Retrospective Single-Center Analysis of 5575 Spinal Surgeries for Complication Associations and Potential Future Use of Generated Data
Abstract
1. Introduction
2. Materials and Methods
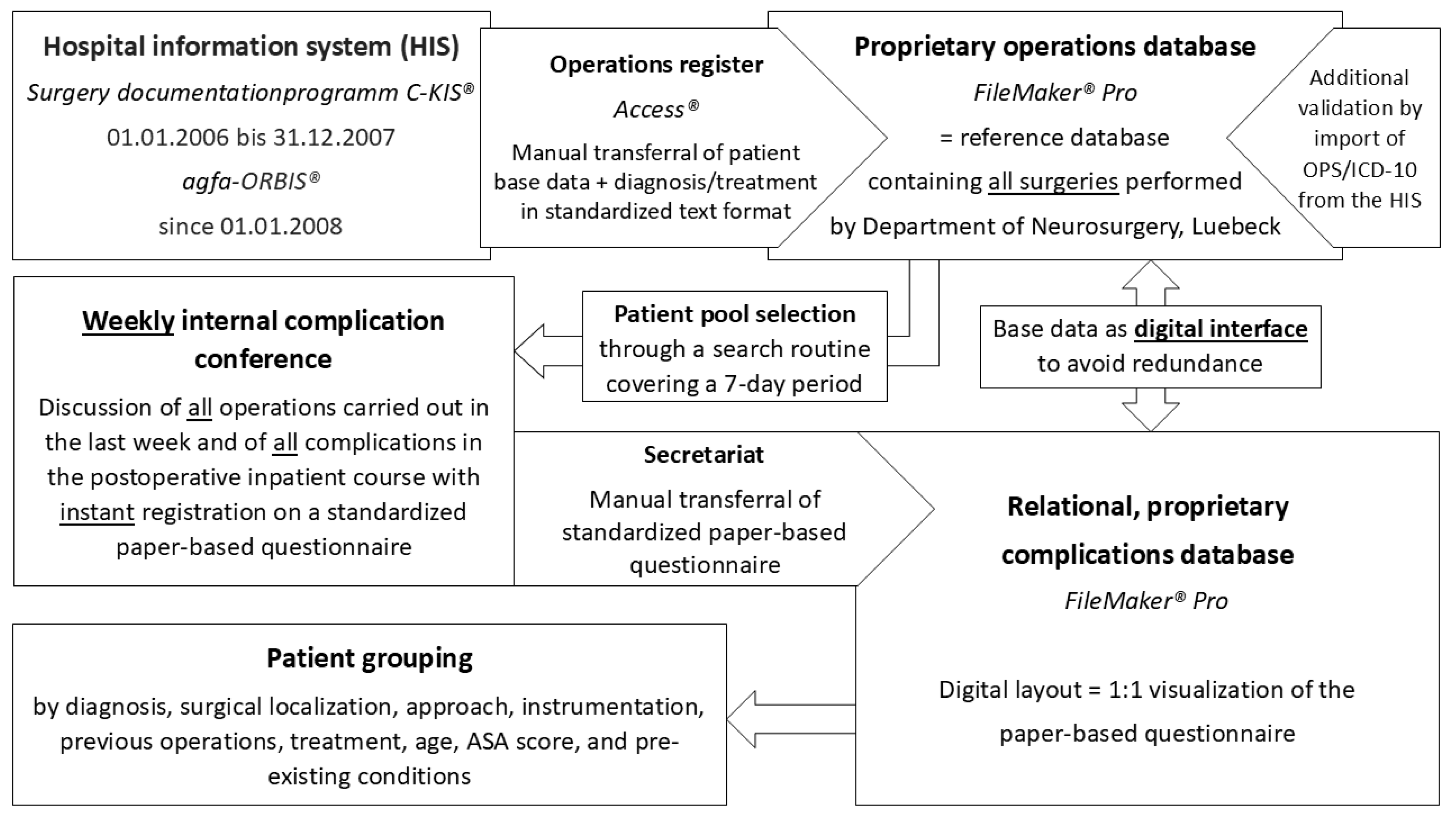
2.1. Data Generation
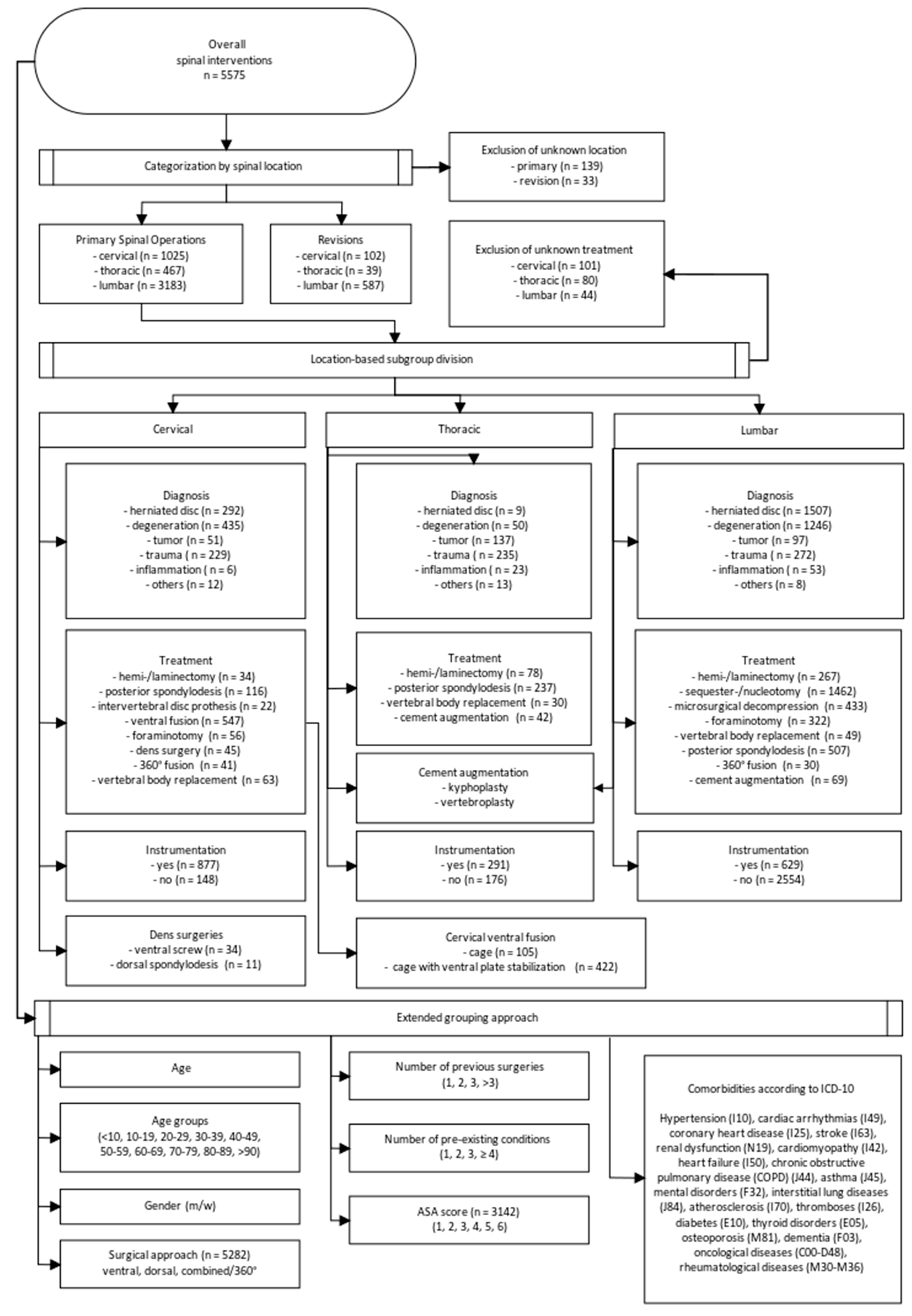
2.2. Patient Grouping
2.3. Complication Definition
2.4. Statistical Evaluation
2.5. Literature Analysis
3. Results
3.1. Overall Cohort Characteristics, Complications, and Mortality
3.2. Identified Associations with Complications
3.3. Age
3.4. Localization
3.5. Instrumentation
3.6. ASA
3.7. Surgical Approach
3.8. Pre-Existing Medical Conditions
3.9. Revision Surgery
4. Discussion
4.1. Risk Stratification and Risk Adjustment
4.2. Future Practical Use of Generated Data
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kohn, L.T.; Corrigan, J.; Donaldson, M.S. To Err Is Human; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Grawe, P. 20 Years After to Err is Human: A Bibliometric Analysis of the IOM Report’s Impact on Research on Patient Safety. Dissertations Theses, Friedrich-Alexander-University Erlangen-Nuernberg (FAU), Erlangen, Germany, 2022. [Google Scholar]
- Bonsanto, M.M.; Tronnier, V.M. Künstliche Intelligenz in der Neurochirurgie. Der Chir. 2020, 91, 229–234. [Google Scholar] [CrossRef]
- von Knebel Doeberitz, M. Menschliche und künstliche Intelligenz in der Medizin. Intell. Theor. Grund. Und Prakt. Anwendungen 2022, 6, 379–392. [Google Scholar] [CrossRef]
- Bonsanto, M.M.; Hamer, J.; Tronnier, V.M.; Kunze, S.A. Complication Conference for Internal Quality Control at the Neurosurgical Department of the University of Heidelberg. In Risk Control and Quality Management in Neurosurgery; Springer: Vienna, Austria, 2001; pp. 139–145. [Google Scholar] [CrossRef]
- Nohara, Y.; Taneichi, H.; Ueyama, K.; Kawahara, N.; Shiba, K.; Tokuhashil, Y.; Tani, T.; Nakahara, S.; Iida, T. Nationwide survey on complications of spine surgery in Japan. J. Orthop. Sci. 2004, 9, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Nasser, R.; Yadla, S.; Maltenfort, M.G.; Harrop, J.S.; Anderson, D.G.; Vaccaro, A.R.; Sharan, A.D.; Ratliff, J.K. Complications in spine surgery. J. Neurosurg. Spine 2010, 13, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Carey, P.A.; Cleveland, A.W.; Bader, J.O.; Bono, C.M. