Risk-Stratified Venous Thromboembolism Chemoprophylaxis After Total Joint Arthroplasty: Evaluation of an Institutional Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Study Objectives, Groups, and Outcome Variables
2.3. VTE Chemoprophylaxis Prescribing Practices During Study Time Frame and Current Institutional Risk Stratification Development
2.4. Data Analyses
3. Results
4. Discussion
4.1. Contextualization with Prior Literature
4.2. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falck-Ytter, Y.; Francis, C.W.; Johanson, N.A.; Curley, C.; Dahl, O.E.; Schulman, S.; Ortel, T.L.; Pauker, S.G.; Colwell, C.W., Jr. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e278S–e325S. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Mont, M.A.; Bozic, K.J.; Della Valle, C.J.; Goodman, S.B.; Lewis, C.G.; Yates, A.C.J., Jr.; Boggio, L.N.; Watters, W.C., 3rd; Turkelson, C.M.; et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty. J. Bone Jt. Surg. Am. 2012, 94, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.C.; Siegal, D.; Lauw, M.N.; Ginsberg, J.S.; Eikelboom, J.W.; Guyatt, G.H.; Hirsh, J. A Systematic Review of Contemporary Trials of Anticoagulants in Orthopaedic Thromboprophylaxis: Suggestions for a Radical Reappraisal. J. Thromb. Thrombolysis 2015, 40, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Muscatelli, S.R.; Charters, M.A.; Hallstrom, B.R. Time for an Update? A Look at Current Guidelines for Venous Thromboembolism Prophylaxis After Hip and Knee Arthroplasty and Hip Fracture. Arthroplast. Today 2021, 10, 105–107. [Google Scholar] [CrossRef] [PubMed]
- The ICM-VTE General Delegates. Recommendations from the ICM-VTE: General. JBJS 2022, 104, 4. [Google Scholar] [CrossRef]
- CRISTAL Study Group; Sidhu, V.S.; Kelly, T.-L.; Pratt, N.; Graves, S.E.; Buchbinder, R.; Adie, S.; Cashman, K.; Ackerman, I.; Bastiras, D.; et al. Effect of Aspirin vs Enoxaparin on Symptomatic Venous Thromboembolism in Patients Undergoing Hip or Knee Arthroplasty: The CRISTAL Randomized Trial. JAMA 2022, 328, 719–727. [Google Scholar] [CrossRef]
