Abstract
Objectives: Despite optimal local control obtained with neoadjuvant chemoradiotherapy (CRT), data on overall survival (OS) and disease-free survival (DFS) of local advanced rectal cancer patients are still equivocal. This meta-analysis aimed to estimate the pathological complete response (pCR), regression rate, DFS, and OS probabilities of rectal cancer patients treated with a second chemotherapy drug added to fluoropyrimidine and long-term radiotherapy. Methods: Computerized bibliographic searches of MEDLINE, PUBMED, Web of Science and the Cochrane Central Register of Controlled Trials databases (1970–2023) were supplemented with hand searches of reference lists. Studies were included if they were randomised controlled trials (RCTs) comparing intensified chemotherapy with CRT to preoperative CRT and if they had patients with resectable, histologically proven rectal adenocarcinoma without metastases. Results: Eighteen RCTs (7695 patients) were analysed. Data on population, intervention, and outcomes were extracted from each RCT, following the intention-to-treat method, by three independent observers and combined using the DerSimonian and Laird methods. A chemotherapy with two drug and long-term radiotherapy CRT, compared to preoperative CRT (fluoropyrimidine and long-term radiotherapy), significantly increases the rate of pathological complete response (OR 1.37 (95% CI, 1.16–1.63) p = 0.0003) and the regression rate (OR 1.57 (95% CI, 1.16–2.14) p < 0.00001). Furthermore, it increases DFS (HR 0.87 (95% CI, 0.79 to 0.95) p = 0.002 and OS HR 0.84 (95% CI, 0.74 to 0.95) p = 0.007). The risk of severe adverse events (≥G3) is increased OR 1.96 (95% CI 1.35–2.85), p = 0.0005. Conclusions: In patients with resectable rectal cancer, intensified chemotherapy can reduce by 13% the risk of disease progression and by 16% the risk of death.
1. Introduction
Worldwide, colorectal cancer (CRC) is widespread. Globocam estimates that the burden of CRC will increase to 3.2 million new cases and 1.6 million deaths by 2040 [1]. Treatment of locally advanced disease, particularly those of the rectum, is a matter of debate. In 2000, a meta-analysis demonstrated that preoperative radiotherapy significantly improved overall survival compared to surgery alone [2,3]. Surgical techniques have also evolved to improve outcomes for these patients, although only recently have these improvements been standardized. The introduction of the total mesorectal excision (TME) [4] technique has significantly improved local recurrence-free survival.
However, preoperative radiotherapy has demonstrated significant therapeutic gain [5,6] in all patients treated surgically with TME, including the elderly [7].
The gold-standard treatment, especially for low rectal tumours, is a neoadjuvant approach with long term radiotherapy, fluoropyrimidine, and TME surgery [8]. Many attempts have been made to improve its effectiveness ulteriorly. If, with this gold standard, only 5% of patients will have a local recurrence disease, there is no demonstrated benefit in decreasing the rate of distant metastasis, with data at five years of 32% [9].
It is necessary to reduce the rate of distant relapse. According to a cancer-centric vision, it is important to destroy as many tumour cells as possible, especially those that have already metastasized. In recent years, oncologists have increased the dosage of chemotherapy and/or radiation therapy to increase their effectiveness in destroying these cells. The results are always contrasting in all areas of application. There is no deterministic relationship between increasing cell lethality and improving outcomes. It is not surprising to find two rectal cancer patients with the same morphology and profile but with two completely opposite therapeutic results.
Evidently, there is something that is not working, and it is necessary to overcome this situation by considering how radiation and chemotherapy drugs interact not only with the tumour but above all with the microenvironment in which the tumour cells grow.
To change the natural history of rectal cancer and increase survival, it is necessary to take advantage of all the treatments used in randomized clinical trials and reinterpret these results with a new vision.
In this setting, the clinical question is if an interaction of long-term radiotherapy with combined chemotherapy drugs can reduce the distant recurrence rate and improve disease-free survival (DFS) and, hopefully, overall survival (OS).
We performed a meta-analysis of randomised clinical trial studies (RCTs) to increase statistical power, including patients with LARC who received different preoperative combined and intensified strategies. Our study achieved the following:
- We estimated the pooled actuarial probabilities of disease-free survival, overall survival, local recurrence and distant metastases in LARC patients treated in these RCTs;
- We analysed variabilities in OS by considering the heterogeneity between studies;
- We identified factors associated with the risk of recurrence and survival.
2. Materials and Methods
2.1. Selection of Trials
This meta-analysis followed the PRISMA statement [10]. A systematic search of MEDLINE and the Cochrane Central Register of Controlled Trials databases was performed for articles published up to 31 December, 2022, with no lower date limit, including the following key “locally advanced rectal cancer”, “neoadjuvant chemoradiotherapy”, “total neoadjuvant treatment”, “induced chemotherapy”, “neoadjuvant radiotherapy”, and “randomised trial and clinical trial”. The reference lists of all retrieved review articles and primary studies were manually searched to identify additional studies. Conference proceedings (ASCO, ASTRO, ESMO, and ESTRO) were manually searched to ensure that the latest oncological data were considered. When the results of a single study were reported in more than one publication, the extrapolated results were used with priority given to those with the longest follow-up. Studies were included in the analysis if they were Phase III RCTs comparing neoadjuvant radiochemotherapy with intensified chemotherapy and long-term radiotherapy to the standard approach and if they had local advanced rectal cancer without extra pelvic disease.
Among the 4803 studies reviewed, 17 (Figure 1) met the inclusion criteria.
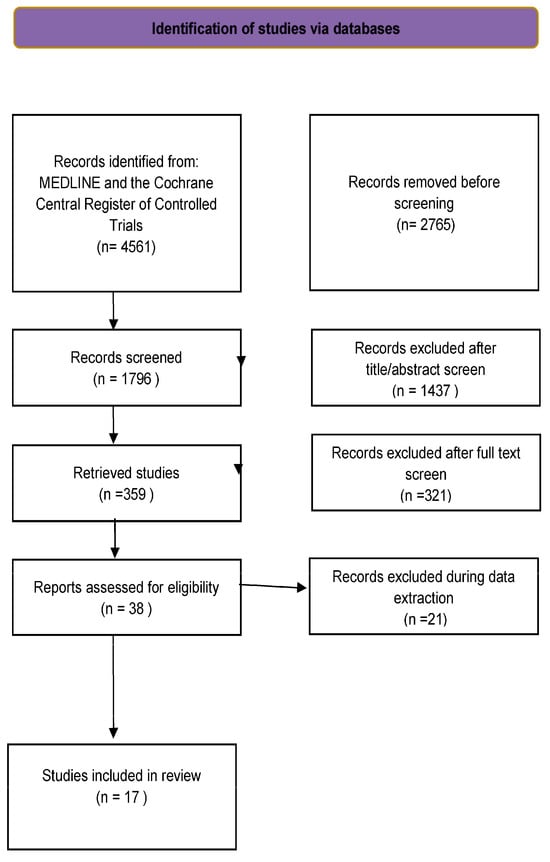
Figure 1.
