Systematic Review and Meta-Analysis of the Application of T-PEP in the Therapeutic Management of COPD Patients
Abstract
1. Introduction
2. Materials and Methods
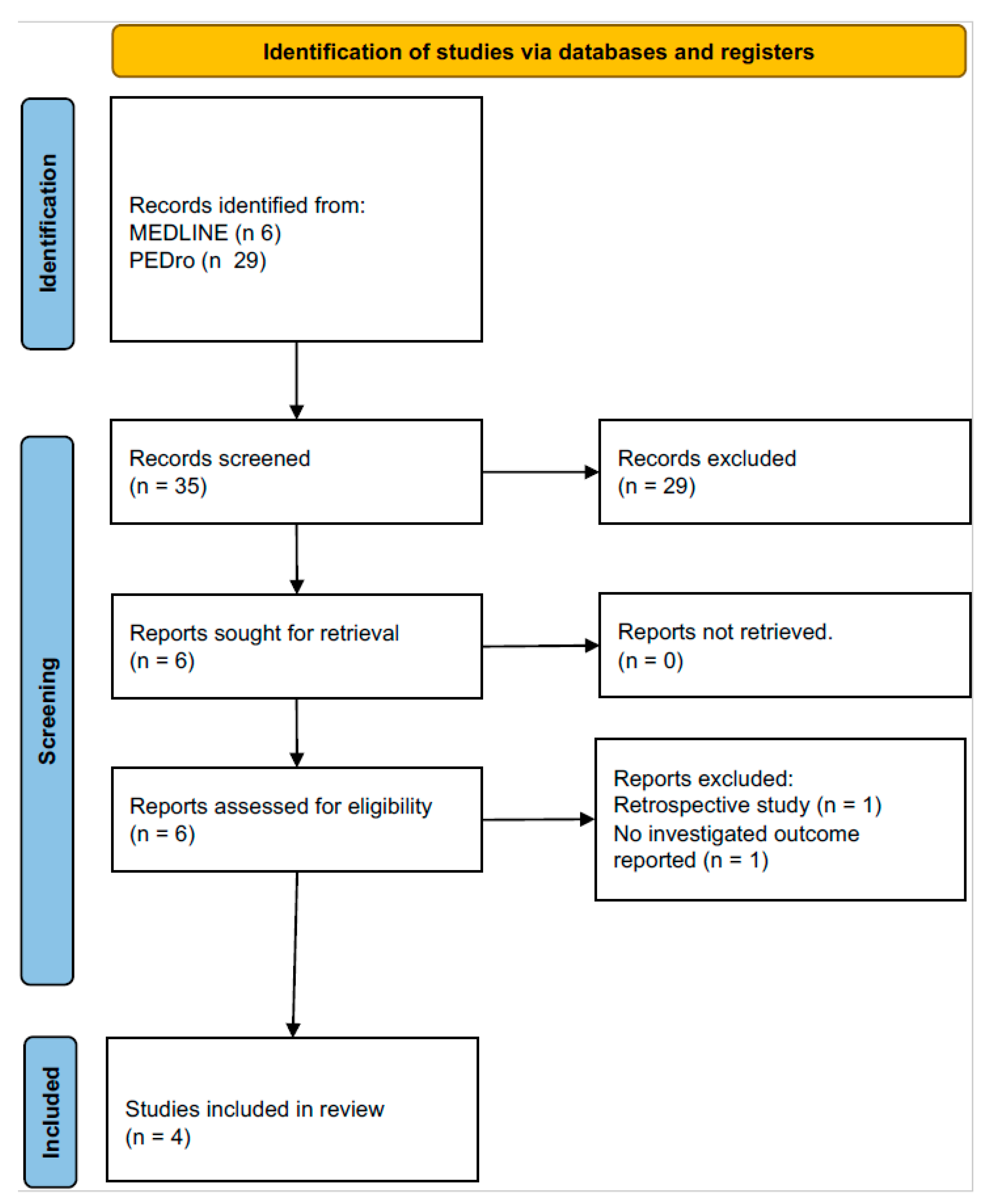
2.1. Search Strategy and Study Eligibility
2.2. Study Selection
2.3. Data Collection
2.4. Endpoint
2.5. Data Synthesis and Analysis
3. Results
3.1. Study Characteristics
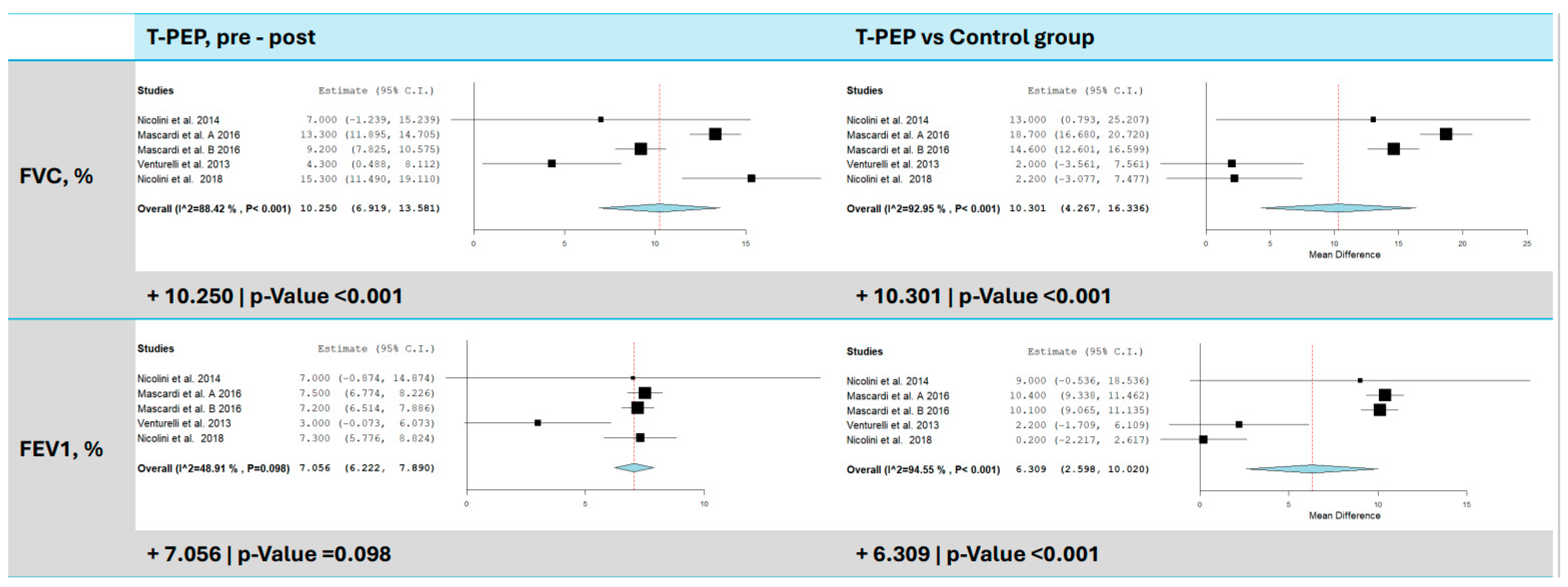
3.2. Dynamic Lung Volumes (FEV1 and FVC)
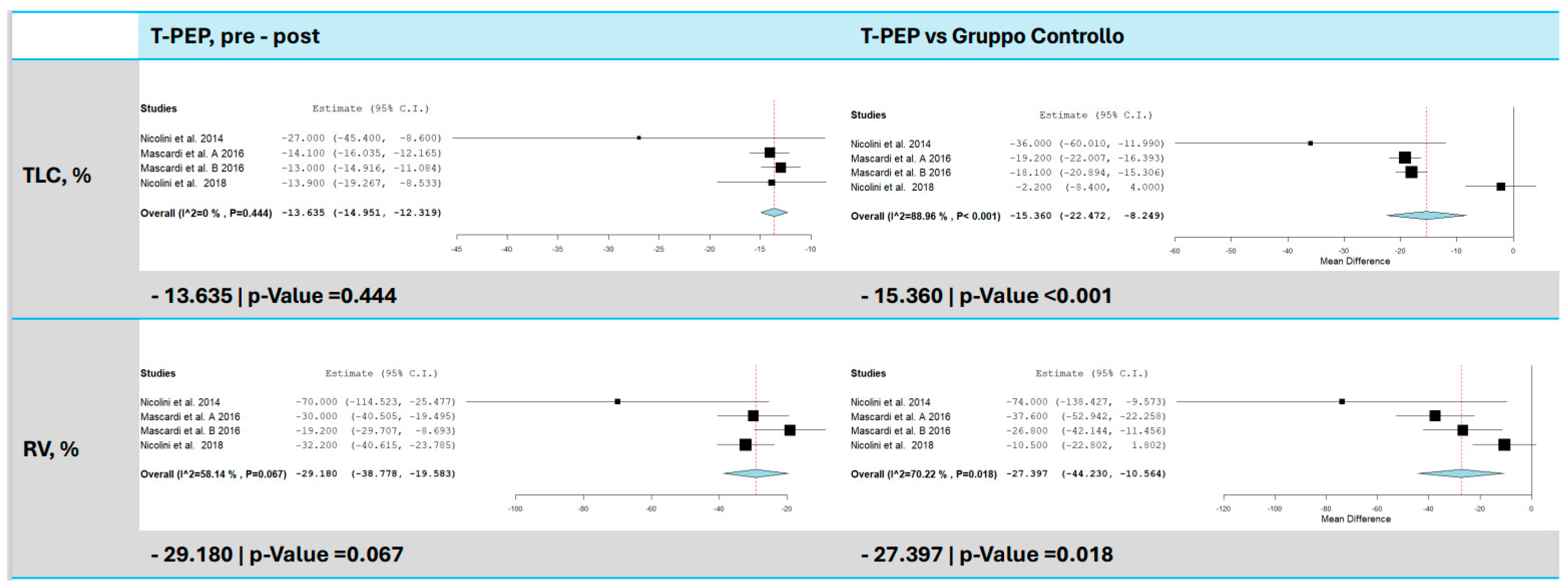
3.3. Total Lung Capacity (TLC)
3.4. Maximum Inspiratory Pressure (MIP) and Maximum Expiratory Pressure (MEP)
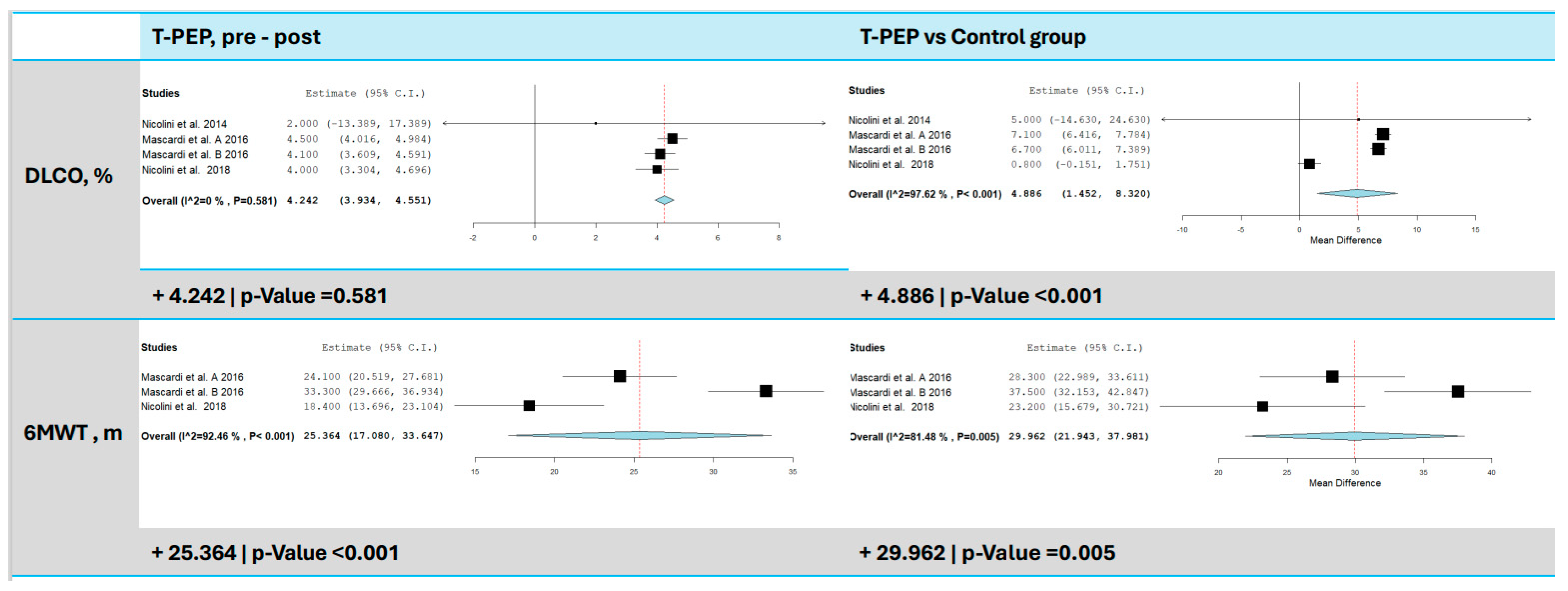
3.5. Diffusing Capacity of the Lungs for Carbon Monoxide (DLCO%)
3.6. Six-Minute Walk Test (6MWT)
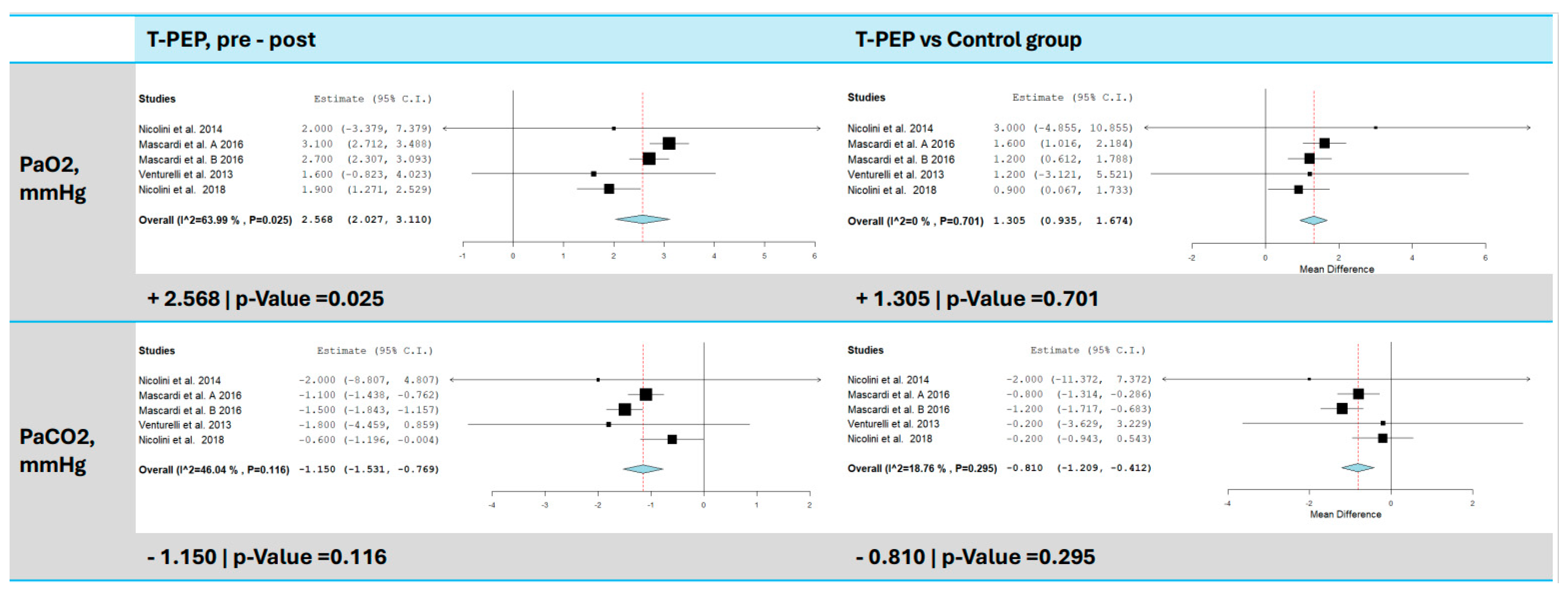
3.7. Blood Gas Analysis (pO2 and pCO2)
3.8. Symptoms and Quality of Life (mMRC, CAT, BCSS)