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: A prognostic study based on 5887 patients. Spine J. 2013, 13, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, J.A.; Kim, C.W. Complications Associated with the Initial Learning Curve of Minimally Invasive Spine Surgery: A Systematic Review. Clin. Orthop. Relat. Res. 2014, 472, 1711–1717. [Google Scholar] [CrossRef]
- Kimmell, K.T.; Algattas, H.; Joynt, P.; Schmidt, T.; Jahromi, B.S.; Silberstein, H.J.; Vates, G.E. Risk Modeling Predicts Complication Rates for Spinal Surgery. Spine 2015, 40, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Sarnthein, J.; Staartjes, V.E.; Regli, L.; Akeret, K.; Bektas, D.; Bellut, D.; Bichsel, O.; Bozinov, O.; Colombo, E.; Dias, S.; et al. Neurosurgery outcomes and complications in a monocentric 7-year patient registry. Brain Spine 2022, 2, 100860. [Google Scholar] [CrossRef]
- Wang, M.C.; Chan, L.; Maiman, D.J.; Kreuter, W.; Deyo, R. Complications and Mortality Associated with Cervical Spine Surgery for Degenerative Disease in the United States. Spine 2007, 32, 342–347. [Google Scholar] [CrossRef]
- Smith, J.S.; Saulle, D.; Chen, C.-J.; Lenke, L.G.; Polly, D.W.; Kasliwal, M.K.; Broadstone, P.A.; Glassman, S.D.; Vaccaro, A.R.; Ames, C.P.; et al. Rates and Causes of Mortality Associated with Spine Surgery Based on 108,419 Procedures. Spine 2012, 37, 1975–1982. [Google Scholar] [CrossRef]
- Goz, V.; Weinreb, J.H.; McCarthy, I.; Schwab, F.; Lafage, V.; Errico, T.J. Perioperative Complications and Mortality After Spinal Fusions. Spine 2013, 38, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Raffo, C.S.; Lauerman, W.C. Predicting Morbidity and Mortality of Lumbar Spine Arthrodesis in Patients in Their Ninth Decade. Spine 2006, 31, 99–103. [Google Scholar] [CrossRef]
- Sobottke, R.; Aghayev, E.; Röder, C.; Eysel, P.; Delank, S.K.; Zweig, T. Predictors of surgical, general and follow-up complications in lumbar spinal stenosis relative to patient age as emerged from the Spine Tango Registry. Eur. Spine J. 2012, 21, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Konodi, M.A.; Cizik, A.M.; Bransford, R.J.; Bellabarba, C.; Chapman, J.R. Risk factors for medical complication after spine surgery: A multivariate analysis of 1591 patients. Spine J. 2012, 12, 197–206. [Google Scholar] [CrossRef]