Study flow chart.
2.2. Review of Studies
The trials were first reviewed using a list of predefined pertinent issues concerning patients’ characteristics and treatments. Study- and patient-level variables were extracted from all studies and entered into a database. Study-level variables included the study name, the first author’s last name, publication year, the region where the study was performed, the number of subjects, the number of centres (single versus multiple), outcomes measured, and study validity. Patient-level variables included mean age, sex, clinical stage, and oncological performance scale. Three independent investigators (CG, MM, and UT) evaluated and classified each RCT. Clinical outcomes were pathological response as complete response (pCR) rate and regression grade (from complete regression to fibrosis and tumour cells with a preponderance of fibrosis), disease-free survival (DFS), overall survival (OS), local recurrence-free survival, and distant metastasis-free survival. Discrepancies between reviewers were infrequent (overall interobserver variations < 10%) and were resolved by discussion. Two independent reviewers (GN, JG) assessed the risk of bias using the Cochrane Risk of Bias table [10]. This tool encompasses six domains: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants/personnel, (4) blinding of outcomes assessors, (5) incomplete outcome data, (6) selective reporting of outcomes, and (7) other potential sources of bias. The study-level assessment was applied for domains 1, 2, 6, and 7, and the outcome-level assessment was applied for domains 3, 4, and 5 of each trial. A third investigator was consulted in case of disagreements (Figure S1).
2.3. Statistical Analyses
Pooled hazard ratios (HRs) for time-to-event outcomes (OS, DFS, local control, and distant metastasis) in the experimental and control arms were either extracted directly from the publications or estimated. The HR estimates were combined into meta-analyses by the inverse variance method. For dichotomous outcomes, crude rates of PCR, downstaging, and safety outcomes, differences observed between the two groups were expressed as the pooled odds ratio (OR), with its 95% confidence interval (CI). Both analyses assessed heterogeneity between studies using the Pearson χ2 test and the I2 statistic [11]. However, all treatment effects on the defined outcome measures were calculated using models based on random effect assumption. Begg’s funnel plots were generated, and Egger’s regression asymmetry test was used to examine potential publication bias related to DFS and OS. All these analyses were computed using (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
More than 4500 records were retrieved from the computerized database search. After removing duplicates, 640 were considered for the title and abstract review. Eighty-two records were selected as potentially relevant publications. After assessing the full text for eligibility, 64 records were excluded. Finally, seventeen studies were included in the pooled analysis (Figure 1).
3.1. Characteristics of the Studies
The main features of the eighteen trials included in this meta-analysis are shown in Table 1. Table 2 shows the treatment details. These studies were published between 2013 and 2022; all are multicentre, nine RCTs are European [12,13,14,15,16,17,18,19,20,21,22,23], three are Chinese [24,25,26,27], two are from the USA [28,29,30], one is Iranian [31], two are Korean [32,33], and another is Australian [34].

Table 1.
The main features of the trials included in the meta-analysis.

Table 2.
Chemotherapy and radiotherapy regimens and their dosage of the trials included in the meta-analysis.
The eighteen RCTs included 7695 patients, 3829 of whom received intensified chemotherapy as part of the preoperative radiochemotherapy approach. The analysed population of each study varied greatly, ranging from 49 [34] to 1266 [28]. In thirteen studies [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] oxaliplatin was associated with standard radiochemotherapy and in five RCTs [22,23,27,33] there was an irinotecan combination.
3.2. Pathological Response
The effect of the different preoperative approaches on the complete pathological response (pCR) rate was reported in all RCTs (7580 patients) and is shown in Figure 2a. Adding an intensified chemotherapy with standard radiochemotherapy enhanced pCR in all RCTs but four [17,21,30,34]. However, a statistically significant difference was found in only three studies [23,24,25,27]. The pooled estimate of the treatment effect on pCR was significant (OR 1.40 (95% CI, 1.18–1.67) p = 0.0001). There was low heterogeneity between studies for pCR, with an I2 = 42%. Globally, 758 patients (20.1%) showed a pCR in intensified chemotherapy CRC versus 599 (15.7%) in the control group.
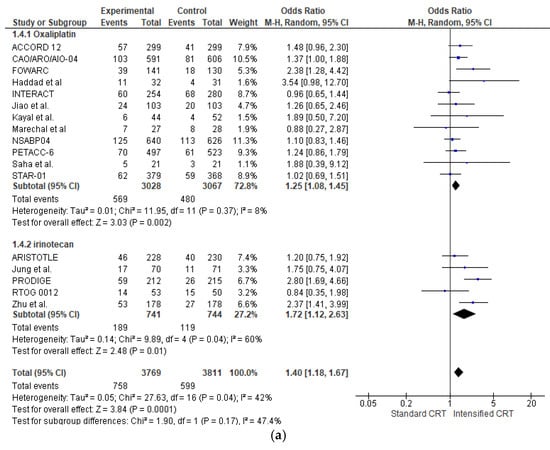
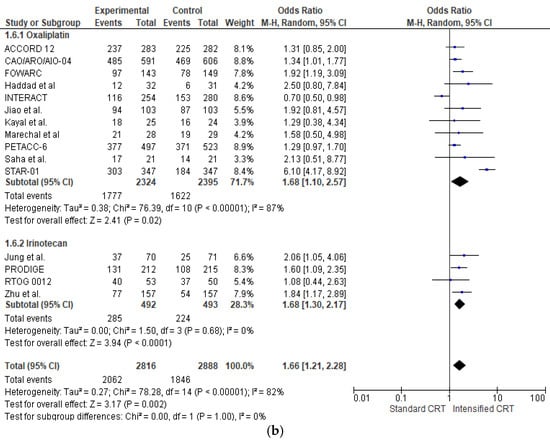
Figure 2.