3.9. Acute Exacerbations of COPD (AECOPD)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| ABG | Arterial blood gas |
| ACTs | Airway clearance techniques |
| AECOPD | Acute exacerbations of COPD |
| BCSS | Breathlessness, Cough, and Sputum Scale |
| CAT | COPD Assessment Test |
| CMH | Chronic mucus hypersecretion |
| COPD | Chronic obstructive pulmonary disease |
| DLCO | Diffusing capacity of the lungs for carbon monoxide |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| IPBB | Intermittent positive pressure breathing |
| MEP | Maximum expiratory pressure |
| mMRC | Modified Medical Research Council Dyspnea Scale |
| MIP | Maximum inspiratory pressure |
| OPEP | Oscillatory Positive Expiratory Pressure |
| pCO2 | Partial pressure of carbon dioxide |
| PEP | Positive expiratory pressure |
| PICO | Patient, intervention, comparison, outcome |
| pO2 | Partial pressure of oxygen |
| PR | Pulmonary rehabilitation |
| PRISMA-P | Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols |
| QoL | Quality of life |
| RCTs | Randomized controlled trials |
| RV | Residual volume |
| 6MWT | Six-Minute Walk Test |
| T-PEP | Temporary positive expiratory pressure |
| TLC | Total lung capacity |
References
- GOLD Report 2024. 2024. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 1 October 2024).
- World Health Organization. The Top 10 Causes of Death. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 1 October 2024).
- Donaldson, G.C.; Hurst, J.R.; Smith, C.J.; Hubbard, R.B.; Wedzicha, J.A. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010, 137, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Frija-Masson, J.; Burgel, P.-R. Targeting mucus hypersecretion: New therapeutic opportunities for COPD? Drugs 2014, 74, 1073–1089. [Google Scholar] [CrossRef]