- Tetreault, L.; Kopjar, B.; Côté, P.; Arnold, P.; Fehlings, M.G. A Clinical Prediction Rule for Functional Outcomes in Patients Undergoing Surgery for Degenerative Cervical Myelopathy. J. Bone Jt. Surg. 2015, 97, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.G.; Yadla, S.; Nasser, R.; Malone, J.; Maltenfort, M.G.; Ratliff, J.K. Patient comorbidity score predicting the incidence of perioperative complications: Assessing the impact of comorbidities on complications in spine surgery. J. Neurosurg. Spine 2012, 16, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Konodi, M.A.; Cizik, A.M.; Weinreich, M.A.; Bransford, R.J.; Bellabarba, C.; Chapman, J. Risk factors for medical complication after cervical spine surgery: A multivariate analysis of 582 patients. Spine 2013, 38, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Hammer, C.; Heller, J.; Kepler, C. Epidemiology and pathophysiology of cervical disc herniation. Semin. Spine Surg. 2016, 28, 64–67. [Google Scholar] [CrossRef]
- Skaf, G.S.; Ayoub, C.M.; Domloj, N.T.; Turbay, M.J.; El-Zein, C.; Hourani, M.H. Effect of Age and Lordotic Angle on the Level of Lumbar Disc Herniation. Adv. Orthop. 2011, 2011, 950576. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.P.; Peul, W.C. Vertebral Body Replacement Systems with Expandable Cages in the Treatment of Various Spinal Pathologies. Neurosurgery 2008, 63, 537–545. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Acosta, F.L.; Ames, C.P. Complications and Outcomes of Lumbar Spine Surgery in Elderly People: A Review of the Literature. J. Am. Geriatr. Soc. 2008, 56, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Conan, Y.; Laurent, E.; Belin, Y.; Lacasse, M.; Amelot, A.; Mulleman, D.; Rosset, P.; Bernard, L.; Grammatico-Guillon, L. Large increase of vertebral osteomyelitis in France: A 2010–2019 cross-sectional study. Epidemiol. Infect. 2021, 149, e227. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Cizik, A.M.; Hamilton, D.; Chapman, J.R. Predicting medical complications after spine surgery: A validated model using a prospective surgical registry. Spine J. 2014, 14, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Uakritdathikarn, T. Perioperative Morbidity and Mortality in Patients Undergoing Spinal Surgery at Vachiraphuket Hospital: Retrospective Review in 300 Cases. Thai J. Anesth. 2023, 49, 109–118. [Google Scholar]