Forest plot of (a) pathological complete response and (b) pathological major response (sum of complete, near-complete, and moderate regression) included in the meta-analysis obtained using a random effects model. In the random model, the sources of error are both within-study and between-study variance.
In the subgroup of RCTs adding oxaliplatin (6095 patients), there was a statistically significant increase in pCR (OR 1.25 (95% CI, 1.08–1.45) p = 0.002) with a very low heterogeneity I2 = 8%. With “robust analyses”, excluding one study at a time and evaluating 11 RCTs, no significant differences in treatment effect estimates were reported.
In the subgroup of RCTs adding irinotecan (1485 patients), there was a statistically significant increase in pCR (OR 1.72 (95% CI, 1.12–2.83) p = 0.04) with a moderate heterogeneity I2 = 60%. With “robust analysis”, a lost statistical significance for pCR omitting the Chinese trial by Zhang [27] or Prodige [23], respectively, was demonstrated.
Analysing the effect of two fluoropyrimidines with different administration in capecitabine trials (3704 patients and 675 pCR), there was a statistically significant increase in pCR in the association (OR 1.54 (95% CI, 1.18–2.02) p = 0.002) with a low heterogeneity I2 = 54%. A similar result is obtained in 5-fluorouracil trials (2610 patients and 444 pCR) (OR 1.33 (95% CI, 1.03–1.71) p = 0.03) with a low heterogeneity I2 = 19%. There was no significant association between pCR and fluoropyrimidine administration, χ2 = 0.96, p = 0.62.
The effect of the different preoperative approaches on tumour regression (defined as the sum of patients with complete, near-complete, and moderate regression [35]) was reported in sixteen RCTs (5704 patients) and is shown in Figure 2b. Adding an intensified chemotherapy with standard radiochemotherapy enhanced tumour regression in all RCTs but three [15,16,21,34]. A statistically significant difference was found in six RCTs [14,19,20,23,24,25,27,33]. The pooled estimate of the treatment effect on tumour regression was significant (OR 1.66 (95% CI, 1.21–2.28) p < 0.00001). There was high heterogeneity between RCTs with an I2 = 82%. In the subgroup of RCTs adding oxaliplatin (4719 patients), there was a statistically significant increase in tumour regression (OR 1.68 (95% CI, 1.10–2.57) p < 0.00001) with a high heterogeneity I2 = 87%. With “robust analyses”, no significant differences in treatment effect estimates were reported. In the subgroup of RCTs adding irinotecan (982 patients), there was a statistically significant increase in tumour regression OR 1.68 (95% CI, 1.30–2.17) p = 0.0001).
Analysing the effect of two fluoropyrimidines with different administration in capecitabine trials (2965 patients and 1898 regressions), there was not a statistically significant increase in pathological regression OR 1.34 (95% CI, 0.99–1.81) p = 0.06) with a moderate heterogeneity I2 = 66%. In 5-fluorouracil trials (2045 patients and 1523 regressions) (OR 1.51 (95% CI, 1.22–1.86) p = 0.0001), with no heterogeneity, there was no significant association between regression and the fluoropyrimidine administration modality, χ2 = 1.17, p = 0.56.
3.3. Disease-Free Survival
Figure 3a shows the HR for DFS (6618 patients) in twelve RCTs and the overall analysis. The HRs for DFS of an intensified chemotherapy were compared to the control arms in all trials. The effect of treatment on DFS significantly favoured intensified chemotherapy added to standard radiochemotherapy in all trials but two [18,33]. However, a statistically significant difference was observed in no study. Our meta-analysis shows a statistically significant benefit obtained with intensified CRT: the pooled estimate of the treatment effect was significant, HR 0.86 (95% CI, 0.79 to 0.94) p = 0.001, corresponding to a 14% reduction of the hazard of disease progression for intensified CRT. No significant heterogeneity was observed between the studies (Χ2 = 2.37), I2 = 0%. The pooled estimate of the treatment effect was significant when using robust analysis. After omitting the largest trial, NSABP, the evaluation of the remaining ten studies did not lose statistical significance, HR 0.87 (95% CI 0.78–0.96) p = 0.006. In RCTs adding oxaliplatin to CRT, there was an 12% reduction of the hazard of disease progression corresponding to a pooled HR of 0.89 (95% CI, 0.80 to 0.97) p = 0.008. Instead, pooling RCTs with irinotecan, the HR was 0.68 (95% CI, 0.49 to 0.93) p = 0.002. There was no significant association between disease-free survival and the fluoropyrimidine administration modality, X2 = 0.28, p = 0.87.
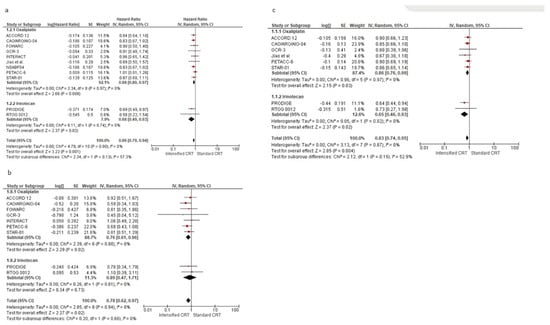
Figure 3.
Forest plot of hazard ratios of (a) disease-free survival, (b) local recurrence-free survival, and (c) distant metastases-free survival of RCTs included in the meta-analysis obtained using a random effects model. In the random model, the sources of error are both within-study and between-study variance.
After analysing postoperative chemotherapy, no statistical difference was found between studies using 5-FU, with HR 0.89 (95% CI, 0.73 to 1.10) p = 0.29. Instead, we found a DFS advantage in studies without a specific recommendation in adjuvant approach, with an HR 0.83 (95% CI, 0.70 to 0.97) p = 0.02, and in studies using FOLFOX or CAPOX, with an HR 0.87 (95% CI, 0.77 to 0.99) p = 0.04.
