A Pilot Case Series on the Use of a Large Mushroom-Shaped Corneal Graft for the Surgical Management of Post-Penetrating Keratoplasty Ectasia and Endothelial Failure
Abstract
:1. Introduction
2. Materials and Methods
3. Technique
3.1. Preoperative Planning
3.2. Donor Graft Preparation
3.3. Recipient Bed Preparation
3.4. Graft Positioning and End of the Procedure
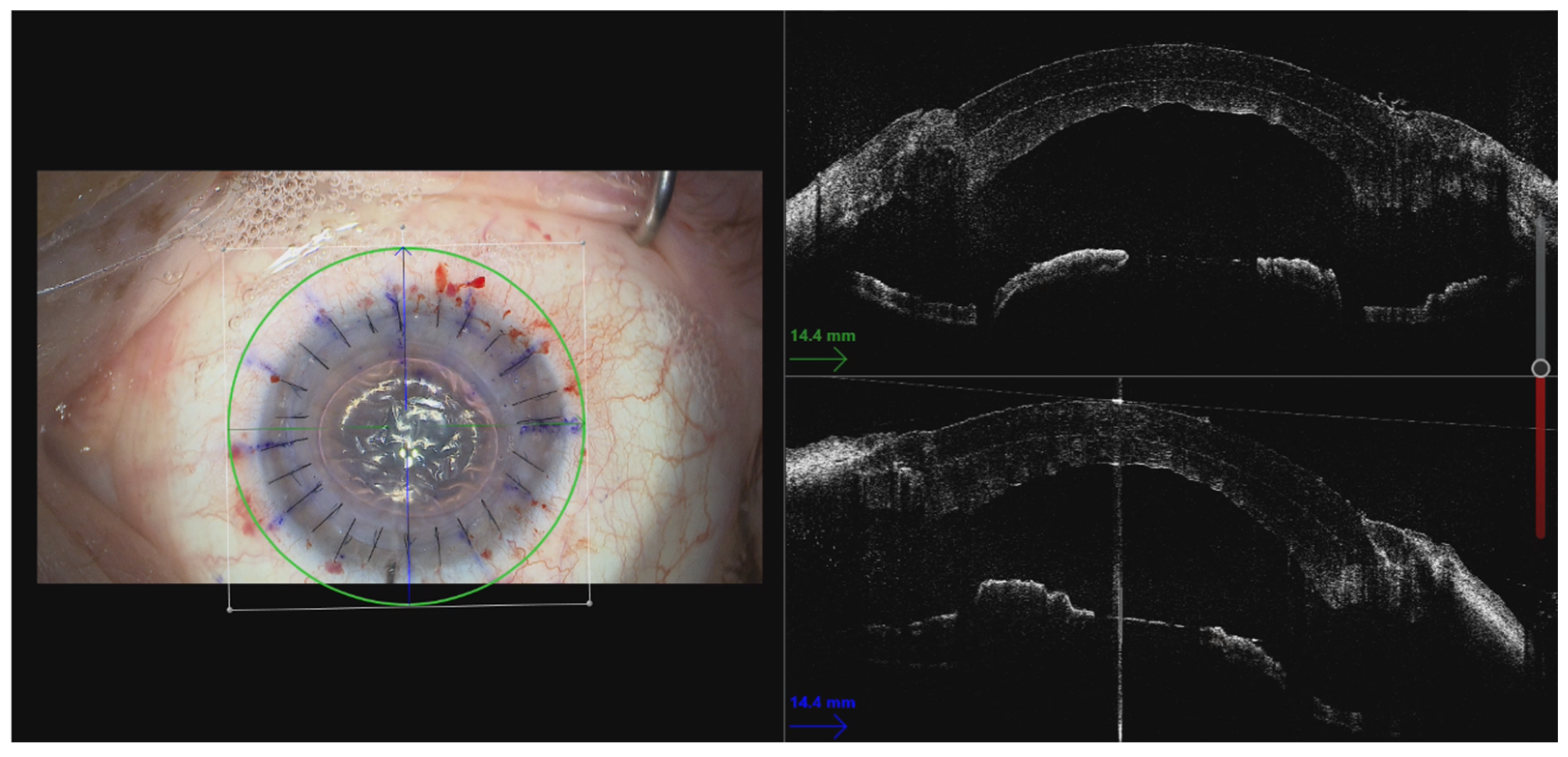
3.5. Use of Intraoperative OCT
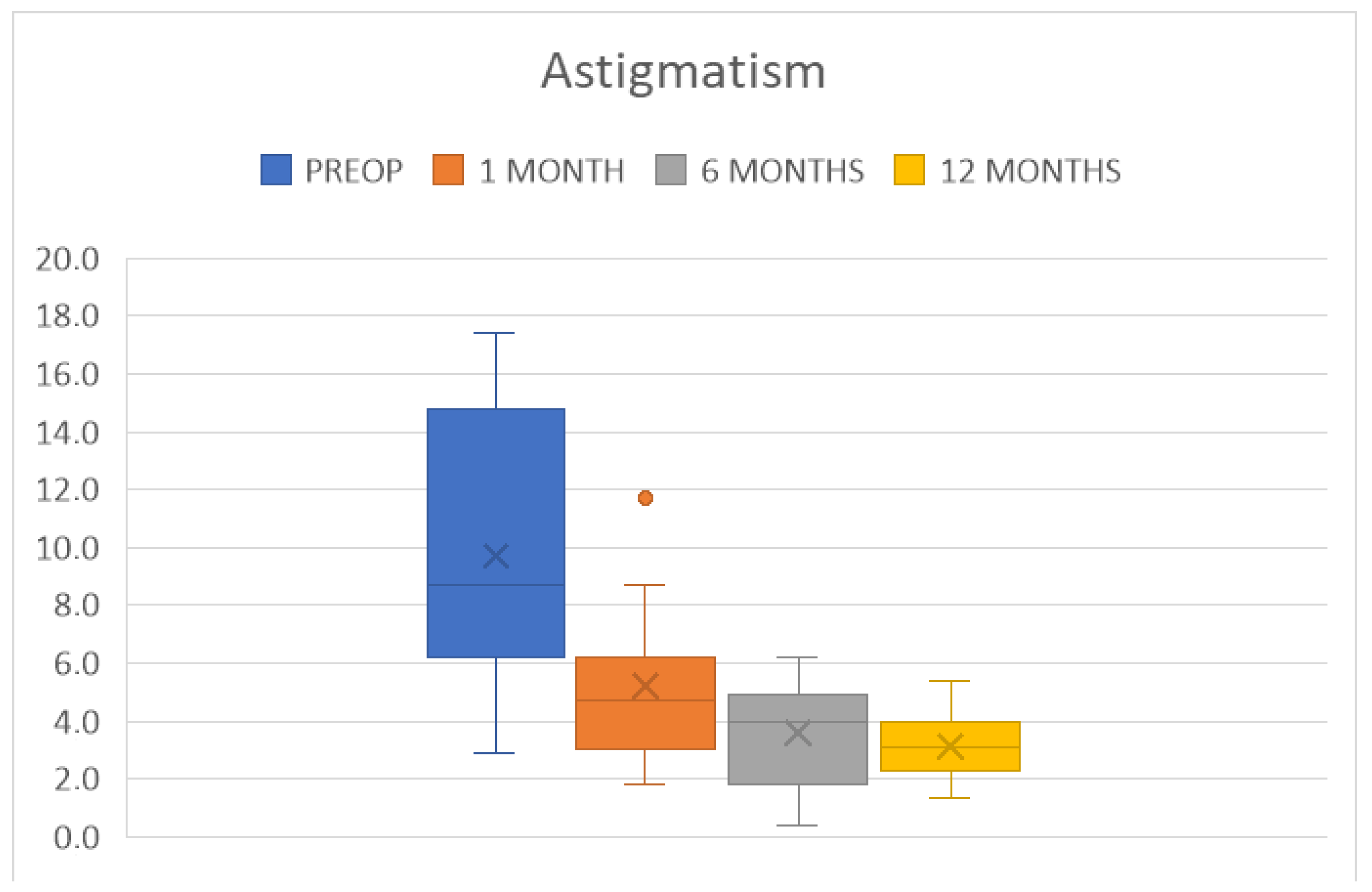
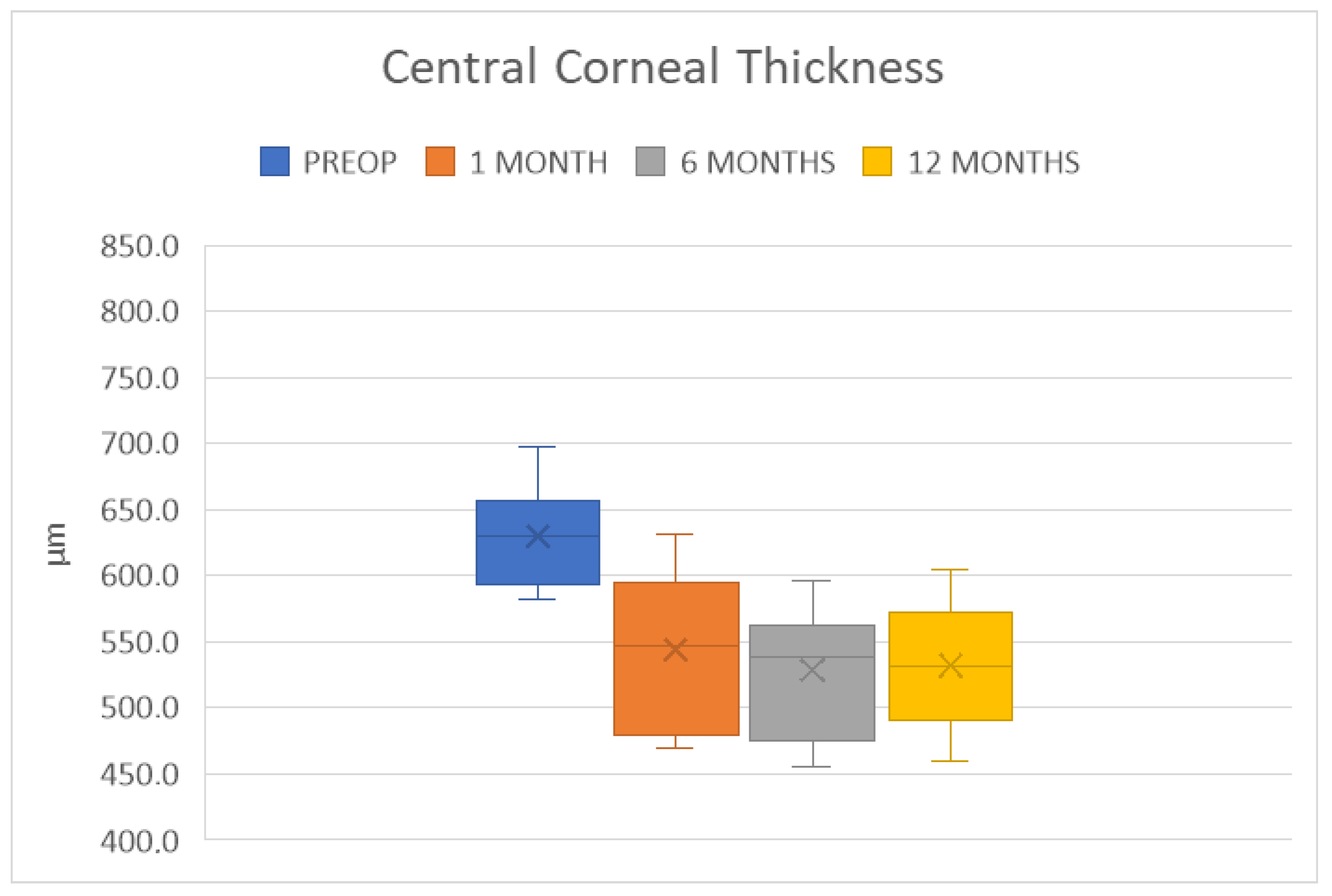
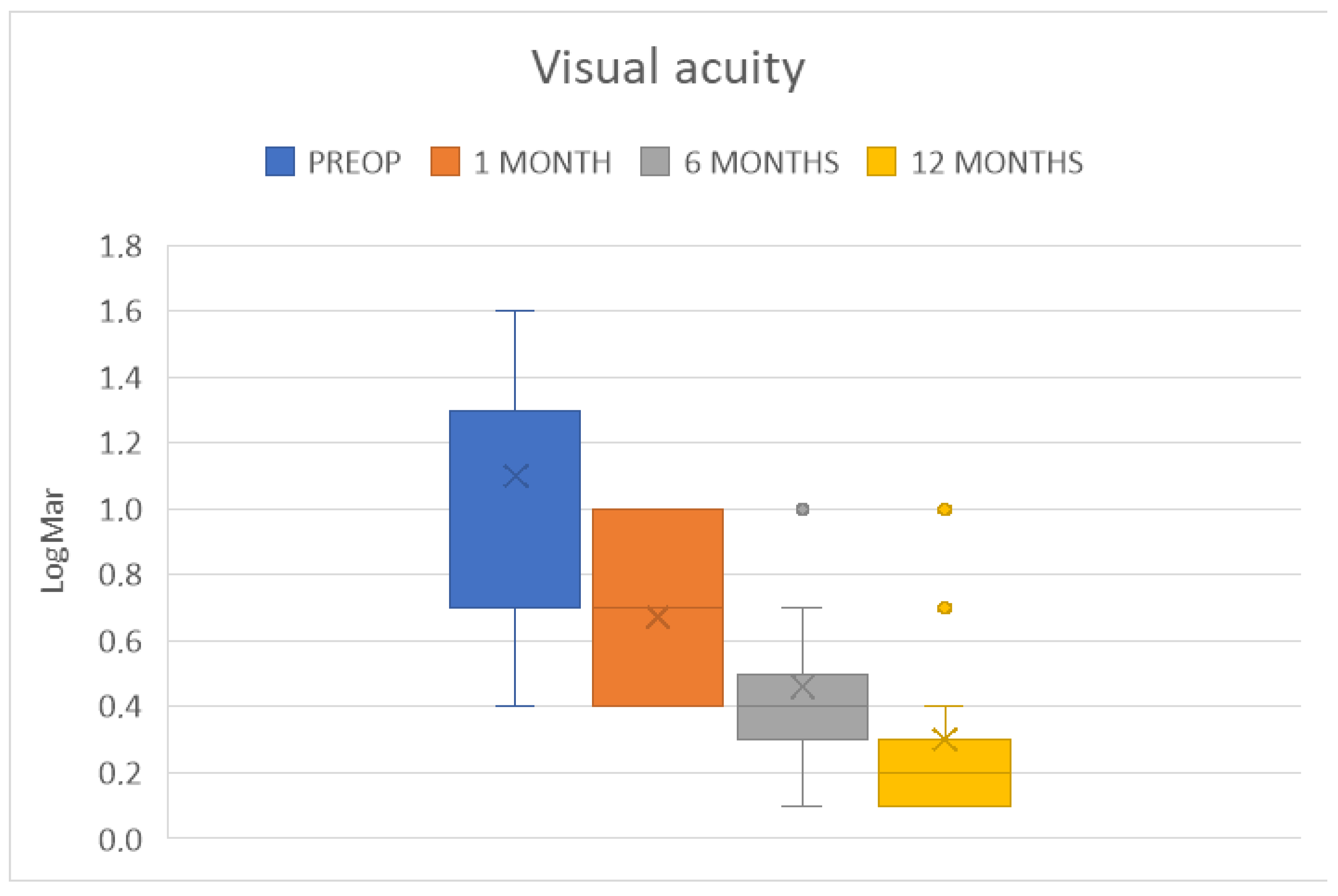
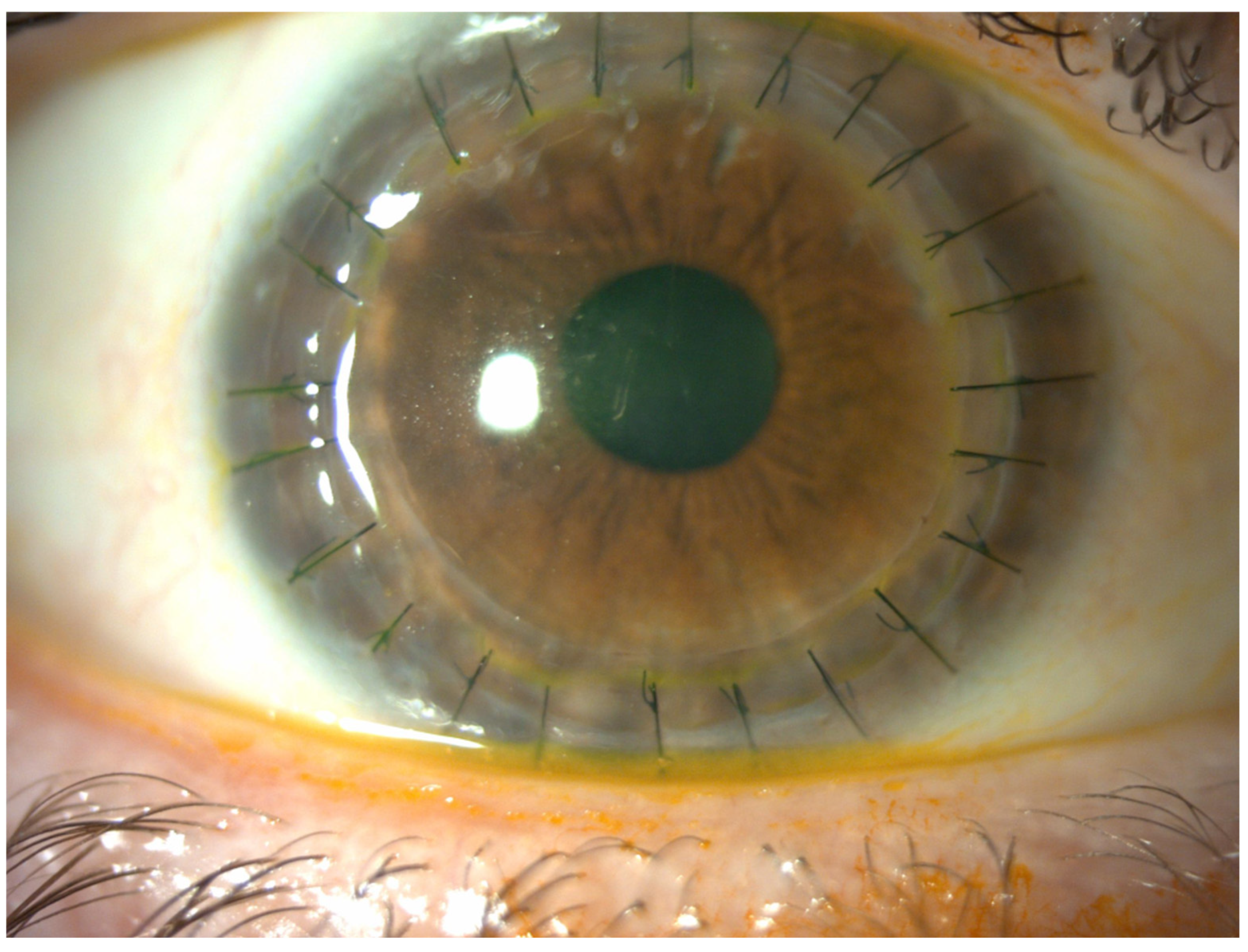
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Moramarco, A.; Gardini, L.; Iannetta, D.; Versura, P.; Fontana, L. Post Penetrating Keratoplasty Ectasia: Incidence, Risk Factors, Clinical Features, and Treatment Options. J. Clin. Med. 2022, 11, 2678. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Murata, H.; Miyai, T.; Shirakawa, R.; Toyono, T.; Yamagami, S.; Usui, T. Characteristics and Risk Factors of Recurrent Keratoconus over the Long Term after Penetrating Keratoplasty. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2377–2383. [Google Scholar] [CrossRef]
- Felipe, A.F.; Hammersmith, K.M.; Nottage, J.M.; Rapuano, C.J.; Nagra, P.K.; Cohen, E.J.; Laibson, P.R. Indications, Visual Outcome, and Ectasia in Clear Corneal Transplants 20 Years Old or More. Cornea 2013, 32, 602–607. [Google Scholar] [CrossRef]
- Raecker, M.E.; Erie, J.C.; Patel, S.V.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. Long-Term Keratometric Changes after Penetrating Keratoplasty for Keratoconus and Fuchs Endothelial Dystrophy. Am. J. Ophthalmol. 2009, 147, 227–233. [Google Scholar] [CrossRef]
- Patel, S.V.; Malta, J.B.; Banitt, M.R.; Mian, S.I.; Sugar, A.; Elner, V.M.; Tester, R.A.; Farjo, Q.A.; Soong, H.K. Recurrent Ectasia in Corneal Grafts and Outcomes of Repeat Keratoplasty for Keratoconus. Br. J. Ophthalmol. 2009, 93, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bourges, J.-L.; Savoldelli, M.; Dighiero, P.; Assouline, M.; Pouliquen, Y.; BenEzra, D.; Renard, G.; Behar-Cohen, F. Recurrence of Keratoconus Characteristics: A Clinical and Histologic Follow-up Analysis of Donor grafts11None of the Authors Has Any Financial Interest in This Study. Ophthalmology 2003, 110, 1920–1925. [Google Scholar] [CrossRef]
- The Evolution of Lamellar Keratoplasty. Available online: https://crstodayeurope.com/articles/2014-feb/the-evolution-of-lamellar-keratoplasty/ (accessed on 3 July 2024).