- Wong, A.P.; Smith, Z.A.; Nixon, A.T.; Lawton, C.D.; Dahdaleh, N.S.; Wong, R.H.; Auffinger, B.; Lam, S.; Song, J.K.; Liu, J.C.; et al. Intraoperative and perioperative complications in minimally invasive transforaminal lumbar interbody fusion: A review of 513 patients. J. Neurosurg. Spine 2015, 22, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Grob, D.; Scheier, H.J.G.; Dvorak, J.; Siegrist, H.; Rubeli, M.; Joller, R. Circumferential fusion of the lumbar and lumbosacral spinet. Arch. Orthop. Trauma Surg. 1991, 111, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Rumalla, K.; Yarbrough, C.K.; Pugely, A.J.; Dorward, I.G. Spinal Fusion for Pediatric Spondylolisthesis: National Trends, Complications, and Short-Term Outcomes. Neurosurgery 2018, 82, 701–709. [Google Scholar] [CrossRef]
- Ayling, O.G.S.; Charest-Morin, R.; Eagles, M.E.; Ailon, T.; Street, J.T.; Dea, N.; McIntosh, G.; Christie, S.D.; Abraham, E.; Jacobs, W.B.; et al. National adverse event profile after lumbar spine surgery for lumbar degenerative disorders and comparison of complication rates between hospitals: A CSORN registry study. J. Neurosurg. Spine 2021, 35, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Gray, D.T.; Kreuter, W.; Mirza, S.; Martin, B.I. United States Trends in Lumbar Fusion Surgery for Degenerative Conditions. Spine 2005, 30, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I.; Kreuter, W.; Goodman, D.C.; Jarvik, J.G. Trends, Major Medical Complications, and Charges Associated with Surgery for Lumbar Spinal Stenosis in Older Adults. JAMA 2010, 303, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Yadla, S.; Maltenfort, M.G.; Ratliff, J.K.; Harrop, J.S. Adult scoliosis surgery outcomes: A systematic review. Neurosurg. Focus 2010, 28, E3. [Google Scholar] [CrossRef]
- Campbell, P.G.; Yadla, S.; Malone, J.; Maltenfort, M.G.; Harrop, J.S.; Sharan, A.D.; Ratliff, J.K. Complications related to instrumentation in spine surgery: A prospective analysis. Neurosurg. Focus 2011, 31, E10. [Google Scholar] [CrossRef]
- Whitmore, R.G.; Stephen, J.H.; Vernick, C.; Campbell, P.G.; Yadla, S.; Ghobrial, G.M.; Maltenfort, M.G.; Ratliff, J.K. ASA grade and Charlson Comorbidity Index of spinal surgery patients: Correlation with complications and societal costs. Spine J. 2014, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Memtsoudis, S.G.; Vougioukas, V.I.; Ma, Y.; Gaber-Baylis, L.K.; Girardi, F.P. Perioperative Morbidity and Mortality After Anterior, Posterior, and Anterior/Posterior Spine Fusion Surgery. Spine 2011, 36, 1867–1877. [Google Scholar] [CrossRef]
