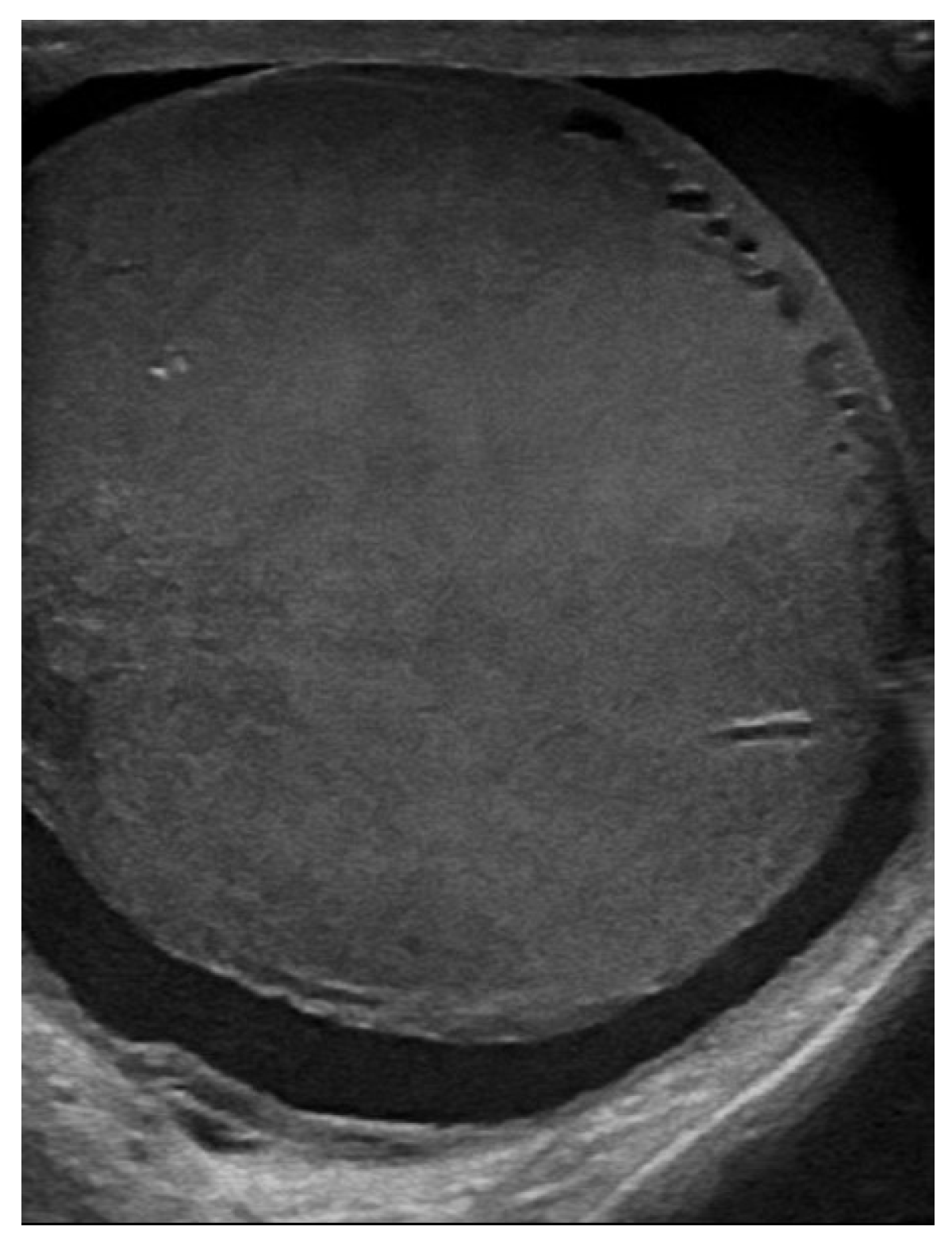
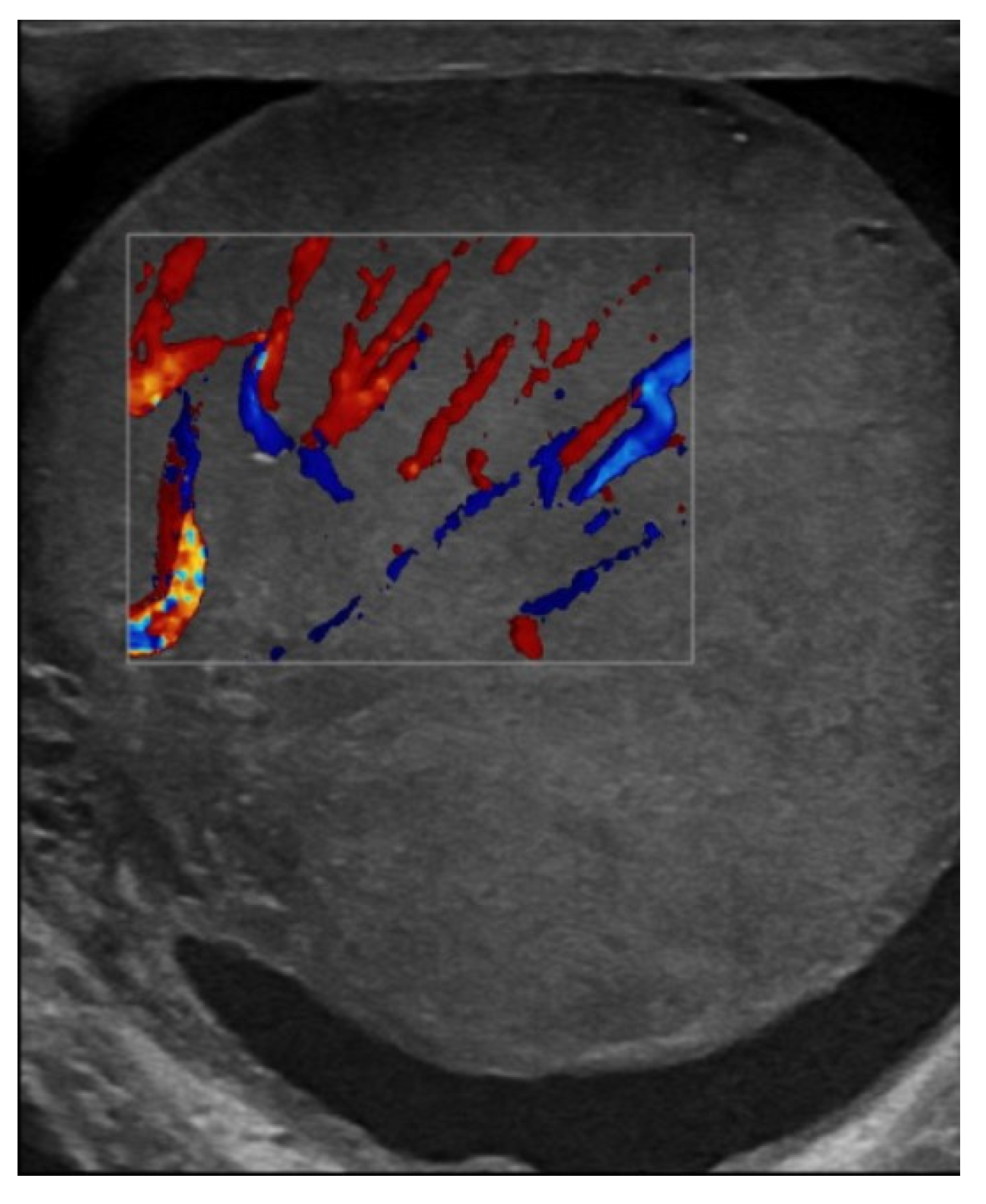
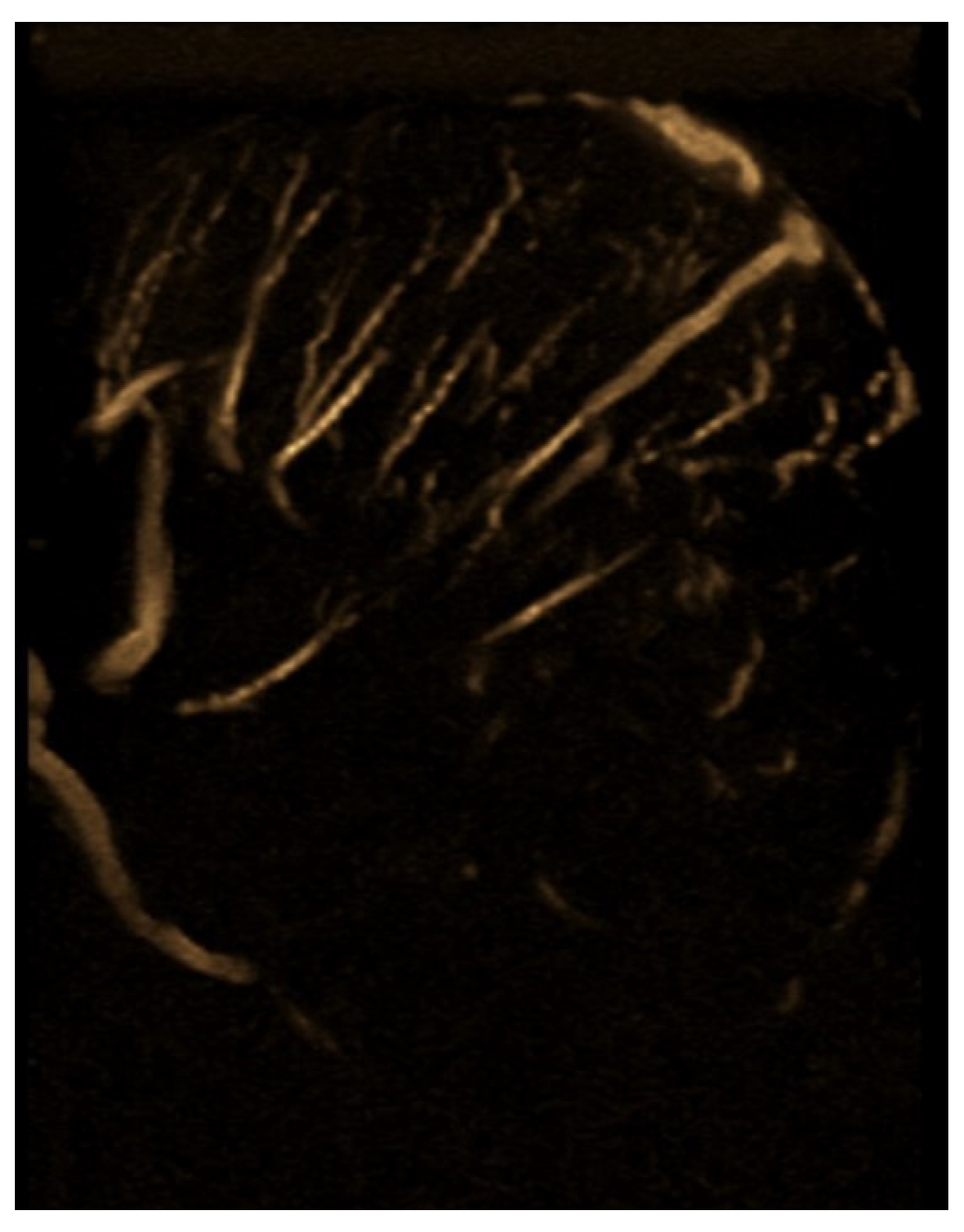
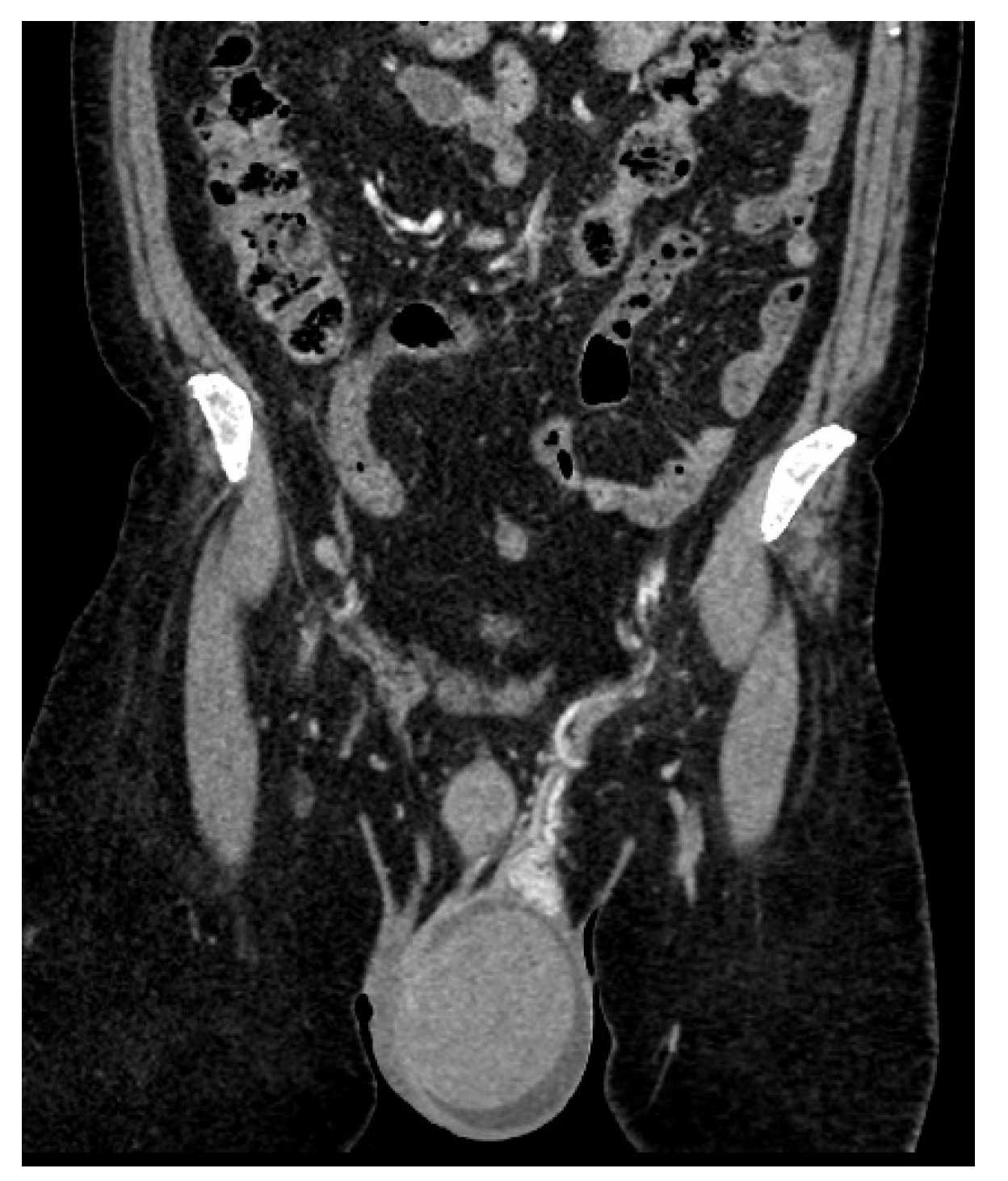

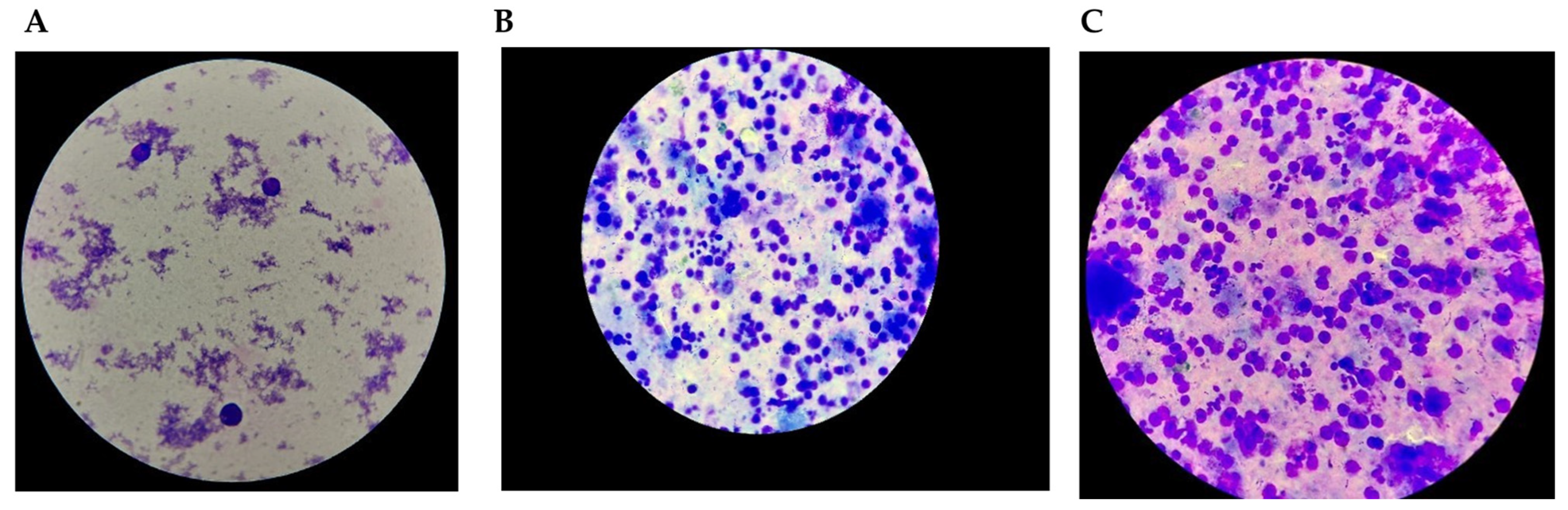
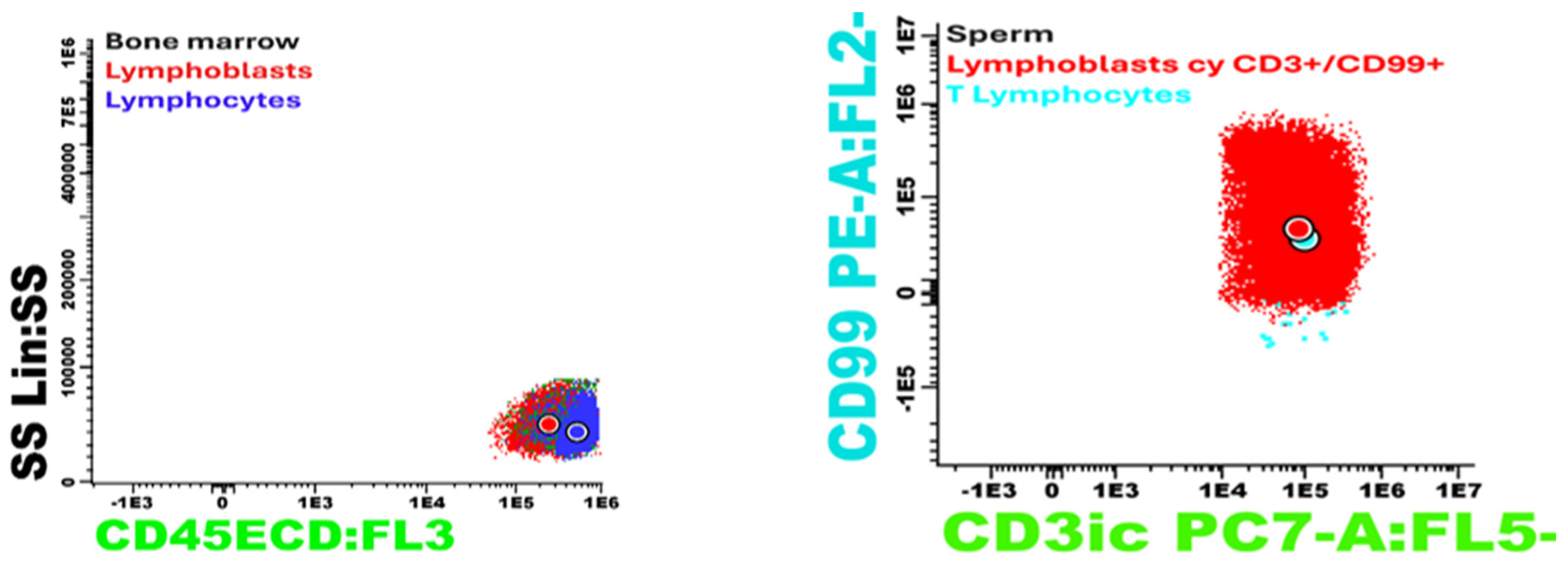
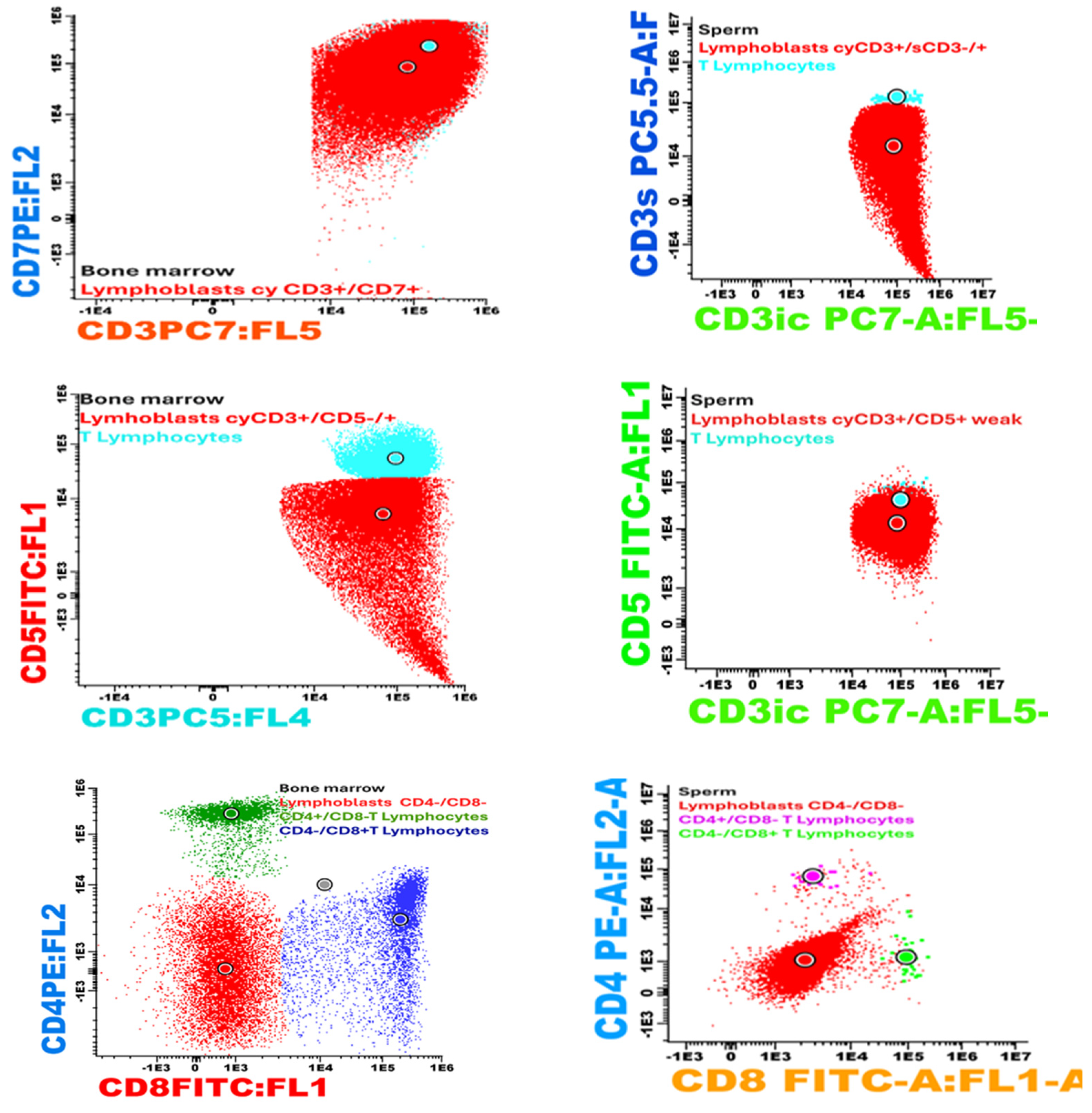
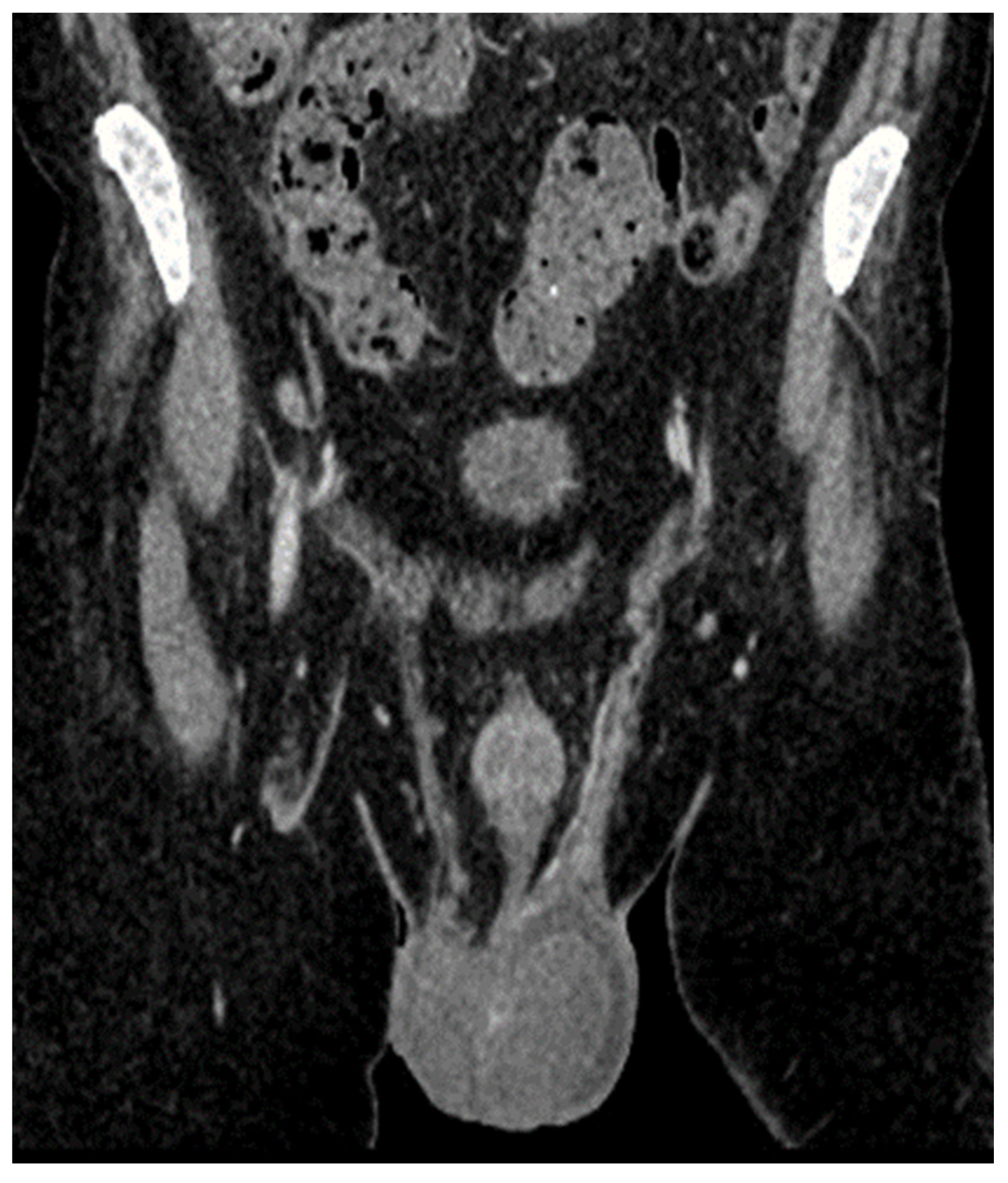
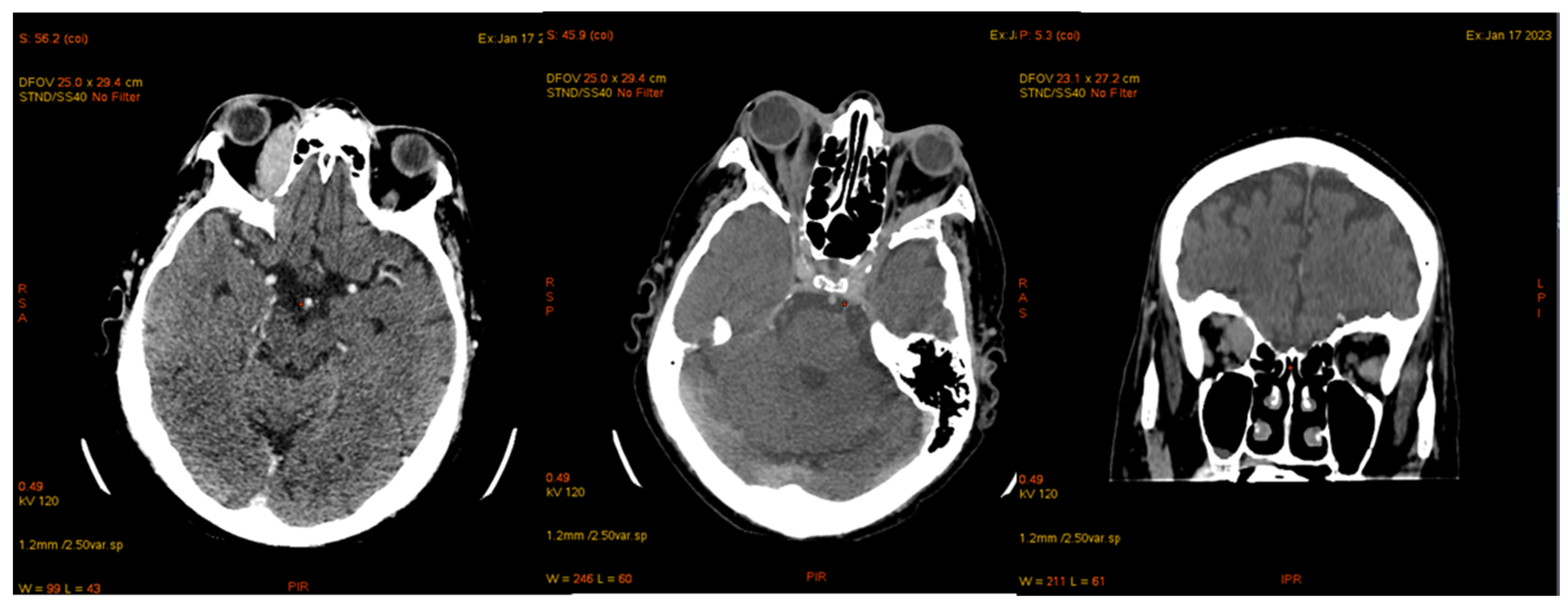

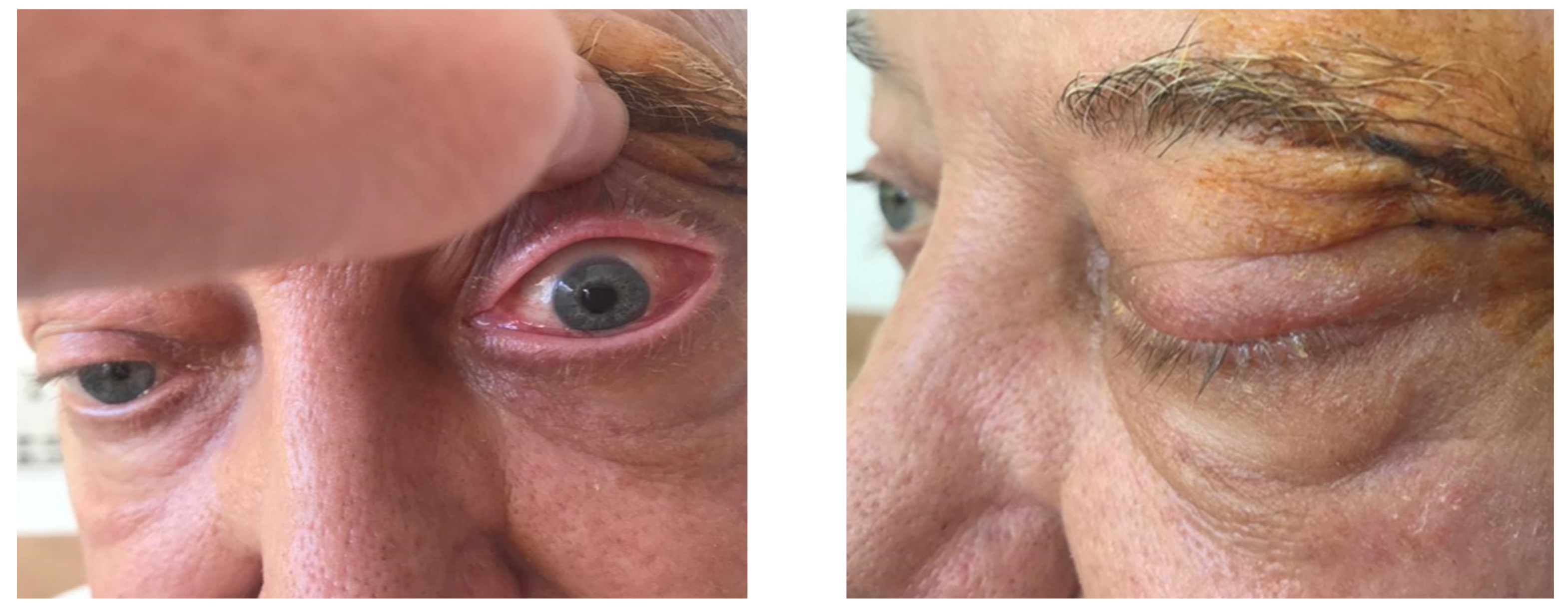
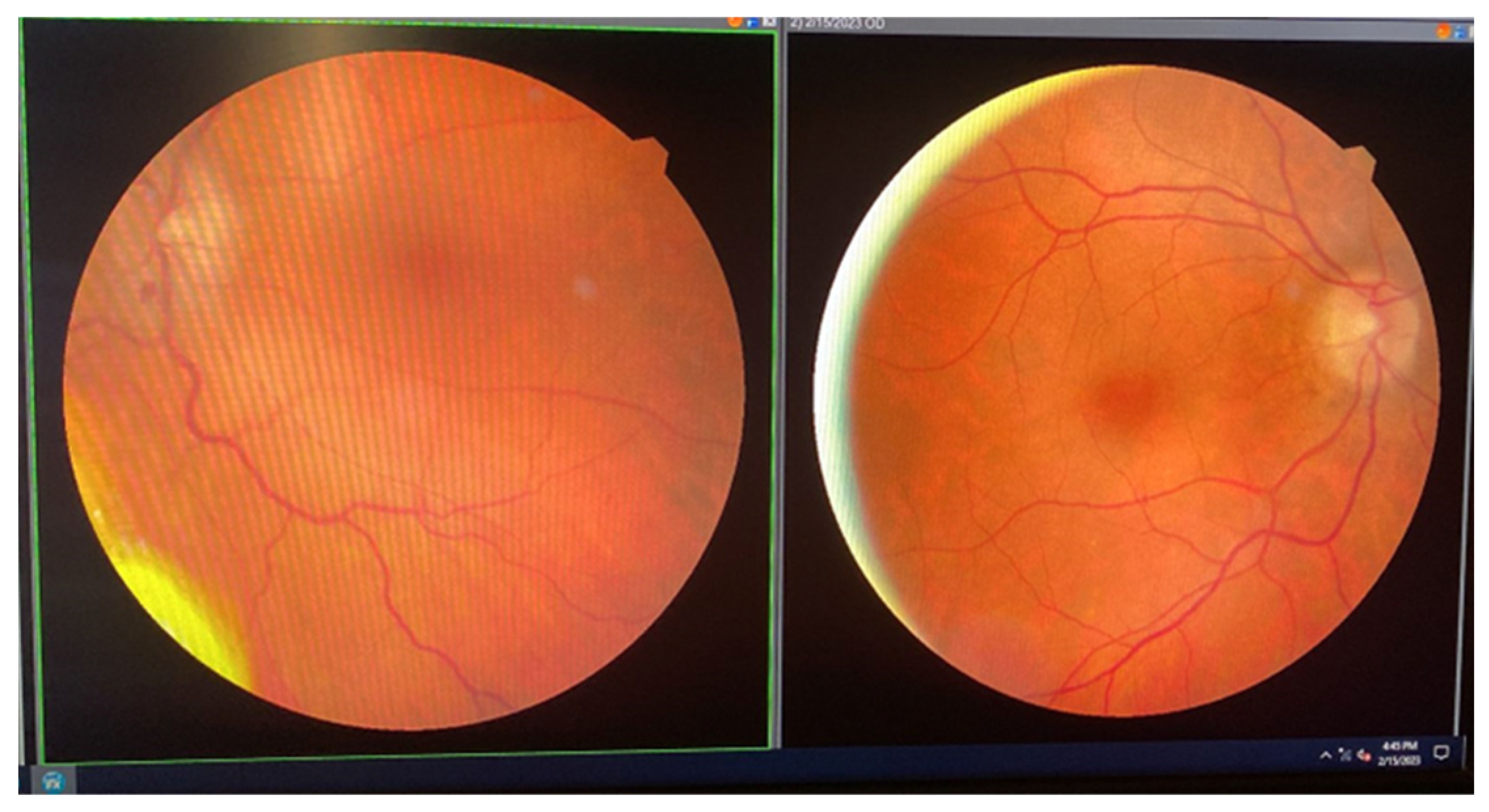
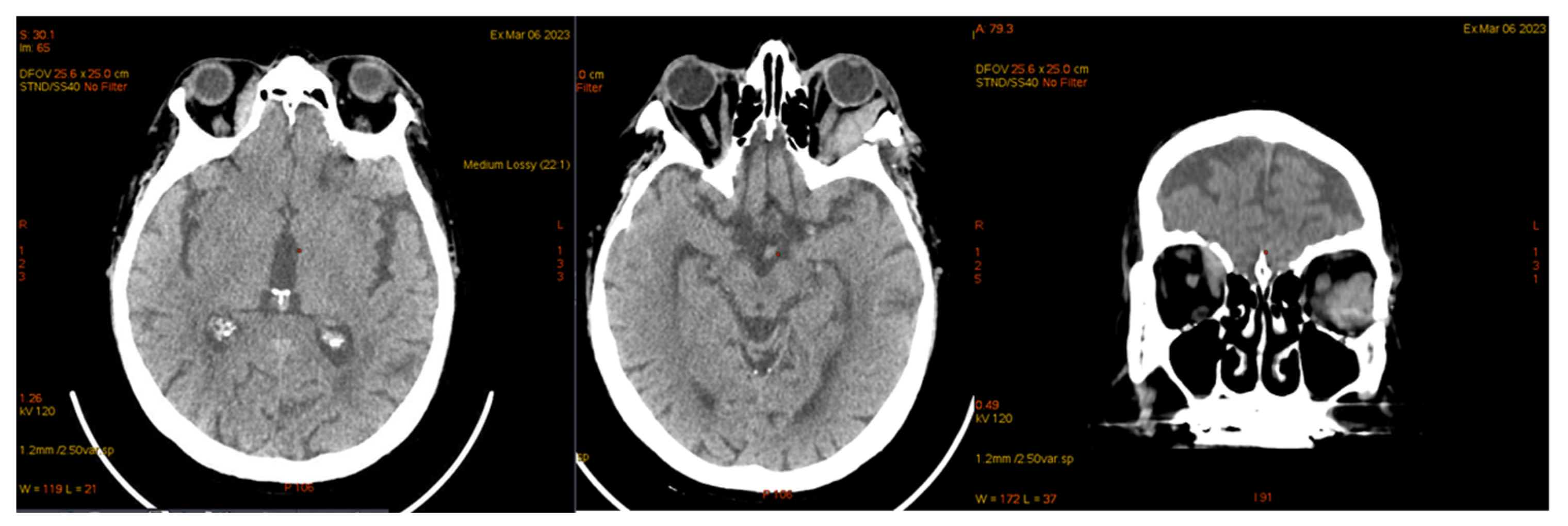


| Laboratory Analyses | Results |
|---|---|
| HLG | Lc = 4930/mm3, 0% neutrophils, 96% lymphocytes cu 50% lymphoblasts. Hb = 12.9 g/dL, Pl = 81,000/mm3 |
| Medullary aspirate | Lymphoblast cells ~56%: Some small cells, with scant cytoplasm, hyperbasophilic-ALL |
| Cytochemistry | PAS (Periodic Acid–Schiff) reaction: 20% positive elements (large, multiple granules). Peroxidase and alpha naphtyl acetate esterase (ANAES) reactions negative |