- Dunican, E.M.; Elicker, B.M.; Henry, T.; Gierada, D.S.; Schiebler, M.L.; Anderson, W.; Barjaktarevic, I.; Barr, R.G.; Bleecker, E.R.; Boucher, R.C.; et al. Mucus Plugs and Emphysema in the Pathophysiology of Airflow Obstruction and Hypoxemia in Smokers. Am. J. Respir. Crit. Care Med. 2021, 203, 957–968. [Google Scholar] [CrossRef]
- Yang, R.; Wu, X.; Gounni, A.S.; Xie, J. Mucus hypersecretion in chronic obstructive pulmonary disease: From molecular mechanisms to treatment. J. Transl. Int. Med. 2023, 11, 312–315. [Google Scholar] [CrossRef]
- Patel, I.S. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002, 57, 759–764. Available online: https://thorax.bmj.com/lookup/doi/10.1136/thorax.57.9.759 (accessed on 21 October 2024). [CrossRef]
- Shah, B.K.; Singh, B.; Wang, Y.; Xie, S.; Wang, C. Mucus Hypersecretion in Chronic Obstructive Pulmonary Disease and Its Treatment. Mediat. Inflamm. 2023, 2023, 8840594. Available online: https://www.hindawi.com/journals/mi/2023/8840594/ (accessed on 9 September 2024). [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit Care Med. 2013, 188, e13–e64. Available online: http://www.atsjournals.org/doi/abs/10.1164/rccm.201309-1634ST (accessed on 1 November 2020). [CrossRef]
- Vogiatzis, I.; Rochester, C.L.; Spruit, M.A.; Troosters, T.; Clini, E.M. Increasing implementation and delivery of pulmonary rehabilitation: Key messages from the new ATS/ERS policy statement. Eur. Respir. J. 2016, 47, 1336–1341. Available online: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.02151-2015 (accessed on 9 September 2024). [CrossRef]
- Belli, S.; Prince, I.; Savio, G.; Paracchini, E.; Cattaneo, D.; Bianchi, M.; Masocco, F.; Teresa Bellanti, M.; Balbi, B. Airway Clearance Techniques: The Right Choice for the Right Patient. Front. Med. 2021, 8, 544826. Available online: https://www.frontiersin.org/articles/10.3389/fmed.2021.544826/full (accessed on 21 October 2024).