- Glassman, S.D.; Alegre, G.; Carreon, L.; Dimar, J.R.; Johnson, J.R. Perioperative complications of lumbar instrumentation and fusion in patients with diabetes mellitus. Spine J. 2003, 3, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, B.T.; Zarrabian, M.; Aleem, I.S.; Fogelson, J.L.; Currier, B.L.; Freedman, B.A.; Bydon, M.; Nassr, A. Incidence of Osteoporosis-Related Complications Following Posterior Lumbar Fusion. Glob. Spine J. 2018, 8, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Linville, D.A.; Bridwell, K.H.; Lenke, L.G.; Vedantam, R.; Leicht, P. Complications in the Adult Spinal Deformity Patient Having Combined Surgery. Spine 1999, 24, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Proietti, L.; Scaramuzzo, L.; Schiro, G.R.; Sessa, S.; Logroscino, C.A. Complications in lumbar spine surgery: A retrospective analysis. Indian J. Orthop. 2013, 47, 340–345. [Google Scholar] [CrossRef]
- Lauterbach, K.W. Evidence-based policy-making—Epidemiology as a key science for quality of life in society. Eur. J. Epidemiol. 2023, 38, 1205–1212. [Google Scholar] [CrossRef]
- Rampersaud, Y.R.; Neary, M.A.; White, K. Spine Adverse Events Severity System. Spine 2010, 35, 790–795. [Google Scholar] [CrossRef]
- Rampersaud, Y.R.; Anderson, P.A.; Dimar, J.R.; Fisher, C.G. Spinal adverse events severity system, version 2 (SAVES-V2): Inter- and intraobserver reliability assessment. J. Neurosurg. Spine 2016, 25, 256–263. [Google Scholar] [CrossRef]
- Gillon, R. Medical ethics: Four principles plus attention to scope. BMJ 1994, 309, 184. [Google Scholar] [CrossRef] [PubMed]
- Parzeller, M.; Wenk, M.; Zedler, B.; Rothschild, M. Aufklärung und Einwilligung bei ärztlichen Eingriffen. Dtsch. Arztebl. 2007, 104, 576–586. [Google Scholar]
- Marewski, J.N.; Gigerenzer, G. Heuristic decision making in medicine. Dialogues Clin. Neurosci. 2012, 14, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Ikwuezunma, I.; Puvanesarajah, V.; Babu, J.; Margalit, A.; Raad, M.; Jain, A. Using Predictive Modeling and Supervised Machine Learning to Identify Patients at Risk for Venous Thromboembolism Following Posterior Lumbar Fusion. Glob. Spine J. 2023, 13, 1097–1103. [Google Scholar] [CrossRef]