- Bechrakis, N.; Blom, M.L.; Stark, W.J.; Green, W.R. Recurrent Keratoconus. Cornea 1994, 13, 73–77. [Google Scholar] [CrossRef]
- Abelson, M.B.; Collin, H.B.; Gillette, T.E.; Dohlman, C.H. Recurrent Keratoconus after Keratoplasty. Am. J. Ophthalmol. 1980, 90, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.M.; Hübner, L.; Kruse, F.E.; Tourtas, T. Characterisation of Ectasia after Penetrating Keratoplasty in Keratoconus Eyes Using Anterior Segment Optical Coherence Tomography. Br. J. Ophthalmol. 2024, 108, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Nirankari, V.S.; Karesh, J.; Bastion, F.; Lakhanpal, V.; Billings, E. Recurrence of Keratoconus in Donor Cornea 22 Years after Successful Keratoplasty. Br. J. Ophthalmol. 1983, 67, 23–28. [Google Scholar] [CrossRef]
- Rubinfeld, R.S.; Traboulsi, E.I.; Arentsen, J.J.; Eagle, R.C. Keratoconus after Penetrating Keratoplasty. Ophthalmic Surg. 1990, 21, 420–422. [Google Scholar] [CrossRef]
- Anshu, A.; Li, L.; Htoon, H.M.; de Benito-Llopis, L.; Shuang, L.S.; Singh, M.J.; Tiang Hwee, T.D. Long-Term Review of Penetrating Keratoplasty: A 20-Year Review in Asian Eyes. Am. J. Ophthalmol. 2021, 224, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Montesel, A.; Sayyad, F.E.; Barraquer, R.I.; Arnalich-Montiel, F.; Barrio, J.L.A.D. Corneal Graft Failure: An Update. Br. J. Ophthalmol. 2021, 105, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Esterman, A.J.; Bartlett, C.; Holland, H.; Hornsby, N.B.; Coster, D.J. How Effective Is Penetrating Corneal Transplantation? Factors Influencing Long-Term Outcome in Multivariate Analysis. Transplantation 2006, 81, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Lowe, M.; Bartlett, C.; Kelly, T.-L.; Coster, D.J. All Contributors Risk Factors for Human Corneal Graft Failure within the Australian Corneal Graft Registry. Transplantation 2008, 86, 1720–1724. [Google Scholar] [CrossRef]
- Ing, J.J.; Ing, H.H.; Nelson, L.R.; Hodge, D.O.; Bourne, W.M. Ten-Year Postoperative Results of Penetrating Keratoplasty. Ophthalmology 1998, 105, 1855–1865. [Google Scholar] [CrossRef]
- Garcia-Ferrer, F.J.; Akpek, E.K.; Amescua, G.; Farid, M.; Lin, A.; Rhee, M.K.; Varu, D.M.; Musch, D.C.; Mah, F.S.; Dunn, S.P.; et al. Corneal Ectasia Preferred Practice Pattern®. Ophthalmology 2019, 126, P170–P215. [Google Scholar] [CrossRef]
- Busin, M.; Madi, S.; Scorcia, V.; Santorum, P.; Nahum, Y. A Two-Piece Microkeratome-Assisted Mushroom Keratoplasty Improves the Outcomes and Survival of Grafts Performed in Eyes with Diseased Stroma and Healthy Endothelium (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2015, 113, T1. [Google Scholar]
- Moramarco, A.; di Geronimo, N.; Airaldi, M.; Gardini, L.; Semeraro, F.; Iannetta, D.; Romano, V.; Fontana, L. Intraoperative OCT for Lamellar Corneal Surgery: A User Guide. J. Clin. Med. 2023, 12, 3048. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Zare, M. Current Approaches for Management of Postpenetrating Keratoplasty Astigmatism. J. Ophthalmol. 2011, 2011, 708736. [Google Scholar] [CrossRef]