- Ren, Y.; Cao, S.-L.; Li, Z.; Luo, T.; Feng, B.; Weng, X.-S. Comparable Efficacy of 100 Mg Aspirin Twice Daily and Rivaroxaban for Venous Thromboembolism Prophylaxis Following Primary Total Hip Arthroplasty: A Randomized Controlled Trial. Chin. Med. J. 2021, 134, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Dunbar, M.; Murnaghan, J.; Kahn, S.R.; Gross, P.; Forsythe, M.; Pelet, S.; Fisher, W.; Belzile, E.; Dolan, S.; et al. Aspirin or Rivaroxaban for VTE Prophylaxis after Hip or Knee Arthroplasty. N. Engl. J. Med. 2018, 378, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, J.L.; Ribeiro, F.N.; Mos, P.A.C.; Reis, J.P.; de Oliveira, H.R.; Miura, B.K. Venous Thromboembolism Prophylaxis after Total Knee Arthroplasty (TKA): Aspirin vs. Rivaroxaban. Rev. Bras. Ortop. 2018, 53, 22–27. [Google Scholar] [CrossRef]
- Krauss, E.S.; Segal, A.; Dengler, N.; Cronin, M.; Pettigrew, J.; Simonson, B.G. Utilization of the Caprini Score for Risk Stratification of the Arthroplasty Patient in the Prevention of Postoperative Venous Thrombosis. Semin. Thromb. Hemost. 2022, 48, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Alameri, M.A.; Syed Sulaiman, S.A.; Ashour, A.M.; Al-Saati, M.F. Venous Thromboembolism Prevention Protocol for Adapting Prophylaxis Recommendations to the Potential Risk Post Total Knee Replacement: A Randomized Controlled Trial. Pharm. Pract. 2020, 18, 2025. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Tamaki, Y.; Nakura, N.; Takayanagi, S.; Saito, A.; Ochiai, S.; Hirakawa, K. Clinical Efficacy of Risk-Stratified Prophylaxis with Low-Dose Aspirin for the Management of Symptomatic Venous Thromboembolism after Total Hip Arthroplasty. J. Orthop. Sci. 2020, 25, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Huang, R.; Rezapoor, M.; Bagheri, B.; Maltenfort, M.G. Individualized Risk Model for Venous Thromboembolism After Total Joint Arthroplasty. J. Arthroplast. 2016, 31, 180–186. [Google Scholar] [CrossRef]
- Kulshrestha, V.; Kumar, S. DVT Prophylaxis after TKA: Routine Anticoagulation vs Risk Screening Approach—A Randomized Study. J. Arthroplast. 2013, 28, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, B.; Nelissen, R.; Arya, R.; Cannegieter, S. Preventing VTE Following Total Hip and Knee Arthroplasty: Is Prediction the Future? J. Thromb. Haemost. 2021, 19, 41–45. [Google Scholar] [CrossRef]