The effect of the different preoperative approaches on local recurrence-free survival was reported in nine RCTs (5005 patients) and is shown in Figure 3b. The pooled estimate of the treatment effect on LRFS was significant, HR 0.78 (95% CI, 0.63 to 0.97) p = 0.003, corresponding to a 22% reduction of the hazard of local recurrence for intensified CRT. Using oxaliplatin increases local control by about 23%, while irinotecan gives an advantage of 11%. No difference was found according to the two fluoropyrimidines with different administration: capecitabine HR 0.81 (95% CI, 0.59 to 1.10) p = 0.17, and 5-fluorouracil HR 0.75 (95% CI, 0.55 to 1.03) p = 0.07.
Seven RCTs (4406 patients) evaluated the control of distant metastases, as shown in Figure 3c. The pooled estimate of the treatment effect on DMFS was significant, HR 0.83 (95% CI, 0.73 to 0.95) p = 0.005, corresponding to a 17% reduction of the hazard of distant metastases for intensified CRT. No heterogeneity was demonstrated I2 = 0%. With “robust analyses”, excluding one study at a time, and therefore evaluating six RCTs, no significant differences in treatment effect estimates were reported.
In the subgroup of RCTs adding oxaliplatin (3876 patients), there was a statistically significant increase in DM control (HR 0.86 (95% CI, 0.75–0.99) p = 0.03). In the subgroup of RCTs adding irinotecan (530 patients), there was a statistically significant increase in DM control (HR 0.65 (95% CI, 0.46–0.93) p = 0.02). According to the two fluoropyrimidines with different administration, and according to the fluoropyrimidine administration, there was an increased distant metastasis control with capecitabine and oxaliplatin/irinotecan, HR 0.82 (95% CI, 0.69 to 0.97), p = 0.02. No difference was seen with 5-fluorouracil, HR 0.85 (95% CI, 0.71 to 1.02) p = 0.54.
3.4. Survival
The effect of adding two chemotherapy drugs to long-term radiotherapy on overall survival (11 studies: 6618 patients) is shown in Figure 4. The treatment effect on OS favoured the two drugs in all but one RCT16; a statistically significant difference was observed in no RCT. The pooled estimate of the treatment effect was significant, with HR 0.84 (95% CI, 0.74 to 0.95) p= 0.007, corresponding to a 16% reduction in the risk of death with intensified therapy. A low heterogeneity was observed between the studies (Χ2 = 13.76), I2 = 20%. The pooled estimate of the treatment effect was significant when robust analysis was used. After removing the largest trial, NSABP, robust analyses showed that all ten remaining studies did not lose statistical significance: HR 0.85 (95% CI 0.73–0.98) p = 0.03. In RCTs adding oxaliplatin to CRT, there was a 13% reduction of the risk of death corresponding to a pooled HR 0.87 (95% CI, 0.75 to 0.99) p = 0.04. Instead, pooling RCTs with irinotecan, the HR was 0.64 (95% CI, 0.45 to 0.92) p = 0.007 (36% reduction of the hazard of disease progression). No difference was found according to the two fluoropyrimidines with different administration: capecitabine had an HR 0.83 (95% CI, 0.68 to 1.00) p = 0.05 and 5-fluorouracil HR 0.90 (95% CI, 0.72 to 1.13) p = 0.37. There was no significant association between overall survival and the fluoropyrimidine administration modality, χ2 = 2.12, p = 0.35. Regarding postoperative chemotherapy, no difference was found between studies without a specific recommendation, with HR 0.76 (95% CI, 0.62 to 0.92) p = 0.005, and studies using 5-FU, with HR 0.79 (95% CI, 0.64 to 0.97) p = 0.02. Instead, we found no OS advantage in studies using FOLFOX or CAPOX as a postoperative treatment, HR 0.97 (95% CI, 0.78 to 1.19) p = 0.74.
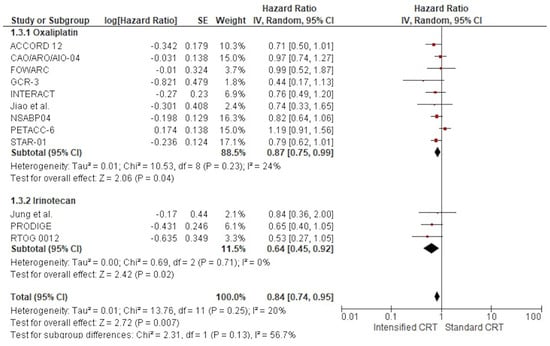
Figure 4.
Forest plots of the hazard ratio of RCTs’ overall survival were included in the meta-analysis and obtained using a random effects model.
3.5. Toxicity
A severe adverse event (≥G3) was described in ten out of eleven studies, corresponding to 53,365,311 patients, 26,252,613 in the intensified treatment and 27,112,698 in the standard treatment. In all RCTs but one [33], there was an increase in severe adverse events in the double-drug chemotherapy group. The pooled estimate of the adverse events (≥G3) was OR 1.962.11 (95% CI 1.3551–2.8593), p < 0.00050001 (Figure 5). Moderate heterogeneity was observed between the studies, with I2 = 84%. 78%.
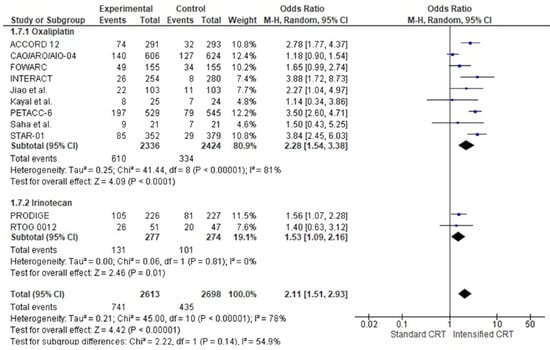
Figure 5.
Forest plots of toxicities of RCTs were included in the meta-analysis and obtained using a random effects model.
Between severe adverse events (≥G3), the most common were gastrointestinal (8% in the standard CRT and 14% in the CRT with more drugs), haematological adverse effects (4.5% vs. 6%), and genitourinary (0.9% vs. 1.4%). Only three trials [12,13,14], 28 analysed the rate of acute and late toxicities (≥G3). Globally, 17.8% of acute and 11.8% of late (≥G3) adverse effects occurred.
In the subgroup of RCTs adding oxaliplatin, the pooled estimate of adverse events was OR 2.4728 (95% CI 1.35–2.8554–3.38), p << 0.00001. In this group, patients treated with intensified chemotherapy with oxaliplatin were two times more likely to have an adverse event ≥ G3.