- Osadnik, C.R.; McDonald, C.F.; Jones, A.P.; E Holland, A. Airway clearance techniques for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012, 2012, CD008328. [Google Scholar] [CrossRef] [PubMed]
- Fagevik Olsén, M.; Lannefors, L.; Westerdahl, E. Positive expiratory pressure-Common clinical applications and physiological effects. Respir. Med. 2015, 109, 297–307. [Google Scholar] [CrossRef]
- de Macedo, J.R.F.F.; Santos, E.d.C.d.; Reychler, G.; Poncin, W. The Impact of Positive Expiratory Pressure Therapy on Hyperinflation in Patients With COPD. Respir. Care 2024, 69, 366–375. Available online: http://rc.rcjournal.com/lookup/doi/10.4187/respcare.11039 (accessed on 11 October 2024). [CrossRef]
- Mascardi, V.; Grecchi, B.; Barlascini, C.; Banfi, P.; Nicolini, A. Effectiveness of temporary positive expiratory pressure (T-PEP) at home and at hospital in patients with severe chronic obstructive pulmonary disease. J. Thorac. Dis. 2016, 8, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.; Mascardi, V.; Grecchi, B.; Ferrari-Bravo, M.; Banfi, P.; Barlascini, C. Comparison of effectiveness of temporary positive expiratory pressure versus oscillatory positive expiratory pressure in severe COPD patients. Clin. Respir. J. 2018, 12, 1274–1282. Available online: https://onlinelibrary.wiley.com/doi/10.1111/crj.12661 (accessed on 17 February 2023). [CrossRef] [PubMed]
- Nicolini, A.; Mollar, E.; Grecchi, B.; Landucci, N. Comparison of intermittent positive pressure breathing and temporary positive expiratory pressure in patients with severe chronic obstructive pulmonary disease. Arch. Bronconeumol. 2014, 50, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, E.; Crisafulli, E.; DeBiase, A.; Righi, D.; Berrighi, D.; Cavicchioli, P.P.; Vagheggini, G.; Dabrosca, F.; Balbi, B.; Paneroni, M.; et al. Efficacy of temporary positive expiratory pressure (TPEP) in patients with lung diseases and chronic mucus hypersecretion. The UNIKO® project: A multicentre randomized controlled trial. Clin. Rehabil. 2013, 27, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. Available online: https://www.acpjournals.org/doi/10.7326/M18-0850 (accessed on 8 October 2023). [CrossRef]
- Herrero-Cortina, B.; Vilaró, J.; Martí, D.; Torres, A.; Miguel-Pagola, M.S.; Alcaraz, V.; Polverino, E. Short-term effects of three slow expiratory airway clearance techniques in patients with bronchiectasis: A randomised crossover trial. Physiotherapy 2016, 102, 357–364. [Google Scholar] [CrossRef]
- Myers, T.R. Positive expiratory pressure and oscillatory positive expiratory pressure therapies. Respir. Care 2007, 52, 1308–1326, discussion 1327. [Google Scholar]
- Ora, J.; Prendi, E.; Attinà, M.L.; Cazzola, M.; Calzetta, L.; Rogliani, P. Efficacy of respiratory tele-rehabilitation in COPD patients: Systematic review and meta-analysis. Monaldi Arch. Chest Dis. 2022, 92. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85137091998&doi=10.4081%2fmonaldi.2022.2105&partnerID=40&md5=3b08ca815f0859e4f41e42725d5a504a (accessed on 1 October 2024). [CrossRef] [PubMed]
- Boucher, R.C. Muco-Obstructive Lung Diseases. N. Engl. J. Med. 2019, 380, 1941–1953. Available online: http://www.nejm.org/doi/10.1056/NEJMra1813799 (accessed on 25 November 2024). [CrossRef] [PubMed]