- Kahn, S.R.; Shivakumar, S. What’s New in VTE Risk and Prevention in Orthopedic Surgery. Res. Pract. Thromb. Haemost. 2020, 4, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Krauss, E.S.; Cronin, M.; Dengler, N.; Simonson, B.G.; Enker, P.; Segal, A. Lessons Learned: Using the Caprini Risk Assessment Model to Provide Safe and Efficacious Thromboprophylaxis Following Hip and Knee Arthroplasty. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620961450. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Stevens, S.M.; Woller, S.C.; Baumann Kreuziger, L.; Doerschug, K.; Geersing, G.-J.; Klok, F.A.; King, C.S.; Murin, S.; Vintch, J.R.E.; Wells, P.S.; et al. Antithrombotic Therapy for VTE Disease. Chest 2024, 166, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Matharu, G.S.; Kunutsor, S.K.; Judge, A.; Blom, A.W.; Whitehouse, M.R. Clinical Effectiveness and Safety of Aspirin for Venous Thromboembolism Prophylaxis After Total Hip and Knee Replacement: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. JAMA Intern. Med. 2020, 180, 376–384. [Google Scholar] [CrossRef]
- Petersen, P.B.; Jørgensen, C.C.; Kehlet, H.; Lundbeck Foundation Centre for Fast-track Hip Knee Replacement Collaborative Group. Venous Thromboembolism despite Ongoing Prophylaxis after Fast-Track Hip and Knee Arthroplasty: A Prospective Multicenter Study of 34,397 Procedures. Thromb. Haemost. 2019, 119, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Dunbar, M.J.; Bohm, E.R.; Belzile, E.; Kahn, S.R.; Zukor, D.; Fisher, W.; Gofton, W.; Gross, P.; Pelet, S.; et al. Aspirin versus Low-Molecular-Weight Heparin for Extended Venous Thromboembolism Prophylaxis after Total Hip Arthroplasty: A Randomized Trial. Ann. Intern. Med. 2013, 158, 800–806. [Google Scholar] [CrossRef]