- Borgia, A.; Romano, V.; Romano, D.; Pagano, L.; Vagge, A.; Giannaccare, G.; Ahmed, M.; Gadhvi, K.; Menassa, N.; Ahmad, M.; et al. Managing Post-Keratoplasty Astigmatism: High-Tech vs. Low-Tech Imaging Techniques for Guiding Suture Manipulation. J. Clin. Med. 2023, 12, 3462. [Google Scholar] [CrossRef] [PubMed]
- Bertelmann, E.; Pleyer, U.; Rieck, P. Risk Factors for Endothelial Cell Loss Post-Keratoplasty. Acta Ophthalmol. Scand. 2006, 84, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Acar, B.T.; Vural, E.T.; Acar, S. Changes in Endothelial Cell Density Following Penetrating Keratoplasty and Deep Anterior Lamellar Keratoplasty. Int. J. Ophthalmol. 2011, 4, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Obata, H.; Ishida, K.; Murao, M.; Miyata, K.; Sawa, M. Corneal Endothelial Cell Damage in Penetrating Keratoplasty. Jpn. J. Ophthalmol. 1991, 35, 411–416. [Google Scholar]
- Malbran, E.S.; Price, F.W.J.; Argañaraz Olivero, J.E.; Malbran, E.J.; Malbran, J.; Malbran, M.; Rogel, L.N.; Price, M.O.; Gordillo, C.H. Peripheral Reconstructive Lamellar Keratoplasty for Late Ectasia After Penetrating Keratoplasty in Keratoconus Eyes. Cornea 2019, 38, 1377. [Google Scholar] [CrossRef]
- Nath, S.; Shen, C.; Koziarz, A.; Banfield, L.; Nowrouzi-Kia, B.; Fava, M.A.; Hodge, W.G. Transepithelial versus Epithelium-off Corneal Collagen Cross-Linking for Corneal Ectasia: A Systematic Review and Meta-Analysis. Ophthalmology 2021, 128, 1150–1160. [Google Scholar] [CrossRef]
- Bizrah, M.; Lin, D.T.C.; Babili, A.; Wirth, M.A.; Arba-Mosquera, S.; Holland, S.P. Topography-Guided Photorefractive Keratectomy for Postkeratoplasty Astigmatism: Long-Term Outcomes. Cornea 2021, 40, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lake, D.; Hamada, S.; Khan, S.; Daya, S.M. Deep Anterior Lamellar Keratoplasty over Penetrating Keratoplasty for Host Rim Thinning and Ectasia. Cornea 2009, 28, 489–492. [Google Scholar] [CrossRef]
- Scorcia, V.; Beltz, J.; Busin, M. Small-Bubble Deep Anterior Lamellar Keratoplasty Technique. JAMA Ophthalmol. 2014, 132, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.J.; Musch, D.C.; Jacobs, D.S.; Lee, W.B.; Kaufman, S.C.; Shtein, R.M. Deep Anterior Lamellar Keratoplasty as an Alternative to Penetrating Keratoplasty. Ophthalmology 2011, 118, 209–218. [Google Scholar] [CrossRef]
- Moramarco, A.; Gardini, L.; Di Mola, I.; di Geronimo, N.; Iannetta, D.; Romano, V.; Hannush, S.B.; Fontana, L. Big-Bubble DALK: A Technique in Evolution. Ocul. Surf. 2024, 34, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.C.; Mattioli, L.; Busin, M. Optimizing Outcomes for Keratoplasty in Ectatic Corneal Disease. Curr. Opin. Ophthalmol. 2020, 31, 268. [Google Scholar] [CrossRef] [PubMed]
- Viola, P.; Neri, E.; Occhipinti, T.; Parekh, M.; Cian, R.; Ponzin, D.; Moramarco, A.; Iovieno, A. Predicting Long-Term Endothelial Cell Loss after Preloaded Descemet Membrane Endothelial Keratoplasty in Fuchs’ Endothelial Corneal Dystrophy: A Mathematical Model. J. Clin. Med. 2024, 13, 877. [Google Scholar] [CrossRef]