- Diaz, A.A.; Orejas, J.L.; Grumley, S.; Nath, H.P.; Wang, W.; Dolliver, W.R.; Yen, A.; Kligerman, S.J.; Jacobs, K.; Manapragada, P.P.; et al. Airway-Occluding Mucus Plugs and Mortality in Patients with Chronic Obstructive Pulmonary Disease. JAMA 2023, 329, 1832. Available online: https://jamanetwork.com/journals/jama/fullarticle/2805343 (accessed on 25 November 2024). [CrossRef] [PubMed]
- D’abrosca, F.; Garabelli, B.; Savio, G.; Barison, A.; Appendini, L.; Oliveira, L.V.; Baiardi, P.; Balbi, B. Comparing airways clearance techniques in chronic obstructive pulmonary disease and bronchiectasis: Positive expiratory pressure or temporary positive expiratory pressure? A retrospective study. Braz. J. Phys. Ther. 2017, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]



| Number Identifier | Study Characteristics | Main Inclusion Criteria | Setting | Therapy Time | Study Duration (Weeks) | Simple Size | Age Years (SD) | Male % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | IG | CG | AIG | IG | CG | AIG | IG | CG | AIG | |||||||
| Nicolini et al., 2014 [17] | Chi CTR-TRC-12002178 | Single-blind randomized trial | 40–80 years old, COPD (FEV1 < 50%) | Day hospital of the Respiratory Medicine Unit | 30 min session twice a day, 5 days per week | 3 weeks | 45 | 15 | 15 | 15 | 73 (6.00) | 70 (6.00) | 70.00 (9.00) | 60 | 53.3 | 60 |
| Mascardi et al., 2016 a [15] | Chi-CTR-TRC 15006662 | Randomized controlled study | COPD (FEV1 < 50%) | Hospital | 30 min session twice a day for 15 days | 15 weeks | 120 | 35 | 35 | 0 | 71.7 (4.60) | 70.7 (6.30) | 0 | 71.8 | 71.8 | 0 |
| Mascardi et al., 2016 b [15] | Chi-CTR-TRC 15006662 | Randomized controlled study | COPD (FEV1 < 50%) | Hospital | 30 min session twice a day for 15 days | 15 weeks | 120 | 34 | 35 | 0 | 70.7 (6.10) | 70.7 (6.30) | 0 | 75 | 71.8 | 0 |
| Venturelli et al., 2013 [18] | NCT00700388 | Single-blind multicenter randomized trial | COPD and/or chronic bronchitis or bronchiectasis with a peak cough expiratory flow > 150 l/min and sputum production > 30 mL/day | Inpatient pulmonary rehabilitation | 15 min session twice a day for 10 days | 10 days | 98 | 44 | 39 | 0 | 70 (10.80) | 71.6 (8.70) | 0 | 54.7 | 77.8 | 0 |
| Nicolini et al., 2018 [16] | Chi-CTR-IPR-16008487 | Randomized controlled study | COPD (FEV1 < 50%), >35 anni | Outpatient Respiratory Unit | 30 min session twice a day for 12 days | 26 weeks | 120 | 35 | 33 | 36 | 72.15 (1.20) | 71.13 (1.90) | 70.67 (2.10) | 72.5 | 75 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepiacci, A.; Starc, N.; Laitano, R.; Pasqua, F.; Rogliani, P.; Ora, J. Systematic Review and Meta-Analysis of the Application of T-PEP in the Therapeutic Management of COPD Patients. J. Clin. Med. 2025, 14, 320. https://doi.org/10.3390/jcm14020320
Sepiacci A, Starc N, Laitano R, Pasqua F, Rogliani P, Ora J. Systematic Review and Meta-Analysis of the Application of T-PEP in the Therapeutic Management of COPD Patients. Journal of Clinical Medicine. 2025; 14(2):320. https://doi.org/10.3390/jcm14020320
Chicago/Turabian StyleSepiacci, Arianna, Nadia Starc, Rossella Laitano, Franco Pasqua, Paola Rogliani, and Josuel Ora. 2025. "Systematic Review and Meta-Analysis of the Application of T-PEP in the Therapeutic Management of COPD Patients" Journal of Clinical Medicine 14, no. 2: 320. https://doi.org/10.3390/jcm14020320
APA StyleSepiacci, A., Starc, N., Laitano, R., Pasqua, F., Rogliani, P., & Ora, J. (2025). Systematic Review and Meta-Analysis of the Application of T-PEP in the Therapeutic Management of COPD Patients. Journal of Clinical Medicine, 14(2), 320. https://doi.org/10.3390/jcm14020320