| Bone marrow immunophenotyping | Population percentage of 30% expressing: CD45+ weak, icCD3+, sCD3−, CD7+, CD99+ weak, CD34−, CD1a−, CD4−, CD8−, CD2−, CD5+ weak, CD123+ weak, MPO−, CD33− Erythroblasts 30%, mature lymphocytes 35%, the vast majority being T lymphocytes [9], The phenotypic profile was suggestive of the diagnosis of acute lymphoblastic leukemia T (pro-T ALL) |
| Bone marrow Histopatological and imunohistochimical | Diffuse marrow infiltration within a lymphoproliferative process with lymphocytes: CD3, CD5—positive in atypical T lymphocytes (90% of the marrow cellular population) CD20—positive in B lymphocytes in a few small lymphoid follicles CD10—negative BCL2—positive in the atypical lymphoid population Ki67 30% |
| Karyotype | 46 XY |
| Molecular Biology | No changes. Negative for: TCF3::PBX1-t(1;19)(q23;p13); KMT2A::AFF1-t(4;11)(q21;q23) BCR::ABL1 p190-t(9;22)(q34;q11); BCR::ABL1 p210-t(9;22)(q34;q11) ETV6::RUNX1-t(12;21)(p13;q22); SIL-TAL 1-del(1)(p32;p32) |
| HLA | Compatible 100% HLA with his sister |
| Date | Medical Events | Therapeutic Behavior and Result |
|---|---|---|
| 2013 | Mediastinal Tumor | Neglected |
| November 2016 | Thymoma 13/14/15 cm | Thymomectomy—December 2016 |