- Bourne, W.M.; Nelson, L.R.; Hodge, D.O. Central Corneal Endothelial Cell Changes over a Ten-Year Period. Investig. Ophthalmol. Vis. Sci. 1997, 38, 779–782. [Google Scholar]
- Bourne, W.M. Cellular Changes in Transplanted Human Corneas. Cornea 2001, 20, 560. [Google Scholar] [CrossRef] [PubMed]
- Barraquer, R.I.; Pareja-Aricò, L.; Gómez-Benlloch, A.; Michael, R. Risk Factors for Graft Failure after Penetrating Keratoplasty. Medicine 2019, 98, e15274. [Google Scholar] [CrossRef]
- Borderie, V.M.; Georgeon, C.; Sandali, O.; Bouheraoua, N. Long-Term Outcomes of Deep Anterior Lamellar versus Penetrating Keratoplasty for Keratoconus. Br. J. Ophthalmol. 2024, 108, 10–16. [Google Scholar] [CrossRef]
- Roberts, H.W.; De Benito-Llopis, L. Comparison of Repeat Penetrating Keratoplasty, DSAEK and DMEK for the Management of Endothelial Failure of Previous PK. Eye 2023, 37, 3596–3601. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.; Bu, J.B.; Gericke, A.; Vossmerbaeumer, U.; Schuster, A.K.; Pfeiffer, N.; Wasielica-Poslednik, J. Comparison of DMEK and DSAEK in Eyes with Endothelial Decompensation After Previous Penetrating Keratoplasty. Cornea 2021, 40, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Martheswaran, T.; Desautels, J.; Majid, M.; Shmunes, K.; Ronquillo, Y.; Hoopes, P. A Contemporary Risk Analysis of Iatrogenic Transmission of Creutzfeldt-Jakob Disease (CJD) via Corneal Transplantation in the United States. Ophthalmol. Ther. 2020, 9, 465–483. [Google Scholar] [CrossRef]
- Malleron, V.; Bloch, F.; Zevering, Y.; Vermion, J.-C.; Semler-Collery, A.; Goetz, C.; Perone, J.-M. Evolution of Corneal Transplantation Techniques and Their Indications in a French Corneal Transplant Unit in 2000–2020. PLoS ONE 2022, 17, e0263686. [Google Scholar] [CrossRef]
- de Sanctis, U.; Alovisi, C.; Bauchiero, L.; Caramello, G.; Girotto, G.; Panico, C.; Vinai, L.; Genzano, F.; Amoroso, A.; Grignolo, F. Changing Trends in Corneal Graft Surgery: A Ten-Year Review. Int. J. Ophthalmol. 2016, 9, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, D.J.; Sit, M.; Naor, J.; Slomovic, A.R. Outcomes of Repeat Penetrating Keratoplasty and Risk Factors for Graft Failure. Cornea 2003, 22, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Al-Mezaine, H.; Wagoner, M.D. Repeat Penetrating Keratoplasty: Indications, Graft Survival, and Visual Outcome. Br. J. Ophthalmol. 2006, 90, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Dickman, M.M.; Spekreijse, L.S.; Dunker, S.L.; Winkens, B.; Berendschot, T.T.J.M.; van den Biggelaar, F.J.H.M.; Kruit, P.J.; Nuijts, R.M.M.A. Long-Term Outcomes of Repeated Corneal Transplantations: A Prospective Dutch Registry Study. Am. J. Ophthalmol. 2018, 193, 156–165. [Google Scholar] [CrossRef]
- Speaker, M.G.; Arentsen, J.J.; Laibson, P.R. Long-Term Survival of Large Diameter Penetrating Keratoplasties for Keratoconus and Pellucid Marginal Degeneration. Acta Ophthalmol. Suppl. 1989, 192, 17–19. [Google Scholar] [CrossRef]