In the subgroup of RCTs adding irinotecan, the pooled estimate of adverse events was not significantly increased, OR 1.0553 (95% CI 0.501.09–2.19), [16], p = 0.89, with no heterogeneity. Analysing the two fluoropyrimidines with different administration, in capecitabine trials (1777 patients and 359 adverse events ≥ G3), there was a statistically significant increase in adverse events ≥G3 in the association with another drug, OR 2.52 (95% CI, 1.77–3.59) p < 0.0001 with a moderate heterogeneity, I2 = 50%. Instead, a marginal statistical difference was obtained in 5-fluorouracil with an OR 1.71 (95% CI, 1–2.93) p = 0.05 with a high heterogeneity of I2 = 80%. There was a significant association between adverse events ≥ G3 and the fluoropyrimidine administration modality, χ2 = 5.94, p < 0.0001.
3.6. Publication Bias
The funnel publication bias plot for the OS (Figure 6) and Egger’s test for publication bias showed that the risk of having missed or overlooked trials was not significant (p = 0.354).
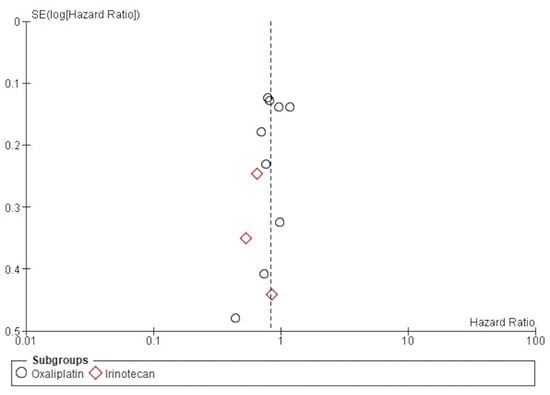
Figure 6.
Funnel plot of publication bias for DFS.
4. Discussion
The results of this meta-analysis demonstrate that adding another chemotherapeutic drug to fluoropyrimidine and long-term radiotherapy can significantly influence all outcomes. Globally, the intensified regimens demonstrated a 16% improvement in OS, a 14% improvement in DFS, a 22% increase in local recurrence control, and a 17% improvement in the control of distant metastases, albeit within a framework of significant heterogeneity among the trials analysed.
At least ten previously published meta-analyses address the benefit of the intensifying chemoradiotherapy approach with another CT added to fluoropyrimidine and RT. Referring to the three most recent [34,35,36], the aggregated data agree, with no difference in overall survival and a statistical increase in distant recurrence control and toxicities. Furthermore, in the most recent 36, it was found to be a significant benefit in terms of DFS. Differing from others, our study also included studies intensifying CRT with irinotecan. Three RCTs had mature data on outcomes: the RTOG 0012 trial, the Prodige trial, and the trial by Jung et al. One by one, these RCTs did not find a statistically significant benefit in OS. Analysing all irinotecan-based studies together, the results reached statistical significance, showing an impressive increase of 36% in OS, 32% in DFS, 11% in local control, and 35% in the reduction of metastatic dissemination.
Furthermore, in this meta-analysis, we have added further studies intensifying with oxaliplatin: the Italian trial INTERACT19. This study considers two approaches: an increase in RT dose (about 10% dose more than standard) and oxaliplatin added to CRT in patients cT2-low-lying/T3 N0-2. The rate of T2 N0 patients is very low, at least 1%, so we can consider this a trial accruing local advanced rectal cancer. Adding this new study and pooling all studies using oxaliplatin, we obtained a statistically significant improvement in OS with an HR 0.87 (95% CI, 0.75–0.99) p = 0.04 with a low grade of heterogeneity between studies, I2 =24%. All oxaliplatin RCTs, excluding one, show an increase in OS despite no statistical significance. The design of every trial was probably not powered to capture small but meaningful differences in true outcomes. The pooled results also reached statistical significance in DFS with an increase over CRT alone of 12%, local control of 24%, and distant metastases control of 14%.
All analysed RCTs compare the intensification of chemotherapy using a long-term radiotherapy approach. The total radiation dose is homogenous, ranging from 45 to 55 Gy, with a daily dose ranging from 1.8 to 2.2 Gy/day. There were differences in fluoropyrimidine administration: some trials used capecitabine orally and some used 5-fluorouracil in bolus or continuous infusion. We wondered if there were differences in the association between different forms of fluoropyrimidine administration and oxaliplatin and/or irinotecan. All outcomes have no statistically significant difference, excluding a marginally statistically significant increase in adverse events ≥ G3 with 5-fluorouracil. We are not made of the thing considering the results of the NSABP trial [26,27] investigating the impact of adding oxaliplatin to either capecitabine or 5-FU continuous infusion in the neoadjuvant setting. The addition of oxaliplatin did not statistically change the outcome of the trials using oral fluoropyrimidine or continuous infusion.
Similar to other meta-analyses, we found a statistical increase in ≥G3 toxicities, with a global incidence of 25.9% versus 17.6% for standard treatment.