| June 2017 | Pro-T ALL | induction and consolidation gmall, 2003 cerebral radiotherapy, 24 gy intrathecal (mtx, ara-c, dexamethasone) result: complete remission |
| February 2018 | allotransplant sister | immunosuppression, 6 months (methotrexate and tacrolimus, then sirolimus, followed by methylprednisolon and sirolimus) |
| March 2022 | SARS-CoV-2 | home treatment |
| June 2022 | first extramedullary relapse left testicular—diagnostic immunophenotyping of semen | HyperCVAD Block A radiotherapy, 24 gy/12 sessions for the scrotal sac and the lymph nodes along the left spermatic vein result: complete metabolic remission petct |
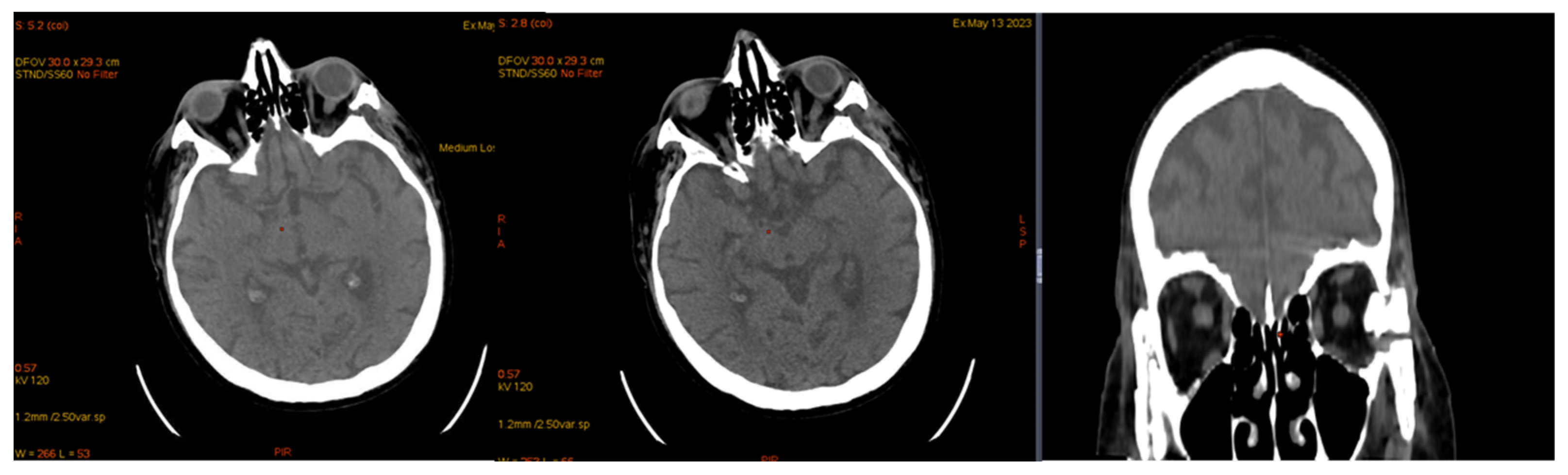
| January 2023 | second extramedullary relapse bilateral ocular left eye clinically, imaging, and biopsy demonstrated right eye demonstrated by imaging | methotrexate, asparaginase, calcium folinate radiotherapy, 30 gy/15 sessions result: imaging complete remission |
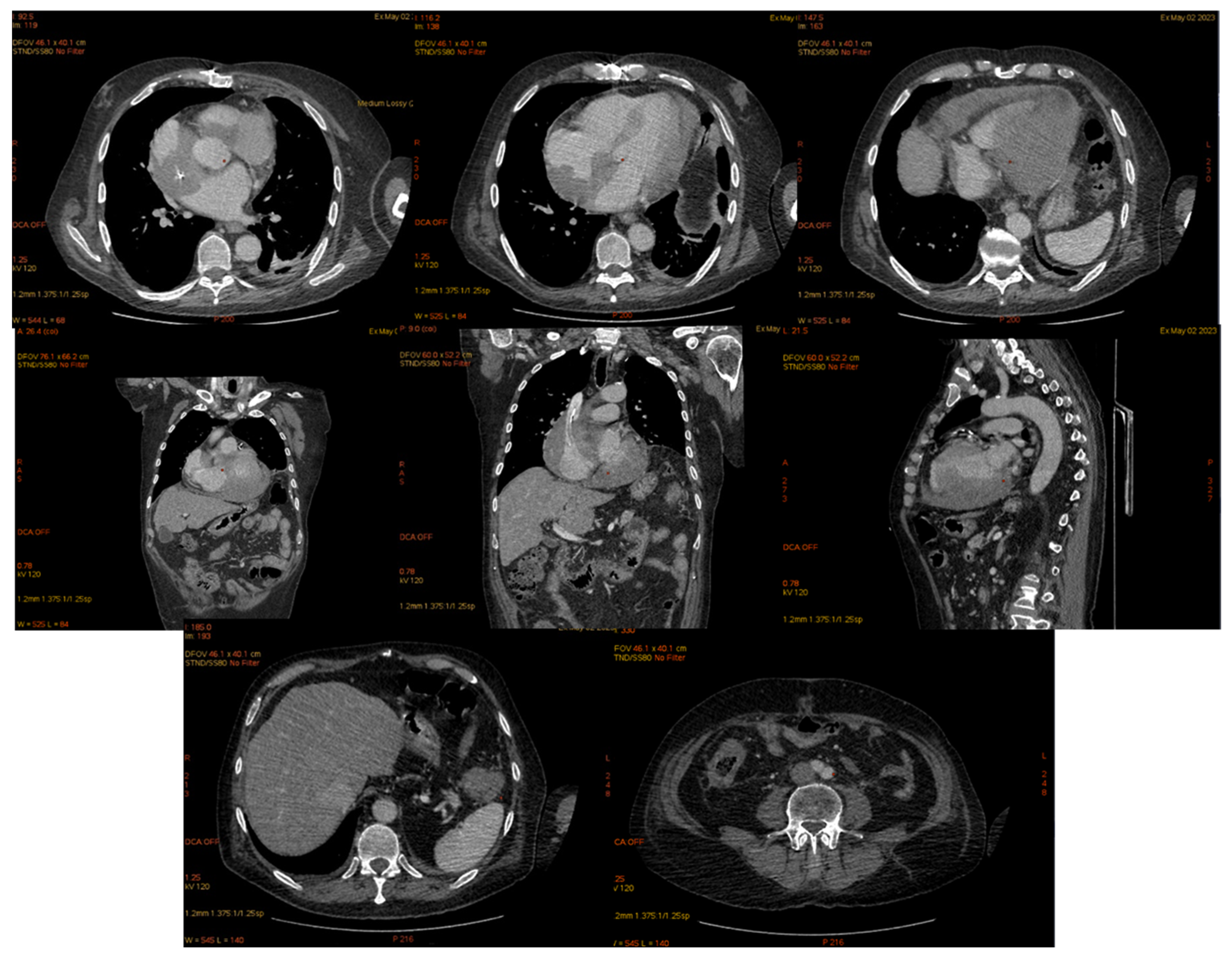
| 2 May 2023 | third emr: cardiac and colonic mass (+cns?) | nelarabine+cyclophosphamide+ etoposide |
| 14 May 2023 | death | msof |