- Saravi, B.; Hassel, F.; Ülkümen, S.; Zink, A.; Shavlokhova, V.; Couillard-Despres, S.; Boeker, M.; Obid, P.; Lang, G. Artificial Intelligence-Driven Prediction Modeling and Decision Making in Spine Surgery Using Hybrid Machine Learning Models. J. Pers. Med. 2022, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Ethikrat, D. Mensch und Maschine—Herausforderungen durch Künstliche Intelligenz—Stellungnahme; Herausgegeben vom Deutschen Ethikrat: Berlin, Germany, 2023; pp. 7–286. [Google Scholar]
- Dao Trong, P.; Olivares, A.; El Damaty, A.; Unterberg, A. Adverse events in neurosurgery: A comprehensive single-center analysis of a prospectively compiled database. Acta Neurochir. 2023, 165, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kashiwazaki, D.; Tomita, T.; Hori, E.; Akioka, N.; Akai, T.; Kuroda, S. Impact of system approach and personal performance on preventable morbidity and mortality events in neurosurgery patients. Acta Neurochir. 2022, 164, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Al Saiegh, F.; Mazza, J.; Hafazalla, K.; Baldassari, M.P.; Theofanis, T.; Ye, D.; Hoelscher, C.; Harrop, J.S.; Evans, J.J.; Jabbour, P.; et al. Developing standardized titles to classify the adverse events in 7418 cranial and spinal neurosurgical procedures. Clin. Neurol. Neurosurg. 2020, 198, 106121. [Google Scholar] [CrossRef] [PubMed]
| Category | Subcategory | Subgroup | p | OR | 95% CI |
|---|---|---|---|---|---|
| Age in years | <0.001 | 1.02 | 1.01–1.02 | ||
| Age group | 60–69 | <0.001 | 3.08 | 1.64–5.78 | |
| 70–79 | 0.002 | 2.75 | 1.47–5.15 | ||
| 80–89 | <0.001 | 3.51 | 1.82–6.74 | ||
| Localization | cervical | <0.001 | 1.91 | 1.54–2.36 | |
| thoracic | <0.001 | 2.15 | 1.63–2.82 | ||
| Diagnosis | cervical | tumor | <0.001 | 4.71 | 2.34–9.5 |
| trauma | 0.001 | 2.31 | 1.39–3.83 | ||
| lumbar | degeneration | <0.001 | 1.68 | 1.27–2.22 | |
| tumor | 0.003 | 2.54 | 1.39–4.64 | ||
| trauma | 0.005 | 1.86 | 1.21–2.87 | ||
| infection | 0.003 | 3.07 | 1.46–6.49 | ||
| Treatment | cervical | hemi-/laminectomy | <0.001 | 4.93 | 2.38–10.22 |
| vertebral body replacement | 0.002 | 2.60 | 1.41–4.8 | ||
| lumbar | hemi-/laminectomy | 0.002 | 2.00 | 1.29–3.1 | |