Many efforts have been made to identify the optimal chemotherapy combination that would increase the cost-effectiveness of therapy. There was a considerable variation in the used chemotherapy schedule, suggesting that a standard oxaliplatin or irinotecan schedule is needed to obtain comparable data on the efficacy and safety profile, particularly regarding the dose and the time of CT infusion. Furthermore, the risk of toxic effects is reduced by identifying patients with a genetic profile with a significant risk of adverse events. As with irinotecan dose-associated toxicities related to a UGT1A1×28 genotype [25], it would be necessary to identify a genotype in which the toxic events are increased with oxaliplatin. However, these toxicities are not a significant obstacle to using an intensified CT in combination with radiotherapy. Toxicities are clinically manageable but require careful monitoring. Globally, relevant and speculative information can be obtained by separately evaluating the local control and distant metastases control. We have demonstrated that the probability of achieving improved systemic control with irinotecan-based intensification was notably higher than with oxaliplatin-based intensification and the contrary for local control. It is known that the LARC presents a spectrum of clinical behaviours and biological profiles [37,38]. Some may evolve with a local progression, while others may principally metastasize. A broad arsenal of treatment approaches can ensure that the intervention will be customized to the specific clinical context. With the two intensification regimens, a different modality of action can be used to tailor treatment strategies to patients’ clinical and pathological profiles. Subgroup analysis reveals differing advantages between oxaliplatin and irinotecan regimens: oxaliplatin-based intensification significantly enhances local control (24% improvement), and it could be particularly suitable for patients at high risk of loco-regional recurrence. Factors such as T4b stage, circumferential resection margin (CRM) involvement on MRI, positive lateral pelvic lymph nodes, or tumours within 5 cm of the anal verge are indicative of a disease characterized by local progression [39]. Instead, irinotecan-based intensification, which has a probable higher systemic control (35% improvement in distant metastasis-free survival), can be a better option for patients with a higher likelihood of metastatic progression. An extramural venous invasion EMVI positivity, N2 stage, high tumour budding, and elevated CEA levels are predictors of a higher propensity to metastatic progression of disease. However, a lack of robust clinical or histological markers to stratify patients limits the ability to make definitive treatment recommendations. Emerging biomarkers like cancer/testis antigens (CTAs) NY-ESO-1 and MAGE-A4 have shown significant potential in identifying aggressive tumour behaviour and guiding therapeutic strategies [40]. In a recent study, CTAs were found to be highly expressed in aggressive soft tissue sarcomas, correlating with poor prognosis and aggressive disease phenotypes [41]. These findings suggest that NY-ESO-1 and MAGE-A4 could serve as predictive markers in soft tissue sarcomas and other malignancies with a high metastatic propensity, such as locally advanced rectal cancer [42].
In addition to the highly favourable response observed in LARC with mismatch repair-deficient tumours, which have shown high sensitivity to single-agent PD-1 blockade [43], modifying the tumour microenvironment could offer a promising avenue to improve outcomes in other subgroups. It is becoming clear that beyond the direct cytotoxic effects, chemotherapy agents can also modulate the tumour microenvironment and immune response [44], which may synergize with radiotherapy. Oxaliplatin promotes immunogenic cell death [45], increases the CTL–Treg cell ratio, and depletes myeloid-derived suppressor cells (MDSCs) [46]. Apparent discrepancies were encountered during irinotecan treatments. An MDSC accumulation was shown in the tumour microenvironment [47]. However, experimental evidence highlighted significantly decreased MDSCs at day six after treatment with irinotecan combined with fluorouracil, leading to enhanced tumour-specific responses [48]. These two chemotherapy drugs can modify the density, composition, localization, and function of tumour-infiltrating lymphoid and myeloid cells, modifying the immune context [49] and improving the results obtained by radiotherapy. Immunosuppression cells have an important role in metastatic growth, limiting the ability of MDSCs to migrate into tumours and suppress the immune response, which can increase local radiation treatment and control metastasizing [50].
Radiotherapy, long considered a local treatment, could also have a systemic effect mediated by immune modulation, further supporting the rationale for combination strategies. These findings suggest treatment efficacy depends on tumour cell lethality and interactions with the immune context and microenvironment, as demonstrated in cancer in other sites [51].
The results of this study are subject to several limitations. Differences in the baseline severity of illness in the population of the RCTs, the dose, the type and the combination of CT may limit the accuracy of this meta-analysis. We analysed these differences by including covariates that described the patients studied and the study design features. Finally, we should be particularly concerned about publication bias in settings where relatively small studies are conducted. However, the risk of having missed or overlooked trials in the setting of studies was insignificant when assessed by tests for publication bias. Therefore, small studies with a small treatment effect are unlikely to remain unpublished.
In patients with resectable rectal carcinoma, the available evidence from the literature data is sufficient to conclude that the addition of more chemotherapy drugs to radiotherapy improves OS, but toxicity is significantly increased by adding other chemotherapy drugs to CRT.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14020345/s1, Figure S1: Quality assessment of included studies.
Author Contributions
validation, all authors; formal analysis, U.T., G.N. and F.F.; data curation, F.F.; writing—original draft preparation, F.F.; writing—review and editing, J.G.; visualization, J.G., F.F. All authors have read and agreed to the published version of the manuscript.