| Cardiac EMR After Allo-HSCT Citation | Age, Years Old (y.o.) | Description of Cardiac Involvement/Injury and Manifestation | |
|---|---|---|---|
| 2002 Wright, T. et al. [44] | 33 y.o. man | 2 years after bone marrow transplant from a matched unrelated donor: isolate intracardiac mass | B-ALL t (11;19) |
| 2003 B C A M Bekkers et al. [42] | 44 y.o.man | After allotransplant: atrial fibrillation, ventricular tachycardia, complete atrioventricular block with an escape rhythm originating from the posterior fascicle, died from ventricular fibrillation the following day At necropsy, extensive cardiac localisation and concomitent relapse of bone marrow | B-ALL |
| 2006 Tsukasa, H. et al. [43] | 14 y.o. Female | 2 stem cell transplantation: 1: using bone marrow; 2: using peripheral blood Isolated cardiac relapse was diagnosed using several non-invasive imaging techniques Developed progressive and fatal hematological disease | T-ALL |
| 2016 Kiju Chang et al. [45] | 42 y.o. man | 7 months after allo-HSCT from a matched sibling donor Surgical LV biopsy revealed diffuse infiltration of ALL blasts in the myocardium and pericardium, complete atrioventricular block, heart failure, multiple heart attacks | B-ALL |
| 2017 Baritussio [46,47] | 38 y.o. man | 9 months after allotransplantation by infiltration in the eyes, myocardium, and pericardium Flocytometric MO and CSF 0.09% T-lymphoblastic lymphocytes were highlighted. He was treated with nelarabine PEV and intrathecal cytosar, with the resolution of the eye and heart tumor | T-ALL |
| 2018 Nadel, J [48] | 38 y.o. | Endomyocardial relapse and PB relapse | B-ALL Ph positive |
| 2021 Sheikh, I. et al. [22] | 11 y.o. man | HSCT with a single unrelated umbilical cord blood unit 8 months post-transplant developed cardiorespiratory failure, BAV, leukemic infiltration of SIV and LV, which required the implantation of a cardiac pacemaker. Later, a cardiac biopsy was performed and chemotherapy was initiated | B-ALL |
| 2023 Cao., Yigeng et al. [49] | 42 y.o. female | Allo-HSCT from a matched, sibling donor CART-cell therapy A cardiac mass was revealed after 11 months allo-HSCT, 5 months after CART | B-ALL/LBL |