- Peng, H.-M.; Chen, X.; Wang, Y.-O.; Bian, Y.-Y.; Feng, B.; Wang, W.; Weng, X.-S.; Qian, W.-W. Risk-Stratified Venous Thromboembolism Prophylaxis after Total Joint Arthroplasty: Low Molecular Weight Heparins and Sequential Aspirin vs Aggressive Chemoprophylaxis. Orthop. Surg. 2021, 13, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.; Dasa, V.; Krause, P.C.; Hall, L.; Chapple, A.G. Hospital Length of Stay Is Associated With Increased Likelihood for Venous Thromboembolism After Total Joint Arthroplasty. Arthroplast. Today 2021, 8, 254–257.e1. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.C.; Whitehouse, M.R.; Blom, A.W.; Kunutsor, S.K. Host-Related Factors for Venous Thromboembolism Following Total Joint Replacement: A Meta-Analysis of 89 Observational Studies Involving over 14 Million Hip and Knee Replacements. J. Orthop. Sci. 2020, 25, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.B.; Kehlet, H.; Jørgensen, C.C.; Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group. Safety of In-Hospital Only Thromboprophylaxis after Fast-Track Total Hip and Knee Arthroplasty: A Prospective Follow-Up Study in 17,582 Procedures. Thromb. Haemost. 2018, 118, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.; Rodrigues, F.B.; Pinto, F.J.; Ferreira, J.J.; Costa, J. Thromboprophylaxis With Apixaban in Patients Undergoing Major Orthopedic Surgery: Meta-Analysis and Trial-Sequential Analysis. Clin. Med. Insights Blood Disord. 2017, 10, 1179545X17704660. [Google Scholar] [CrossRef]
- Suen, K.; Westh, R.N.; Churilov, L.; Hardidge, A.J. Low-Molecular-Weight Heparin and the Relative Risk of Surgical Site Bleeding Complications: Results of a Systematic Review and Meta-Analysis of Randomized Controlled Trials of Venous Thromboprophylaxis in Patients After Total Joint Arthroplasty. J. Arthroplast. 2017, 32, 2911–2919.e6. [Google Scholar] [CrossRef]
- Hyland, S.J.; Kramer, B.J.; Fada, R.A.; Lucki, M.M. Clinical Pharmacist Service Associated With Improved Outcomes and Cost Savings in Total Joint Arthroplasty. J. Arthroplast. 2020, 35, 2307–2317.e1. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A Simulation Study of the Number of Events per Variable in Logistic Regression Analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Khatkar, H.; Elahi, Z.; See, A.; McDonald, S.; Neal-Smith, G. Preventing Venous Thromboembolism after Elective Total Hip Arthroplasty Surgery—Are the Current Guidelines Appropriate? Venous Thromboembolism Prophylaxis in Elective Total Hip Arthroplasty Surgery. J. Clin. Orthop. Trauma 2022, 26, 101782. [Google Scholar] [CrossRef]
- The ICM-VTE Hip & Knee Delegates. Recommendations from the ICM-VTE: Hip & Knee. J. Bone Joint Surg. Am. 2022, 104, 180–231. [Google Scholar] [CrossRef]
- Moisander, A.M.; Pamilo, K.; Eskelinen, A.; Huopio, J.; Kautiainen, H.; Kuitunen, A.; Paloneva, J. Venous Thromboembolism Is Rare after Total Hip and Knee Joint Arthroplasty with Long Thromboprophylaxis in Finnish Fast-Track Hospitals. Arch. Orthop. Trauma. Surg. 2023, 143, 5623–5629. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.B.; Andersen, I.T.; Overgaard, S.; Fenstad, A.M.; Lie, S.A.; Gjertsen, J.-E.; Furnes, O. Optimal Duration of Anticoagulant Thromboprophylaxis in Total Hip Arthroplasty: New Evidence in 55,540 Patients with Osteoarthritis from the Nordic Arthroplasty Register Association (NARA) Group. Acta Orthop. 2019, 90, 298–305. [Google Scholar] [CrossRef]
- Liu, K.C.; Bagrodia, N.; Richardson, M.K.; Piple, A.S.; Kusnezov, N.; Wang, J.C.; Lieberman, J.R.; Heckmann, N.D. Risk Factors Associated with Thromboembolic Complications after Total Hip Arthroplasty: An Analysis of 1129 Pulmonary Emboli. J. Am. Acad. Orthop. Surg. 2024, 32, e706–e715. [Google Scholar] [CrossRef] [PubMed]
- Lavu, M.S.; Porto, J.R.; Hecht, C.J.; Acuña, A.J.; Kaelber, D.C.; Parvizi, J.; Kamath, A.F. Low-Dose Aspirin Is the Safest Prophylaxis for Prevention of Venous Thromboembolism after Total Knee Arthroplasty across All Patient Risk Profiles. J. Bone Jt. Surg. Am. 2024, 106, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-H.; Wang, C.-Y. Is It Time to Comprehensively Utilize Low-Dose Aspirin for Preventing Venous Thromboembolism after Total Knee Arthroplasty? Commentary on an Article by Monish S. Lavu, MHM; et al.: “Low-Dose Aspirin Is the Safest Prophylaxis for Prevention of Venous Thromboembolism after Total Knee Arthroplasty across All Patient Risk Profiles”. J. Bone Joint Surg. Am. 2024, 106, e29. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.S.; Haut, E.R. Should Aspirin Be Routinely Used for Venous Thromboembolism Prophylaxis After Total Knee Arthroplasty? Even the Authors of This Commentary Cannot Agree. JAMA Surg. 2019, 154, 72–73. [Google Scholar] [CrossRef]
- Jørgensen, C.C.; Petersen, P.B.; Reed, M.; Kehlet, H. Recommendations on Thromboprophylaxis in Major Joint Arthroplasty—Many Guidelines, Little Consensus? J. Thromb. Haemost. 2019, 17, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, V.D., Jr.; Eikelboom, J.; McCollister Evarts, C.; Franklin, P.D.; Goldhaber, S.Z.; Iorio, R.; Lambourne, C.A.; Magaziner, J.S.; Magder, L.S.; Steering Committee of The PEPPER Trial. Selection Bias, Orthopaedic Style: Knowing What We Don’t Know About Aspirin. J. Bone Jt. Surg. Am. 2020, 102, 631–633. [Google Scholar] [CrossRef]
- Matharu, G.S.; Garriga, C.; Whitehouse, M.R.; Rangan, A.; Judge, A. Is Aspirin as Effective as the Newer Direct Oral Anticoagulants for Venous Thromboembolism Prophylaxis After Total Hip and Knee Arthroplasty? An Analysis From the National Joint Registry for England, Wales, Northern Ireland, and the Isle of Man. J. Arthroplast. 2020, 35, 2631–2639.e6. [Google Scholar] [CrossRef] [PubMed]
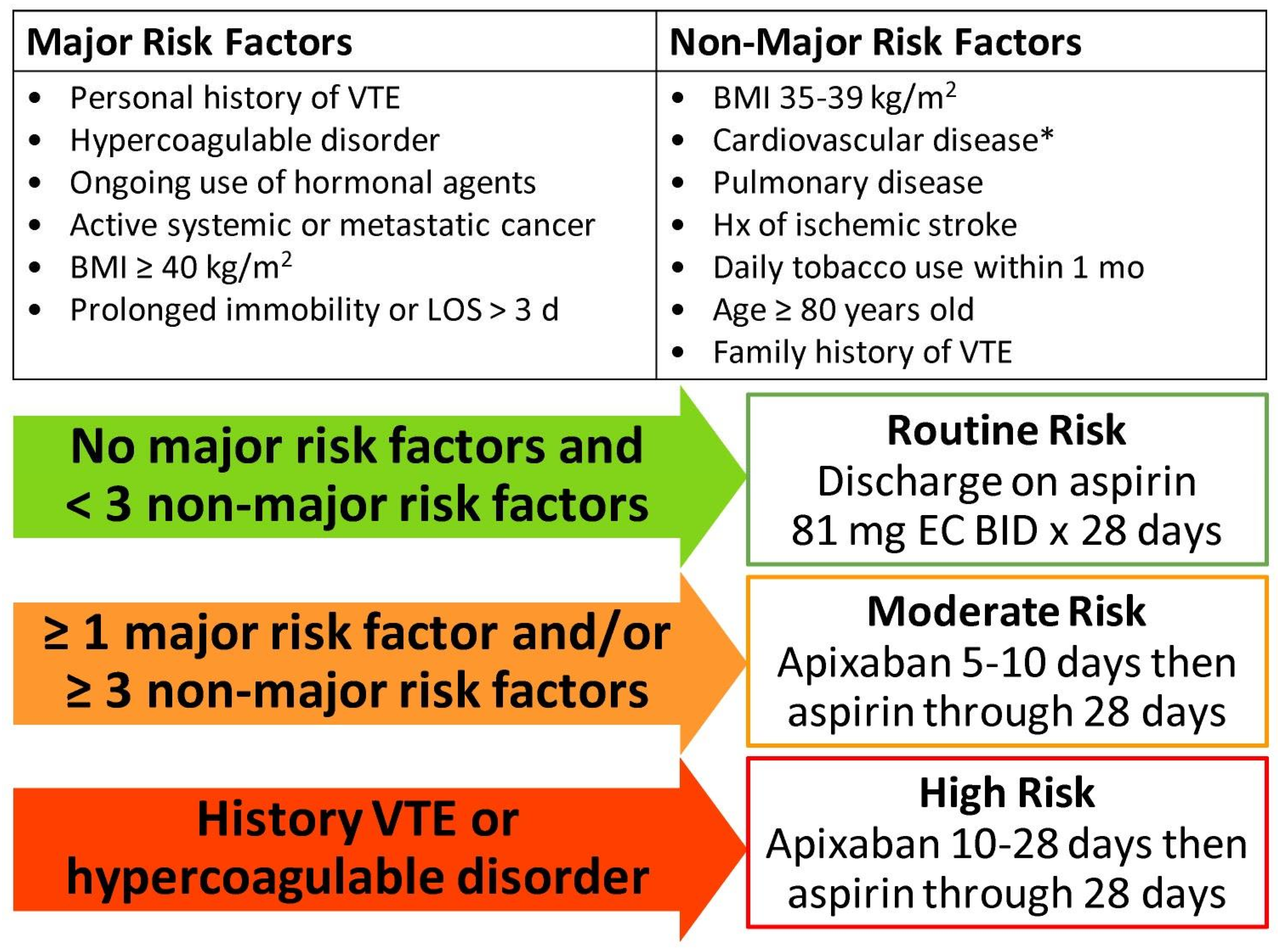
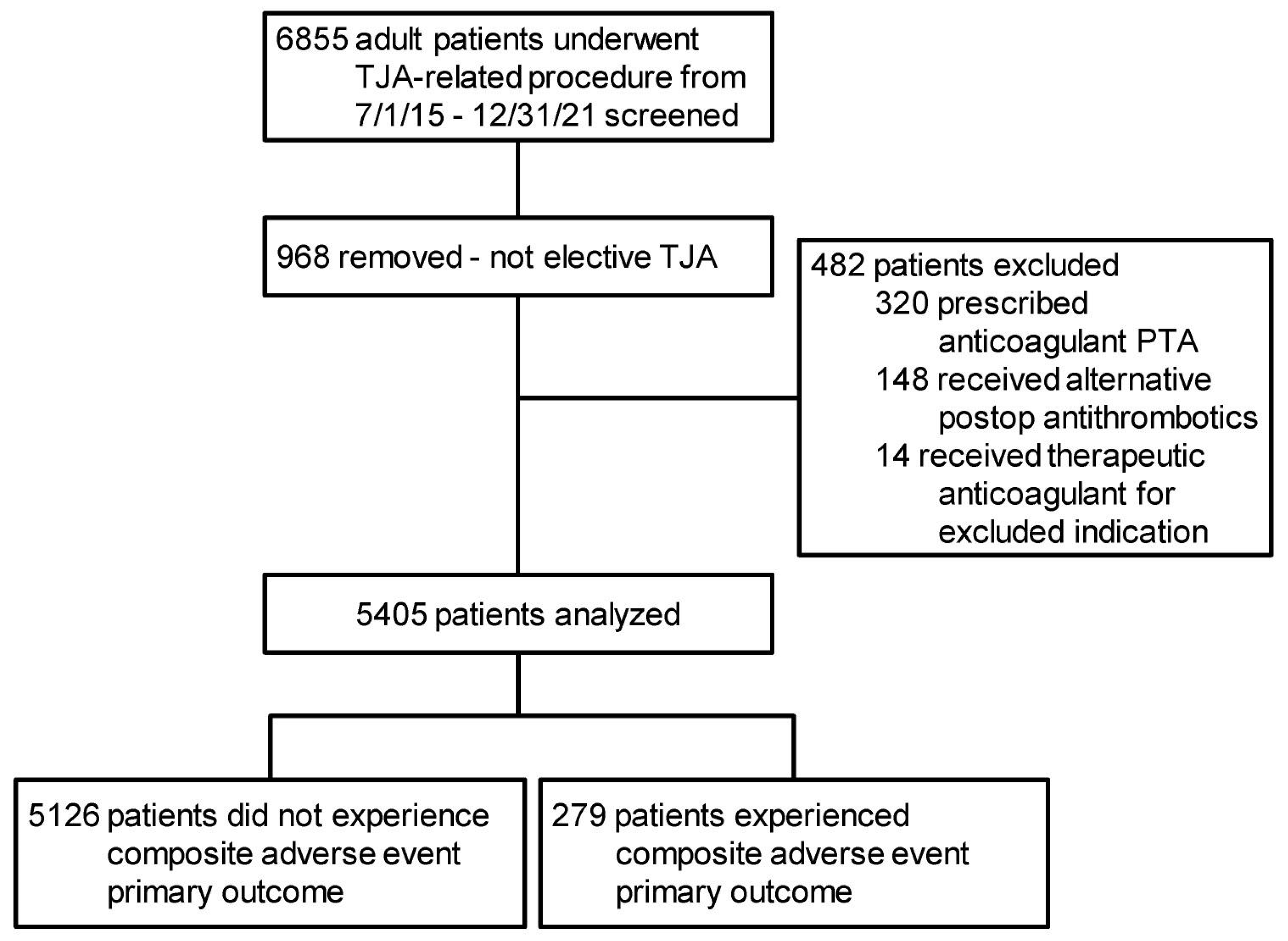
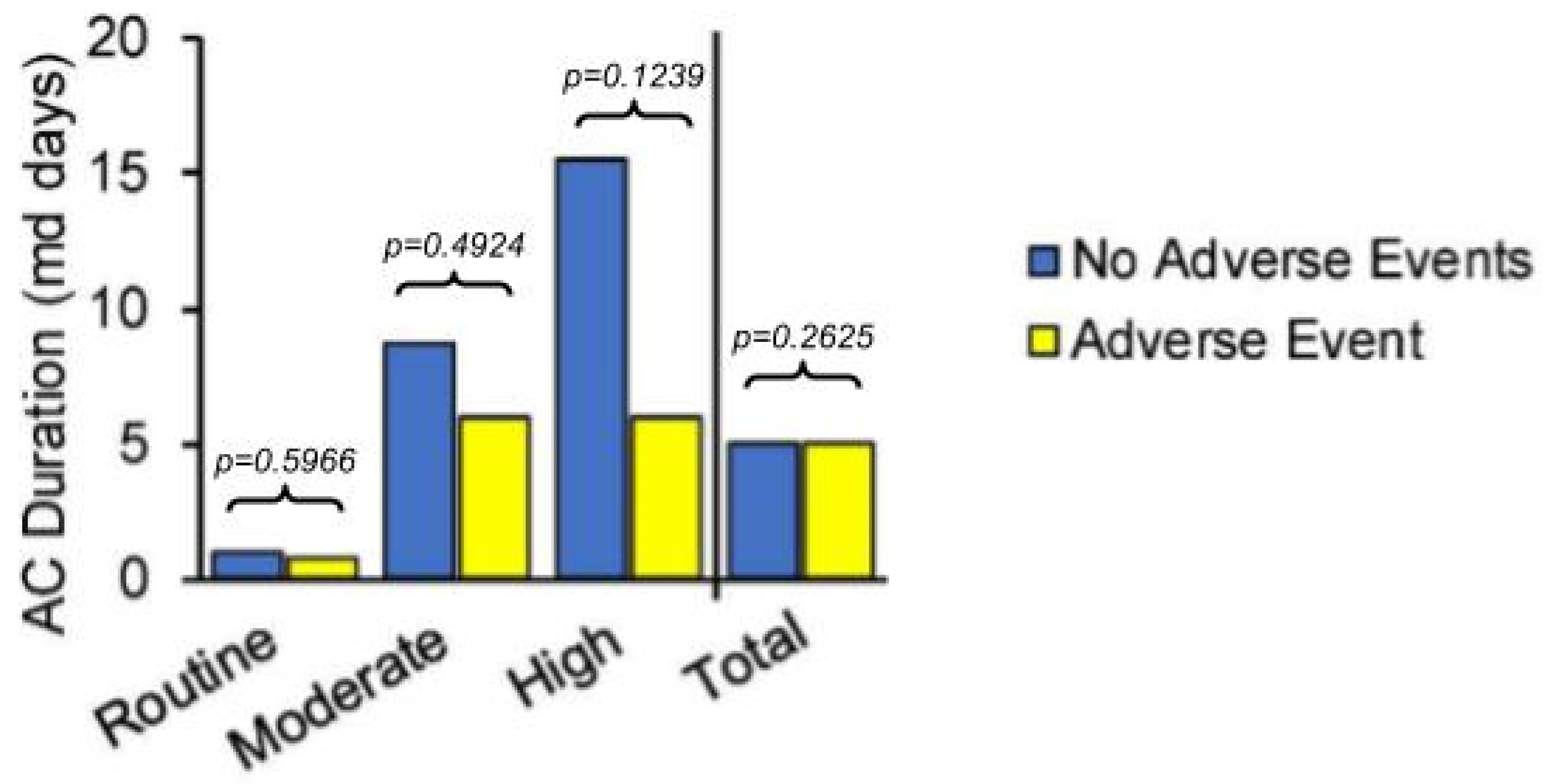
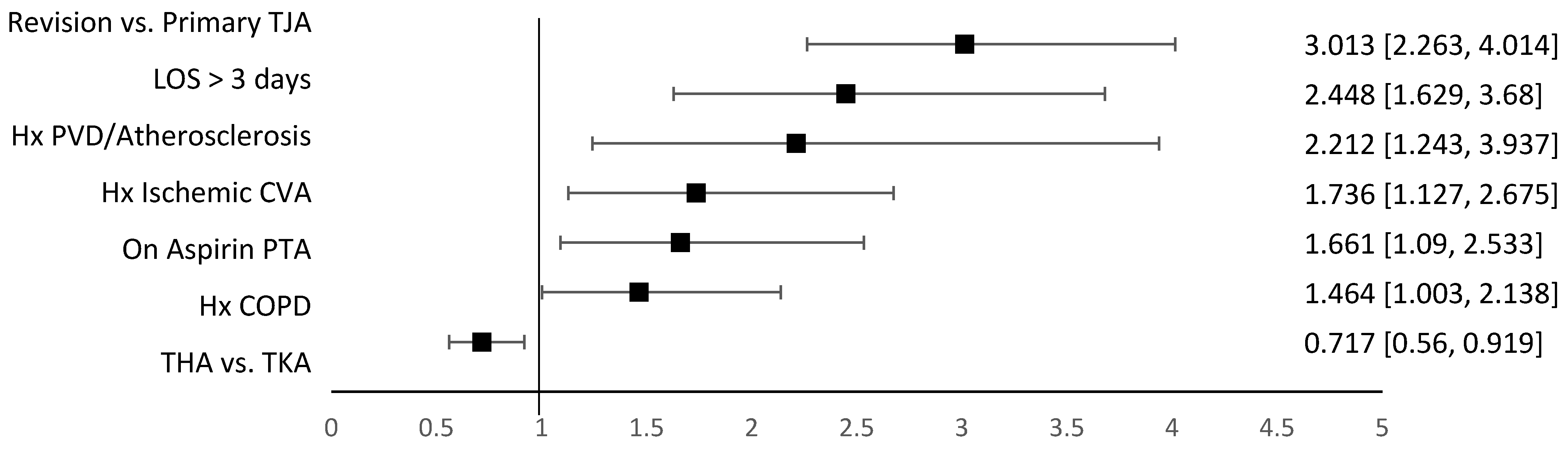
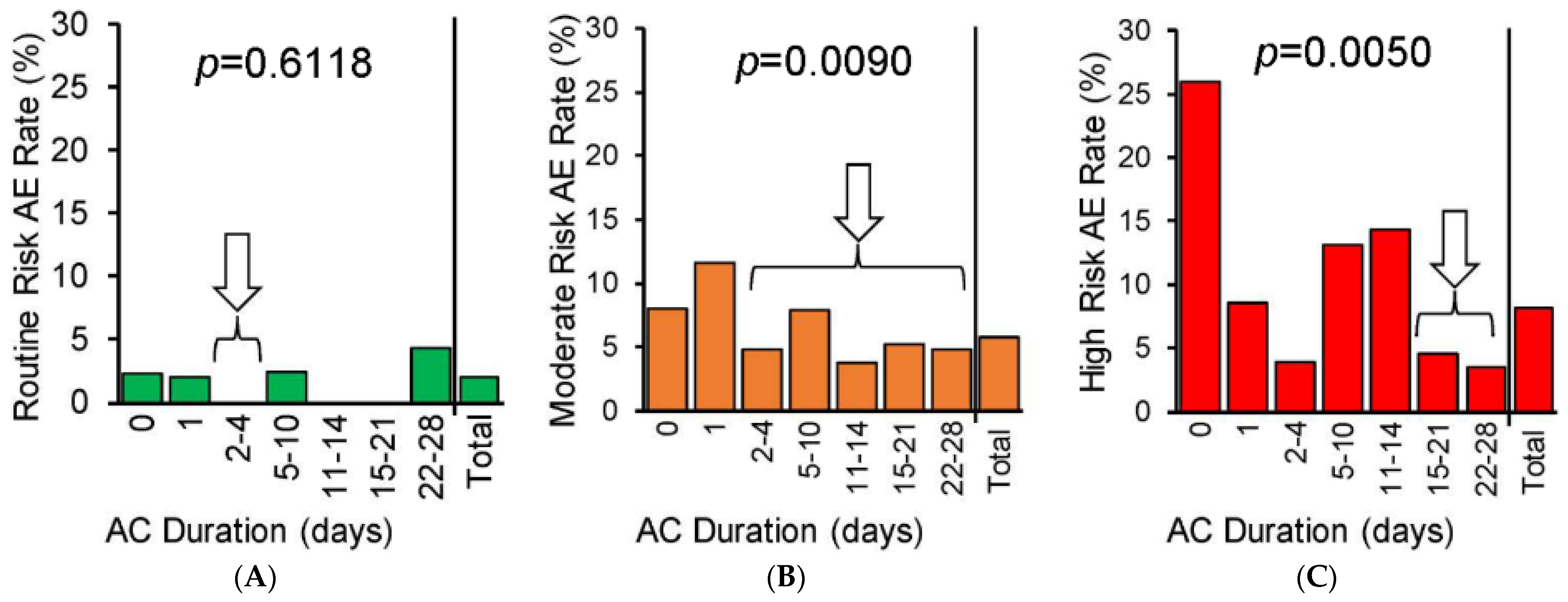
| Thrombotic Adverse Events | Hemorrhagic/Wound Adverse Events |
|---|---|
|
|
| Variable | N | % of Total Population N = 5405 | % of Complication Population n = 279 |
|---|---|---|---|
| Any VTE | 48 | 0.9 | 17.2 |
| DVT | 38 | 0.7 | 13.6 |
| PE | 10 | 0.2 | 3.6 |
| Any arterial thrombotic event | 23 | 0.4 | 8.2 |
| CVA | 3 | 0.06 | 1.1 |
| MI | 4 | 0.07 | 1.4 |
| Other arterial thrombosis | 17 | 0.3 | 6.1 |
| Readmission for wound complication or any-site bleeding | 82 | 1.5 | 29.4 |
| Reoperation for wound complication or bleeding | 82 | 1.5 | 29.4 |
| Blood transfusion 2+ units PRBCs | 106 | 2 | 38 |