- Romano, D.; Aiello, F.; Parekh, M.; Levis, H.J.; Gadhvi, K.A.; Moramarco, A.; Viola, P.; Fontana, L.; Semeraro, F.; Romano, V. Incidence and Management of Early Postoperative Complications in Lamellar Corneal Transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3097–3111. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.C.; Spena, R.; Fusco, F.; Dondi, R.; Myerscough, J.; Fabbri, F.; Bovone, C.; Busin, M. Long-Term Outcomes of Two-Piece Mushroom Keratoplasty for Traumatic Corneal Scars. Am. J. Ophthalmol. 2022, 236, 20–31. [Google Scholar] [CrossRef]
- Scorcia, V.; Busin, M. Survival of Mushroom Keratoplasty Performed in Corneas with Postinfectious Vascularized Scars. Am. J. Ophthalmol. 2012, 153, 44–50.e1. [Google Scholar] [CrossRef] [PubMed]
- Busin, M.; Beltz, J.; Scorcia, V. Mushroom Keratoplasty in Pediatric Patients. Saudi J. Ophthalmol. 2011, 25, 269–274. [Google Scholar] [CrossRef]
- Liu, C.; Mehta, J.S.; Liu, Y.-C. Femtosecond Laser-Assisted Corneal Transplantation. Taiwan J. Ophthalmol. 2023, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, F.; Wiggermann, A.; Maier, P.C.; Böhringer, D.; Reinhard, T. Clinical Results of 123 Femtosecond Laser-Assisted Penetrating Keratoplasties. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Levinger, E.; Trivizki, O.; Levinger, S.; Kremer, I. Outcome of “Mushroom” Pattern Femtosecond Laser–Assisted Keratoplasty Versus Conventional Penetrating Keratoplasty in Patients with Keratoconus. Cornea 2014, 33, 481. [Google Scholar] [CrossRef]
- Fung, S.S.M.; Aiello, F.; Maurino, V. Outcomes of Femtosecond Laser-Assisted Mushroom-Configuration Keratoplasty in Advanced Keratoconus. Eye 2016, 30, 553–561. [Google Scholar] [CrossRef]
- Bahar, I.; Kaiserman, I.; McAllum, P.; Rootman, D. Femtosecond Laser-Assisted Penetrating Keratoplasty: Stability Evaluation of Different Wound Configurations. Cornea 2008, 27, 209. [Google Scholar] [CrossRef]
- Malta, J.B.; Soong, H.K.; Shtein, R.; Banitt, M.; Musch, D.C.; Sugar, A.; Mian, S.I. Femtosecond Laser-Assisted Keratoplasty: Laboratory Studies in Eye Bank Eyes. Curr. Eye Res. 2009, 31, 18–25. [Google Scholar] [CrossRef]
- Plamann, K.; Aptel, F.; Arnold, C.L.; Courjaud, A.; Crotti, C.; Deloison, F.; Druon, F.; Georges, P.; Hanna, M.; Legeais, J.-M.; et al. Ultrashort Pulse Laser Surgery of the Cornea and the Sclera. J. Opt. 2010, 12, 084002. [Google Scholar] [CrossRef]
- Crotti, C.; Deloison, F.; Alahyane, F.; Aptel, F.; Kowalczuk, L.; Legeais, J.-M.; Peyrot, D.A.; Savoldelli, M.; Plamann, K. Wavelength Optimization in Femtosecond Laser Corneal Surgery. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3340–3349. [Google Scholar] [CrossRef]
- Bénard, A.; Sitta, R.; Brezin, A.P.; Cochener, B.; Monnet, D.; Denis, P.; Pisella, P.-J.; Hayes, N.; Schweitzer, C. FEMCAT Study Group Cost Utility and Value of Information Analysis of Femtosecond Laser-Assisted Cataract Surgery. JAMA Ophthalmol. 2023, 141, 625–629. [Google Scholar] [CrossRef]
- van den Biggelaar, F.J.H.M.; Cheng, Y.Y.Y.; Nuijts, R.M.M.A.; Schouten, J.S.A.G.; Wijdh, R.-J.; Pels, E.; van Cleynenbreugel, H.; Eggink, C.A.; Rijneveld, W.J.; Dirksen, C.D. Economic Evaluation of Endothelial Keratoplasty Techniques and Penetrating Keratoplasty in The Netherlands. Am. J. Ophthalmol. 2012, 154, 272–281.e2. [Google Scholar] [CrossRef]