Funding
No funds were received to support this article.
Conflicts of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Abbreviations
LARC, locally advanced rectal cancer; CRT, chemoradiotherapy; 5-FU, 5 fluorouracil; RCT, randomised controlled trial; pCR, pathological complete response; DFS, disease-free survival; OS, overall survival.
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Cammà, C.; Giunta, M.; Fiorica, F.; Pagliaro, L.; Craxì, A.; Cottone, M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA 2000, 284, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J.; Ryall, R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, F.; Cartei, F.; Carau, B.; Berretta, S.; Spartà, D.; Tirelli, U.; Santangelo, A.; Maugeri, D.; Luca, S.; Leotta, C.; et al. Adjuvant radiotherapy on older and oldest elderly rectal cancer patients. Arch. Gerontol. Geriatr. 2009, 49, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.D.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, F.; Cartei, F.; Licata, A.; Enea, M.; Ursino, S.; Colosimo, C.; Cammà, C. Can chemotherapy concomitantly delivered with radiotherapy improve survival of patients with resectable rectal cancer? A meta-analysis of literature data. Cancer Treat. Rev. 2010, 36, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; the PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 349, 7647. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Gérard, J.P.; Azria, D.; Gourgou-Bourgade, S.; Martel-Laffay, I.; Hennequin, C.; Etienne, P.L.; Vendrely, V.; François, E.; de La Roche, G.; Bouché, O.; et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J. Clin. Oncol. 2010, 28, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Gérard, J.P.; Azria, D.; Gourgou-Bourgade, S.; Martel-Lafay, I.; Hennequin, C.; Etienne, P.L.; Vendrely, V.; François, E.; de La Roche, G.; Bouché, O.; et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4558–4565. [Google Scholar] [CrossRef]
- Rödel, C.; Liersch, T.; Becker, H.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Graeven, U.; Arnold, D.; Lang-Welzenbach, M.; Raab, H.R.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012, 13, 679–687. [Google Scholar] [CrossRef]
- Maréchal, R.; Vos, B.; Polus, M.; Delaunoit, T.; Peeters, M.; Demetter, P.; Hendlisz, A.; Demols, A.; Franchimont, D.; Verset, G.; et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: A randomized multicentric phase II study. Ann. Oncol. 2012, 23, 1525–1530. [Google Scholar] [CrossRef]
- Schmoll, H.-J.; Stein, A.; Van Cutsem, E.; Price, T.; Hofheinz, R.D.; Nordlinger, B.; Daisne, J.-F.; Janssens, J.; Brenner, B.; Reinel, H.; et al. Pre- and Postoperative Capecitabine Without or with Oxaliplatin in Locally Advanced Rectal Cancer: PETACC 6 Trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J. Clin. Oncol. 2021, 39, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Aschele, C.; Cionini, L.; Lonardi, S.; Pinto, C.; Cordio, S.; Rosati, G.; Artale, S.; Tagliagambe, A.; Ambrosini, G.; Rosetti, P.; et al. Primary Tumor Response to Preoperative Chemoradiation with or Without Oxaliplatin in Locally Advanced Rectal Cancer: Pathologic Results of the STAR-01 Randomized Phase III Trial. J. Clin. Oncol. 2011, 29, 2773–2780. [Google Scholar] [CrossRef]
- Aschele, C.; Lonardi, S.; Cionini, L.; Pinto, C.; Cordio, S.S.; Rosati, G.; Bianchi, A.S.; Tagliagambe, A.; Frisinghelli, M.; Zagonel, V.; et al. Final Results of STAR-01: A Randomized Phase III Trial Comparing Preoperative Chemoradiation with or without Oxaliplatin in Locally Advanced Rectal Cancer. J. Clin. Oncol. 2016, 34, 3521. [Google Scholar] [CrossRef]
- Valentini, V.; Gambacorta, M.A.; Cellini, F.; Aristei, C.; Coco, C.; Barbaro, B.; Alfieri, S.; D’Ugo, D.; Persiani, R.; Deodato, F.; et al. The INTERACT Trial: Long-term results of a randomised trial on preoperative capecitabine-based radiochemotherapy intensified by concomitant boost or oxaliplatin, for cT2 (distal)–c3 rectal cancer. Radiother. Oncol. 2019, 134, 110–118. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Adams, R.; Gollins, S.; Samuel, L.M.; Glynne-Jones, R.; Harte, R.; West, N.; Quirke, P.; Myint, A.S.; Bach, S.P.; et al. ARISTOTLE: A phase III trial comparing concurrent capecitabine with capecitabine and irinotecan (Ir) chemoradiation as preoperative treatment for MRI-defined locally advanced rectal cancer (LARC). J. Clin. Oncol. 2020, 38, 4101. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Modified FOLFOX6 with or Without Radiation Versus Fluorouracil and Leucovorin with Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J. Clin. Oncol. 2016, 34, 3300–3307. [Google Scholar] [CrossRef]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Neoadjuvant Modified FOLFOX6 with or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J. Clin. Oncol. 2019, 37, 3223–3233. [Google Scholar] [CrossRef]
- Jiao, D.; Zhang, R.; Gong, Z.; Liu, F.; Chen, Y.; Yu, Q.; Sun, L.; Duan, H.; Zhu, S.; Liu, F.; et al. Fluorouracil-based preoperative chemoradiotherapy with or without oxaliplatin for stage II/III rectal cancer: A 3-year follow-up study. Chin. J. Cancer Res. 2015, 27, 9. [Google Scholar]
- Iyer, L.; Das, S.; Janisch, L.; Wen, M.; Ramírez, J.; Karrison, T.; Fleming, G.F.; Vokes, E.E.; Schilsky, R.L.; Ratain, M.J. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenom. J. 2002, 2, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Allegra, C.J.; Yothers, G.; O’Connell, M.J.; Beart, R.W.; Wozniak, T.F.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; Arora, A.; et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation with or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. JNCI J. Natl. Cancer Inst. 2015, 107, djv248. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Colangelo, L.H.; Beart, R.W.; Petrelli, N.J.; Allegra, C.J.; Sharif, S.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; et al. Capecitabine and Oxaliplatin in the Preoperative Multimodality Treatment of Rectal Cancer: Surgical End Points From National Surgical Adjuvant Breast and Bowel Project Trial R-04. J. Clin. Oncol. 2014, 32, 1927–1934. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Paulus, R.; Mitchell, E.; Hanna, N.; Yuen, A.; Nichols, R.; Yalavarthi, S.; Hayostek, C.; Willett, C. 5-Year Updated Results of Rtog-0012 Randomized Phase II Study Of Neoadjuvant Combined Modality Chemoradiation for Distal Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Dastidar, A.; Mahata, A.; Das, A.; Sarkar, R.; Kayal, P. A Randomized Comparative Study between Neoadjuvant 5-Fluorouracil and Leukovorin versus 5-Fluorouracil and Cisplatin along with Concurrent Radiation in Locally Advanced Carcinoma Rectum. Clin. Cancer Investig. J. 2014, 4, 32. [Google Scholar] [CrossRef]