| Total Population N = 5405 | Composite Complication Primary Outcome | |||
|---|---|---|---|---|
| No n = 5126 | Yes n = 279 | p-Value | ||
| VTE Risk Strata, n (%) | <0.001 | |||
| Routine | 1084 (20) | 1062 (20.7) | 22 (7.9) | |
| Moderate | 3993 (74) | 3764 (73.4) | 229 (82.1) | |
| High | 327 (6) | 300 (5.9) | 27 (9.7) | |
| Exposure Groups *, n (%) | 0.0003 | |||
| Aspirin Only | 639 (11.8) | 605 (11.8) | 34 (12.2) | |
| Enoxaparin then Aspirin | 3207 (59.3) | 3011 (58.7) | 196 (70.3) | |
| Apixaban then Aspirin | 1461 (27) | 1416 (27.6) | 45 (16.1) | |
| Total Population N = 5405 | Composite Complication Primary Outcome | |||
|---|---|---|---|---|
| No n = 5126 | Yes n = 279 | p-Value | ||
| Demographics | ||||
| Age, mean (SD) | 62.1 (10.2) | 62 (56–68) | 63 (54–73) | 0.1773 |
| Age ≥ 80, n (%) | 247 (4.6) | 227 (4.4) | 20 (8.7) | 0.0328 |
| Sex, n (%) | 0.3740 | |||
| Female | 3141 (58.1) | 2986 (58.3) | 155 (55.6) | |
| Male | 2264 (41.9) | 2140 (41.7) | 124 (44.4) | |
| Weight (kg), mean (SD) | 92.8 (20.1) | 92.8 (20) | 93.7 (22.2) | 0.4644 |
| BMI (kg/m2), median (IQR) | 32 (28–36) | 32 (28–36) | 32.1 (27–36) | 0.8407 |
| BMI 35–39 (kg/m2), n (%) | 1163 (21.5) | 1101 (21.5) | 62 (22.2) | 0.7685 |
| BMI ≥ 40 (kg/m2), n (%) | 510 (9.4) | 478 (9.3) | 32 (11.5) | 0.2327 |
| Medical History, n (%) | ||||
| Any VTE | 310 (5.7) | 284 (5.5) | 26 (9.3) | 0.0082 |
| DVT | 309 (5.7) | 283 (5.5) | 26 (9.3) | 0.0078 |
| PE | 1 (<0.1) | 1 (<0.1) | 0 (0) | 1.0000 |
| Hypercoagulable Disorder | 44 (0.8) | 41 (0.8) | 3 (1) | 0.4946 |
| Active Cancer | 2 (<0.1) | 2 (<0.1) | 0 (0) | 1.0000 |
| CAD/Ischemic Disease | 468 (8.7) | 428 (8.3) | 40 (14.3) | 0.0005 |
| Heart Failure | 124 (2.3) | 110 (2.1) | 14 (5) | 0.0018 |
| Atrial Fibrillation | 150 (2.8) | 137 (2.6) | 13 (4.7) | 0.0491 |
| Cardiomyopathy | 50 (0.9) | 47 (0.9) | 3 (1.1) | 0.7428 |
| Valvular Disease | 48 (0.9) | 43 (0.8) | 5 (1.8) | 0.0996 |
| Varicose Veins | 4 (<0.1) | 3 (<0.1) | 1 (0.4) | 0.1911 |
| PVD/Atherosclerosis | 113 (2.1) | 98 (1.9) | 15 (5.4) | <0.0001 |
| COPD | 271 (5) | 353 (6.9) | 36 (12.9) | <0.0001 |