| posterior spondylodesis | <0.001 | 2.83 | 2.04–3.92 | ||
| 360° fusion | <0.001 | 4.81 | 2.01–11.52 | ||
| Instrumentation | lumbar | yes | <0.001 | 2.39 | 1.83–3.12 |
| ASA score | 3 | <0.001 | 2.66 | 1.73–4.1 | |
| 4 | <0.001 | 6.39 | 2.63–15.53 | ||
| Surgical approach | primary | ventral | 0.001 | 1.48 | 1.17–1.87 |
| combined/360° | 0.002 | 2.02 | 1.69–3.60 | ||
| revision–lumbar | combined/360° | <0.001 | 8.39 | 2.72–25.89 | |
| Pre-existing | 2 | <0.001 | 1.68 | 1.32–2.13 | |
| medical conditions | 3 | <0.001 | 2.52 | 1.91–3.31 | |
| ≥4 | <0.001 | 2.06 | 1.42–3.00 | ||
| comorbidities | hypertension | 0.034 * | 1.22 | 1.02–1.46 | |
| osteoporosis | 0.037 * | 1.33 | 1.02–1.74 | ||
| arrhythmias | 0.005 * | 1.57 | 1.15–2.14 | ||
| oncological | 0.025 * | 1.61 | 1.06–2.43 | ||
| renal dysfunction | 0.022 * | 1.69 | 1.08–2.64 | ||
| stroke history | <0.001 * | 3.09 | 1.81–5.29 | ||
| thrombosis | 0.028 * | 2.13 | 1.08–4.18 |
| Average ± SD | Complication n (%) | p | OR | 95% CI | |
|---|---|---|---|---|---|
| Age in years | 63.19 ± 15.54 | 610 (10.9%) | <0.001 | 1.02 | 1.01–1.02 |
| Age groups in years | Overall n (%) | ||||
| <9 | 31 (0.56%) | 6 (19.35%) | 0.006 | 4.54 | 1.54–13.33 |
| 10–19 | 75 (1.35%) | 1 (1.33%) | 0.195 | 0.26 | 0.03–2.01 |
| 20–29 | 219 (3.93%) | 11(5.02%) | Ref | - | - |
| 30–39 | 474 (8.5%) | 31 (6.54%) | 0.438 | 1.32 | 0.65–2.68 |
| 40–49 | 827 (14.83%) | 62 (7.50%) | 0.204 | 1.53 | 0.79–2.96 |
| 50–59 | 1007 (18.06%) | 96 (9.53%) | 0.035 | 1.99 | 1.05–3.79 |
| 60–69 | 1113 (19.96%) | 156 (14.02%) | <0.001 | 3.08 | 1.64–5.78 |
| 70–79 | 1331 (23.87%) | 169 (12.70%) | 0.002 | 2.75 | 1.47–5.15 |
| 80–89 | 486 (8.72%) | 76 (15.64%) | <0.001 | 3.51 | 1.82–6.74 |
| >90 | 12 (0.22%) | 2 (16.67%) | 0.111 | 3.78 | 0.74–19.40 |
| Overall n (%) | Complication n (%) | p | OR | 95% CI | |
|---|---|---|---|---|---|
| ASA Score | 3142 (100%) | 363 (11.55%) | |||
| 1 | 377 (12.0%) | 26 (6.9%) | Ref | - | - |
| 2 | 1741 (55.41%) | 164 (9.42%) | 0.122 | 1.4 | 0.91–2.16 |
| 3 | 996 (31.7%) | 164 (16.47%) | <0.001 | 2.66 | 1.73–4.10 |
| 4 | 28 (0.89%) | 9 (32.14%) | <0.001 | 6.39 | 2.63–15.53 |
| Pre-Existing- Condition | Overall n (%) | Complication n (%) | p | OR | 95% CI |
|---|---|---|---|---|---|
| Number | 5575 (100%) | 610 (10.94%) | |||