- Saha, A.; Ghosh, S.K.; Roy, C.; Saha MLChoudhury, K.B.; Chatterjee, K. A Randomized Controlled Pilot Study to Compare Capecitabine-Oxaliplatin with 5-FU-Leucovorin as Neoadjuvant Concurrent Chemoradiation in Locally Advanced Adenocarcinoma of Rectum. J. Cancer Res. Ther. 2015, 11, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.; Miraie, M.; Farhan, F.; Fazeli, M.S.; Alikhassi, A.; Maddah-Safaei, A.; Aghili, M.; Kalaghchi, B.; Babaei, M. Addition of oxaliplatin to neoadjuvant radiochemotherapy in MRI-defined T3, T4 or N+ rectal cancer: A randomized clinical trial. Asia Pac. J. Clin. Oncol. 2017, 13, 416–422. [Google Scholar] [CrossRef]
- Jung, M.; Shin, S.J.; Koom, W.S.; Jung, I.; Keum, K.C.; Hur, H.; Min, B.S.; Baik, S.H.; Kim, N.K.; Kim, H.; et al. A Randomized Phase 2 Study of Neoadjuvant Chemoradiaton Therapy with 5-Fluorouracil/Leucovorin or Irinotecan/S-1 in Patients with Locally Advanced Rectal Cancer. Int. J. Radiat. Oncol. 2015, 93, 1015–1022. [Google Scholar] [CrossRef]
- Kim, C.W.; Kang, B.M.; Kim, I.Y.; Kim, J.Y.; Park, S.J.; Park, W.C.; Bae, K.B.; Bae, B.-N.; Baek, S.K.; Baik, S.H.; et al. Korean Society of Coloproctology (KSCP) trial of cONsolidation Chemotherapy for Locally advanced mid or low rectal cancer after neoadjUvant concurrent chemoraDiothErapy: A multicenter, randomized controlled trial (KONCLUDE). BMC Cancer 2018, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-L.; Fang, Z.; Shu, L.-H.; Tao, G.-Q.; Wang, J.-Q.; Rui, Z.-L.; Zhang, Y.-J.; Tian, Z.-Q. Meta-analysis of oxaliplatin-based versus fluorouracil-based neoadjuvant chemoradiotherapy and adjuvant chemotherapy for locally advanced rectal cancer. Oncotarget 2017, 8, 34340–34351. [Google Scholar] [CrossRef]
- Hüttner, F.J.; Probst, P.; Kalkum, E.; Hackbusch, M.; Jensen, K.; Ulrich, A.; Debus, J.; Jäger, D.; Diener, M.K. Addition of Platinum Derivatives to Fluoropyrimidine-Based Neoadjuvant Chemoradiotherapy for Stage II/III Rectal Cancer: Systematic Review and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2019, 111, 887–902. [Google Scholar] [CrossRef]
- Des Guetz, G.; Landre, T.; Bollet, M.A.; Mathonnet, M.; Quéro, L. Is There a Benefit of Oxaliplatin in Combination with Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer? An Updated Meta-Analysis. Cancers 2021, 13, 6035. [Google Scholar] [CrossRef]
- Siddiqui, M.R.S.; Simillis, C.; Hunter, C.; Chand, M.; Bhoday, J.; Garant, A.; Vuong, T.; Artho, G.; Rasheed, S.; Tekkis, P.; et al. A Meta-Analysis Comparing the Risk of Metastases in Patients with Rectal Cancer and MRI-Detected Extramural Vascular Invasion (MrEMVI) vs MrEMVI-Negative Cases. Br. J. Cancer 2017, 116, 1513–1519. [Google Scholar] [CrossRef]
- Battersby, N.J.; How, P.; Moran, B.; Stelzner, S.; West, N.P.; Branagan, G.; Strassburg, J.; Quirke, P.; Tekkis, P.; Pedersen, B.G.; et al. Prospective Validation of a Low Rectal Cancer Magnetic Resonance Imaging Staging System and Development of a Local Recurrence Risk Stratification Model: The MERCURY II Study. Ann. Surg. 2016, 263, 751–760. [Google Scholar] [CrossRef]
- Lord, A.C.; Corr, A.; Chandramohan, A.; Hodges, N.; Pring, E.; Airo-Farulla, C.; Moran, B.; Jenkins, J.T.; Di Fabio, F.; Brown, G. Assessment of the 2020 NICE Criteria for Preoperative Radiotherapy in Patients with Rectal Cancer Treated by Surgery Alone in Comparison with Proven MRI Prognostic Factors: A Retrospective Cohort Study. Lancet Oncol. 2022, 23, 793–801. [Google Scholar] [CrossRef]
- Meng, X.; Sun, X.; Liu, Z.; He, Y. A Novel Era of Cancer/Testis Antigen in Cancer Immunotherapy. Int. Immunopharmacol. 2021, 98, 107889. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Nishimura, S.; Ito, T.; Akagi, M. Clinicopathological Assessment of Cancer/Testis Antigens NY-ESO-1 and MAGE-A4 in Highly Aggressive Soft Tissue Sarcomas. Diagnostics 2022, 12, 733. [Google Scholar] [CrossRef]
- Ishihara, M.; Kageyama, S.; Miyahara, Y.; Ishikawa, T.; Ueda, S.; Soga, N.; Naota, H.; Mukai, K.; Harada, N.; Ikeda, H.; et al. MAGE-A4, NY-ESO-1 and SAGE MRNA Expression Rates and Co-Expression Relationships in Solid Tumours. BMC Cancer 2020, 20, 606. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Kepp, O.; Schlemmer, F.; Adjemian, S.; Tailler, M.; Shen, S.; Michaud, M.; Menger, L.; Gdoura, A.; Tajeddine, N.; et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 2011, 30, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aparicio, M.; Alzuguren, P.; Mauleon, I.; Medina-Echeverz, J.; Hervas-Stubbs, S.; Mancheno, U.; Berraondo, P.; Crettaz, J.; Gonzalez-Aseguinolaza, G.; Prieto, J.; et al. Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice. Gut 2011, 60, 341–349. [Google Scholar] [CrossRef]
- Kanterman, J.; Sade-Feldman, M.; Biton, M.; Ish-Shalom, E.; Lasry, A.; Goldshtein, A.; Hubert, A.; Baniyash, M. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014, 74, 6022–6035. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, H.-M.; Park, J.-S.; Sohn, H.-J.; Kim, S.-G.; Kim, H.-J.; Oh, S.-T.; Kim, T.-G. Dendritic cell vaccine in addition to FOLFIRI regimen improve antitumor effects through the inhibition of immunosuppressive cells in murine colorectal cancer model. Vaccine 2010, 28, 7787–7796. [Google Scholar] [CrossRef]
- Kalanxhi, E.; Meltzer, S.; Schou, J.V.; Larsen, F.O.; Dueland, S.; Flatmark, K.; Jensen, B.V.; Hole, K.H.; Seierstad, T.; Redalen, K.R.; et al. Systemic immune response induced by oxaliplatin-based neoadjuvant therapy favours survival without metastatic progression in high-risk rectal cancer. Br. J. Cancer 2018, 118, 1322–1328. [Google Scholar] [CrossRef]
- Wang, L.; Dou, X.; Chen, S.; Yu, X.; Huang, X.; Zhang, L.; Chen, Y.; Wang, J.; Yang, K.; Bugno, J.; et al. YTHDF2 inhibition potentiates radiotherapy antitumor efficacy. Cancer Cell 2023, 41, 1294–1308.e8. [Google Scholar] [CrossRef]
- Fiorica, F.; Tebano, U.; Gabbani, M.; Perrone, M.; Missiroli, S.; Berretta, M.; Giuliani, J.; Bonetti, A.; Remo, A.; Pigozzi, E.; et al. Beyond Abscopal Effect: A Meta-Analysis of Immune Checkpoint Inhibitors and Radiotherapy in Advanced Non-Small Cell Lung Cancer. Cancers 2021, 13, 2352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).