| OSA | 1009 (18.7) | 937 (18.3) | 72 (25.8) | 0.0017 |
| Ischemic CVA | 274 (5) | 247 (4.8) | 27 (9.7) | 0.0003 |
| Tobacco Use | 832 (15.4) | 776 (15.1) | 56 (20) | 0.0262 |
| Hemophilia | 5 (<0.1) | 5 (<0.1) | 0 (0) | 1.0000 |
| On Aspirin PTA | 300 (5.5) | 272 (5.3) | 28 (10) | 0.0008 |
| On Hormonal Agent PTA | 57 (1.1) | 56 (1.1) | 1 (0.4) | 0.3679 |
| Procedure/Hospitalization Data | ||||
| Operative Joint, n (%) | 0.0129 | |||
| THA | 1967 (36.4) | 1846 (36) | 121 (43.4) | |
| TKA | 3438 (63.6) | 3280 (64) | 158 (56.6) | |
| Procedure Type, n (%) | <0.0001 | |||
| Primary | 4837 (89.5) | 4632 (90.4) | 205 (73.5) | |
| Revision | 568 (10.5) | 494 (9.6) | 74 (26.5) | |
| LOS (days), median (IQR) | 3.4 (3.1–4.3) | 3.4 (3.1–4.3) | 4.4 (3.4–5.5) | <0.0001 |
| LOS > 3 days, n (%). | 4133 (76.5) | 3881 (75.7) | 252 (90.3) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyland, S.J.; Fada, M.J.; Secic, M.; Fada, R.A.; Lockhart, M.M.; Parrish, R.H., II. Risk-Stratified Venous Thromboembolism Chemoprophylaxis After Total Joint Arthroplasty: Evaluation of an Institutional Approach. J. Clin. Med. 2025, 14, 366. https://doi.org/10.3390/jcm14020366
Hyland SJ, Fada MJ, Secic M, Fada RA, Lockhart MM, Parrish RH II. Risk-Stratified Venous Thromboembolism Chemoprophylaxis After Total Joint Arthroplasty: Evaluation of an Institutional Approach. Journal of Clinical Medicine. 2025; 14(2):366. https://doi.org/10.3390/jcm14020366
Chicago/Turabian StyleHyland, Sara J., Maria J. Fada, Michelle Secic, Robert A. Fada, Marie M. Lockhart, and Richard H. Parrish, II. 2025. "Risk-Stratified Venous Thromboembolism Chemoprophylaxis After Total Joint Arthroplasty: Evaluation of an Institutional Approach" Journal of Clinical Medicine 14, no. 2: 366. https://doi.org/10.3390/jcm14020366
APA StyleHyland, S. J., Fada, M. J., Secic, M., Fada, R. A., Lockhart, M. M., & Parrish, R. H., II. (2025). Risk-Stratified Venous Thromboembolism Chemoprophylaxis After Total Joint Arthroplasty: Evaluation of an Institutional Approach. Journal of Clinical Medicine, 14(2), 366. https://doi.org/10.3390/jcm14020366