| Colon EMR after allo-HSCT citation | Age, years old (y.o.) | Description of colonic involvement/injury | |
| 2019 Hathorn, K [50] | 57 y.o. man | 27 months from transplant relapse PB and BM transverse colon infiltrate | (Ph+) ALL |
| Issak, A [51] | 61 y.o. | Sigmoid infiltrated and BM infiltrated | B-ALL |
| 2022 Ifthikar, Zainab [52] | 30 y.o. male | Allo-HSCT from matched sibling Second allo-HSCT from a different matched sibling donor Acute lower GI bleeding Extramedullary relapse of ALL in the ascending colon+ relapse BM and PB | B-ALL |
| Orbital EMR after allo-HSCT citation | Age, years old (y.o.) | Description of orbital involvement/injury | |
| 1995 A, Colombini [41] | 13 y.o. girl | 3 years after BMT A tumor at the inferomedial part of the orbit infiltrating the maxillary and ethmoid sinuses and nasal cavities and also involving the rectus muscles | B-ALL |
| 2020 Willier, S [38] | 3 y.o. boy | Two allogeneic hematopoietic stem cell transplantations (HSCT) and previous CD19-CAR T-cell therapy. On day +16 after second transplant, episcleral and intraocular infiltration ALL relapse, and after few days, PB and BM relapse | pro-B ALL |