| 0 | 2526 (45.31%) | 210 (8.31%) | Ref | - | - |
| 1 | 1437 (25.78%) | 156 (10.86%) | 0.008 | 1.34 | 1.08–1.67 |
| 2 | 908 (16.29%) | 120 (13.22%) | <0.001 | 1.68 | 1.32–2.13 |
| 3 | 463 (8.3%) | 86 (18.57%) | <0.001 | 2.52 | 1.91–3.31 |
| ≥4 | 241 (4.32%) | 38 (15.77%) | <0.001 | 2.06 | 1.42–3.00 |
| Overall n (%) | Complication n (%) | p | OR | 95% CI | |
|---|---|---|---|---|---|
| Pre- Existing- Condition | 5575 (100%) | 610 (10,94%) | |||
| Art. hypertension | 2284 (40.97%) | 296 (12.96%) | 0.034 | 1.22 | 1.02–1.46 |
| Diabetes mellitus | 749 (13.43%) | 102 (13.62%) | 0.406 | 1.11 | 0.87–1.41 |
| Coronary heart disease | 506 (9.08%) | 70 (13.83%) | 0.660 | 1.07 | 0.80–1.42 |
| Osteoporosis | 487 (8.74%) | 73 (14.95%) | 0.037 | 1.33 | 1.02–1.74 |
| Thyroid disorder | 375 (6.73%) | 56 (14.93%) | 0.147 | 1.25 | 0.92–1.70 |
| Arrythmia | 318 (5.70%) | 60 (18.87%) | 0.005 | 1.57 | 1.15–2.14 |
| Oncologic condition | 169 (3.03%) | 29 (17.16%) | 0.025 | 1.61 | 1.06–2.43 |
| Renal dysfunction | 133 (2.39%) | 27 (20.3%) | 0.022 | 1.69 | 1.08–2.64 |
| Stroke in history | 68 (1.22%) | 20 (29.41%) | <0.001 | 3.09 | 1.81–5.29 |
| Thrombosis | 48 (0.86%) | 12 (25.0%) | 0.028 | 2.13 | 1.08–4.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Materlik, Y.; Tronnier, V.M.; Bonsanto, M.M. Retrospective Single-Center Analysis of 5575 Spinal Surgeries for Complication Associations and Potential Future Use of Generated Data. J. Clin. Med. 2025, 14, 312. https://doi.org/10.3390/jcm14020312
Materlik Y, Tronnier VM, Bonsanto MM. Retrospective Single-Center Analysis of 5575 Spinal Surgeries for Complication Associations and Potential Future Use of Generated Data. Journal of Clinical Medicine. 2025; 14(2):312. https://doi.org/10.3390/jcm14020312
Chicago/Turabian StyleMaterlik, Yoram, Volker Martin Tronnier, and Matteo Mario Bonsanto. 2025. "Retrospective Single-Center Analysis of 5575 Spinal Surgeries for Complication Associations and Potential Future Use of Generated Data" Journal of Clinical Medicine 14, no. 2: 312. https://doi.org/10.3390/jcm14020312
APA StyleMaterlik, Y., Tronnier, V. M., & Bonsanto, M. M. (2025). Retrospective Single-Center Analysis of 5575 Spinal Surgeries for Complication Associations and Potential Future Use of Generated Data. Journal of Clinical Medicine, 14(2), 312. https://doi.org/10.3390/jcm14020312