- Adeyoju, J.; Konstantopoulos, A.; Mehta, J.S.; Hossain, P. Femtolaser-Assisted Keratoplasty: Surgical Outcomes and Benefits. J. EuCornea 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Tran, T.M.; Farid, M. Update on Femtosecond Laser–Enabled Keratoplasty. Cornea 2023, 42, 395. [Google Scholar] [CrossRef] [PubMed]
- Lucisano, A.; Lionetti, G.; Yu, A.C.; Giannaccare, G.; D’Angelo, S.; Busin, M.; Scorcia, V. Outcomes of Conventional 8.0-Mm Versus Large 9.0-Mm Diameter Deep Anterior Lamellar Keratoplasty for Keratoconus. Cornea 2023, 42, 815. [Google Scholar] [CrossRef]
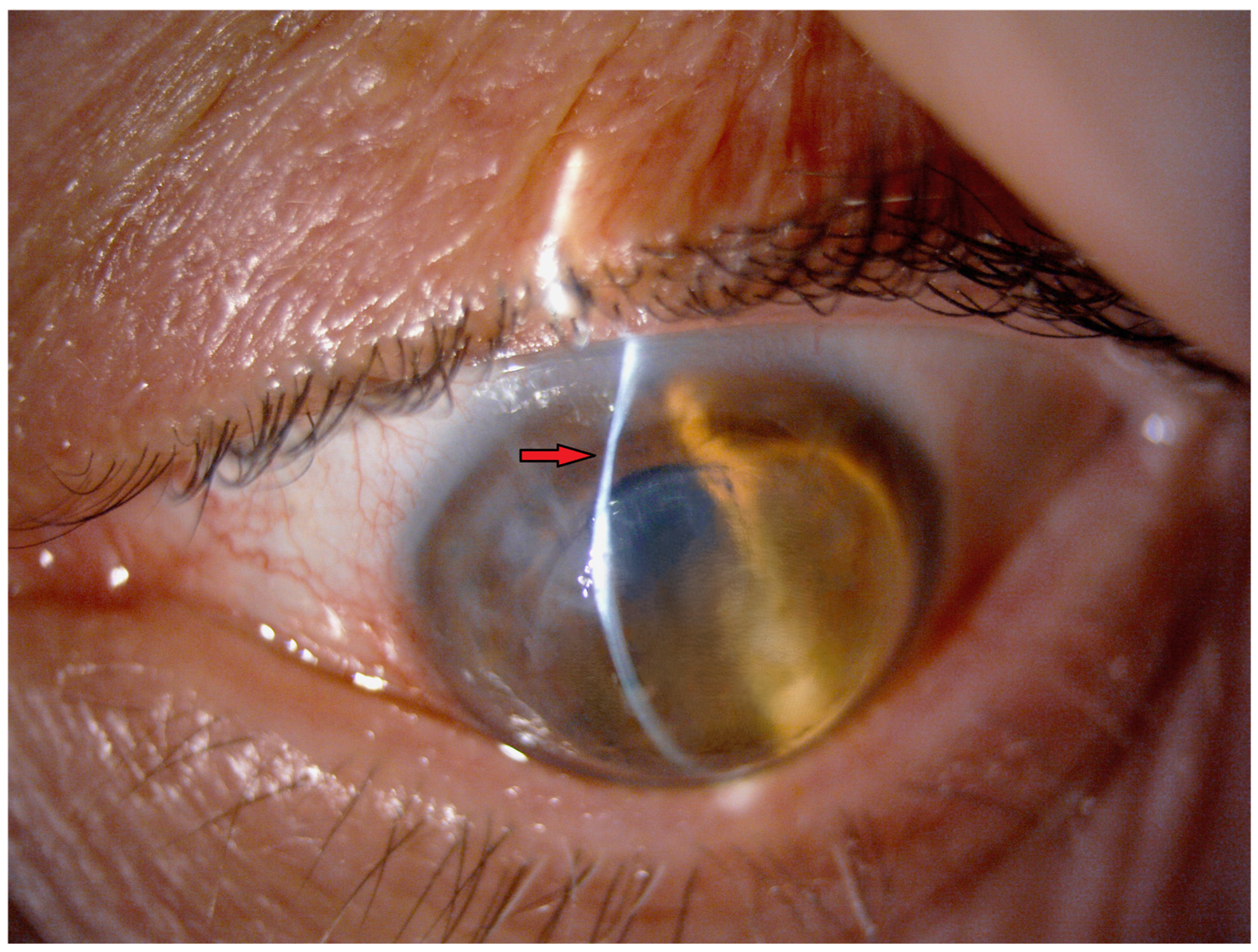
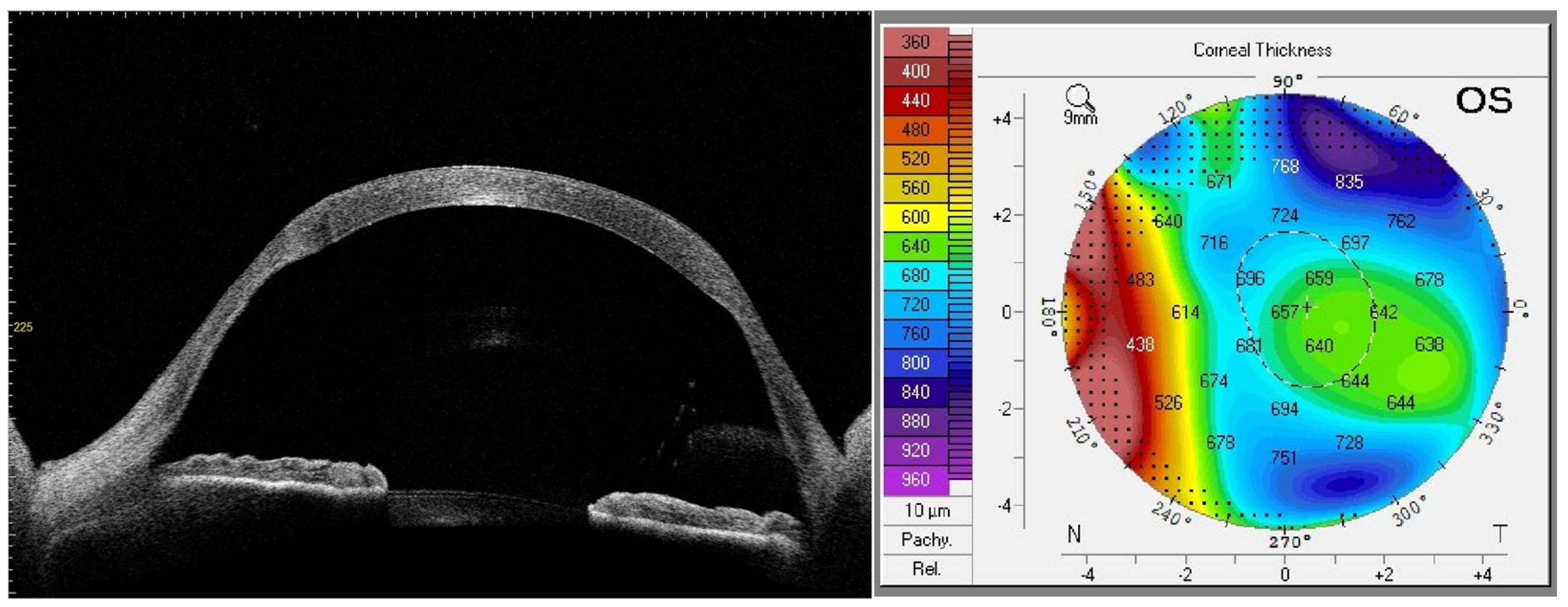

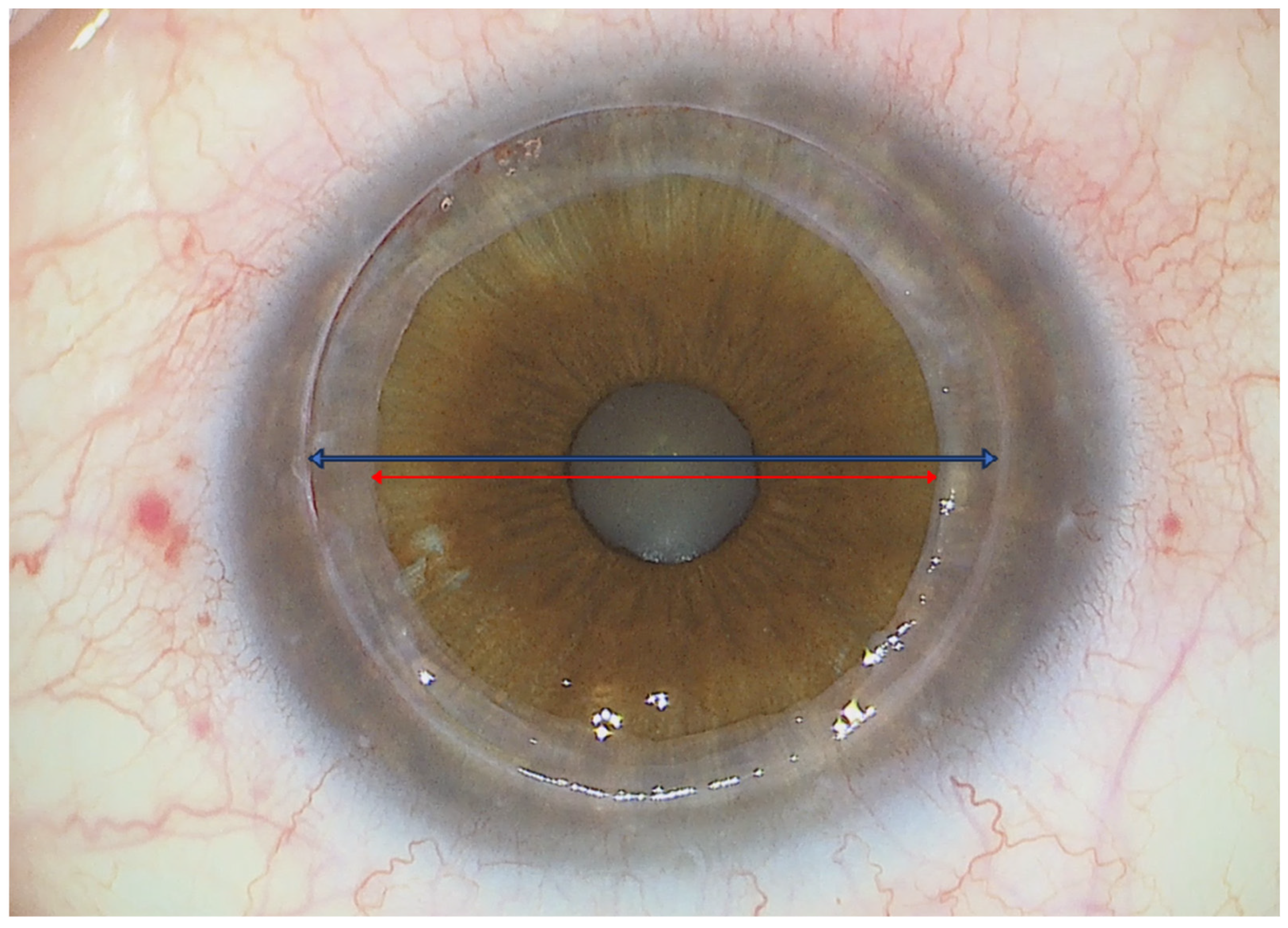

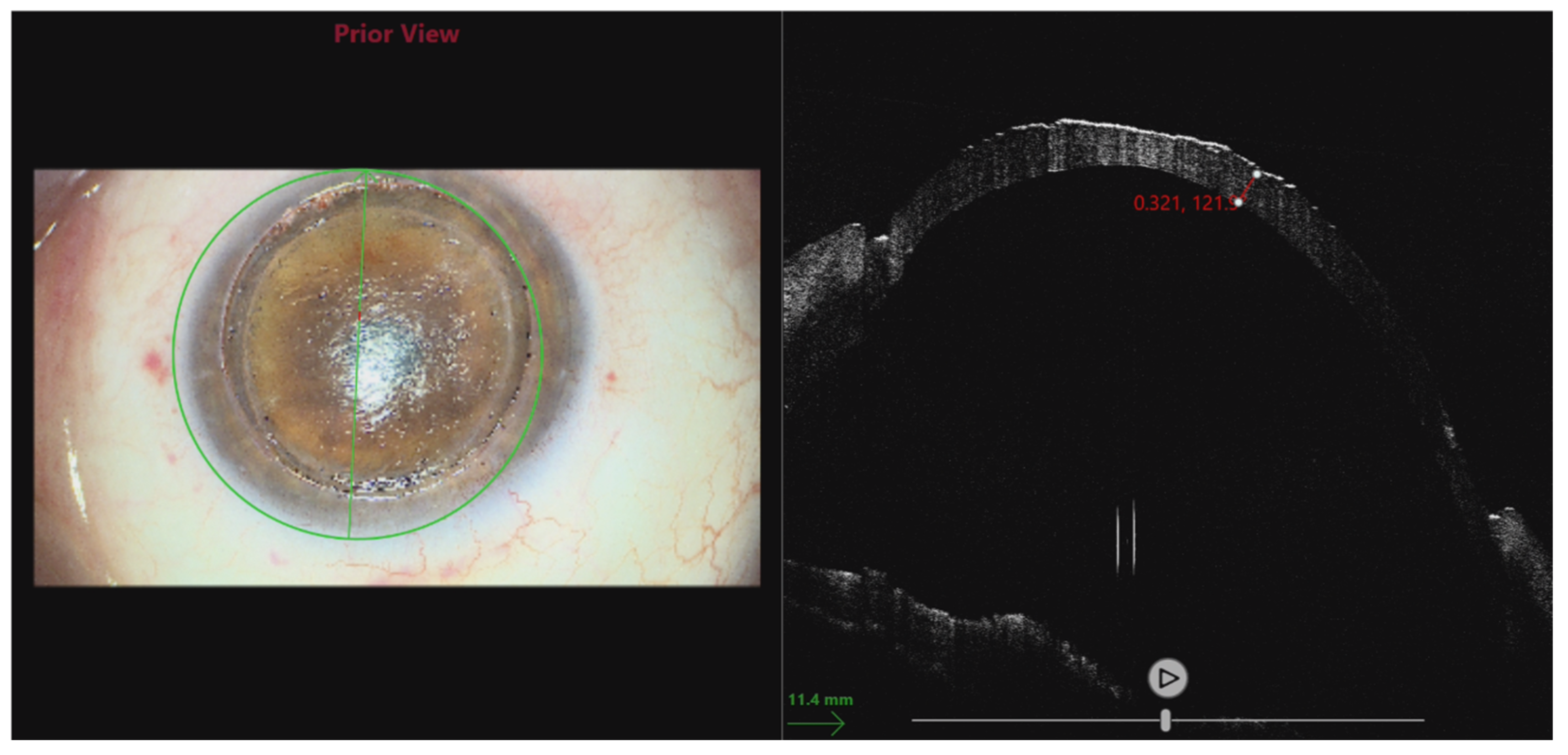
| AGE (years) | 58 ± 9 |
| GENDER | 14 M; 1 F |
| EYE | 7 OD; 8 OS |
| YEARS FROM PRIMARY PK | 28.5 ± 13.3 |
| BCVA (LogMAR) | 1.1 ± 0.4 |
| CCT (µm) | 629 ± 39 |
| ECD (cells/mm2) | not measurable * |
| KMIN (D) | 46.8 ± 4.9 |
| KMAX (D) | 56.5 ± 5.1 |
| AVK (D) | 51.1 ± 4.5 |
| Parameter Measured | 6 Months | 12 Months | ||
|---|---|---|---|---|
| BCVA (LogMAR) | 0.5 ± 0.3 | p < 0.0001 | 0.3 ± 0.2 | p < 0.0001 |
| CCT (µm) | 528 ± 46 | p < 0.0001 | 532 ± 45 | p = 0.0001 |
| ECD (cells/mm2) | 2181 ± 190 | 1926 ± 199 | ||
| KMIN (D) | 41.7 ± 1.8 | p < 0.001 | 42.0 ± 1.2 | p = 0.001 |
| KMAX (D) | 45.3 ± 1.5 | p < 0.0001 | 45.1 ± 1.2 | p < 0.0001 |
| AVK (D) | 43.5 ± 1.4 | p < 0.0001 | 43.5 ± 1.1 | p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moramarco, A.; Fontana, L.; di Geronimo, N.; Rapezzi, G.; Savini, G.; Viola, P.; Mete, M.; Romano, V. A Pilot Case Series on the Use of a Large Mushroom-Shaped Corneal Graft for the Surgical Management of Post-Penetrating Keratoplasty Ectasia and Endothelial Failure. J. Clin. Med. 2025, 14, 343. https://doi.org/10.3390/jcm14020343
Moramarco A, Fontana L, di Geronimo N, Rapezzi G, Savini G, Viola P, Mete M, Romano V. A Pilot Case Series on the Use of a Large Mushroom-Shaped Corneal Graft for the Surgical Management of Post-Penetrating Keratoplasty Ectasia and Endothelial Failure. Journal of Clinical Medicine. 2025; 14(2):343. https://doi.org/10.3390/jcm14020343
Chicago/Turabian StyleMoramarco, Antonio, Luigi Fontana, Natalie di Geronimo, Giulio Rapezzi, Giacomo Savini, Pietro Viola, Maurizio Mete, and Vito Romano. 2025. "A Pilot Case Series on the Use of a Large Mushroom-Shaped Corneal Graft for the Surgical Management of Post-Penetrating Keratoplasty Ectasia and Endothelial Failure" Journal of Clinical Medicine 14, no. 2: 343. https://doi.org/10.3390/jcm14020343
APA StyleMoramarco, A., Fontana, L., di Geronimo, N., Rapezzi, G., Savini, G., Viola, P., Mete, M., & Romano, V. (2025). A Pilot Case Series on the Use of a Large Mushroom-Shaped Corneal Graft for the Surgical Management of Post-Penetrating Keratoplasty Ectasia and Endothelial Failure. Journal of Clinical Medicine, 14(2), 343. https://doi.org/10.3390/jcm14020343