| TESTIS-adult EMR after allo-HSCT citation | Age, years old (y.o.) | Descrition of testis involvement/injury | |
| 2019 Jayakrishnan, T. [36] | 80 y.o. man | 5 years post-allogeneic matched unrelated donor Peripheral blood stem cell transplant | B-ALL Ph+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cătană, A.C.; Vlădoiu, M.-G.; Sandu, M.; Olteanu, A.; Mocanu, L.; Mihai, E.; Teodoru, M.; Matei, C.; Zahu, R.; Varady, Z.; et al. A Unique Case of Extramedullary Relapse in Acute Lymphoblastic Leukemia: Testicular to Ocular, Cardiac, and Colonic Involvement and the Role of Sperm Phenotyping in Diagnosis—Case Report and Literature Review. J. Clin. Med. 2025, 14, 405. https://doi.org/10.3390/jcm14020405
Cătană AC, Vlădoiu M-G, Sandu M, Olteanu A, Mocanu L, Mihai E, Teodoru M, Matei C, Zahu R, Varady Z, et al. A Unique Case of Extramedullary Relapse in Acute Lymphoblastic Leukemia: Testicular to Ocular, Cardiac, and Colonic Involvement and the Role of Sperm Phenotyping in Diagnosis—Case Report and Literature Review. Journal of Clinical Medicine. 2025; 14(2):405. https://doi.org/10.3390/jcm14020405
Chicago/Turabian StyleCătană, Alina Camelia, Maria-Gabriela Vlădoiu, Mariana Sandu, Ariela Olteanu, Liliana Mocanu, Elena Mihai, Minodora Teodoru, Claudiu Matei, Renata Zahu, Zsofia Varady, and et al. 2025. "A Unique Case of Extramedullary Relapse in Acute Lymphoblastic Leukemia: Testicular to Ocular, Cardiac, and Colonic Involvement and the Role of Sperm Phenotyping in Diagnosis—Case Report and Literature Review" Journal of Clinical Medicine 14, no. 2: 405. https://doi.org/10.3390/jcm14020405
APA StyleCătană, A. C., Vlădoiu, M.-G., Sandu, M., Olteanu, A., Mocanu, L., Mihai, E., Teodoru, M., Matei, C., Zahu, R., Varady, Z., Mondoc, L., Noor, C., Moicean, A., & Mera, G. (2025). A Unique Case of Extramedullary Relapse in Acute Lymphoblastic Leukemia: Testicular to Ocular, Cardiac, and Colonic Involvement and the Role of Sperm Phenotyping in Diagnosis—Case Report and Literature Review. Journal of Clinical Medicine, 14(2), 405. https://doi.org/10.3390/jcm14020405







