Structural Equation Modelling of Retinopathy of Prematurity Treatment Integrating Both Physical and Clinical Effects
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Ophthalmologic Examination
2.3. Categorisation of the Risk Factors for ROP
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Univariate Analysis of the Association Between Treated ROP and Risk Factors
3.3. Decision Tree Analysis of ROP Treatment
3.4. Multivariate Logistic Regression Analysis of the Association Between Treated ROP and Risk Factors
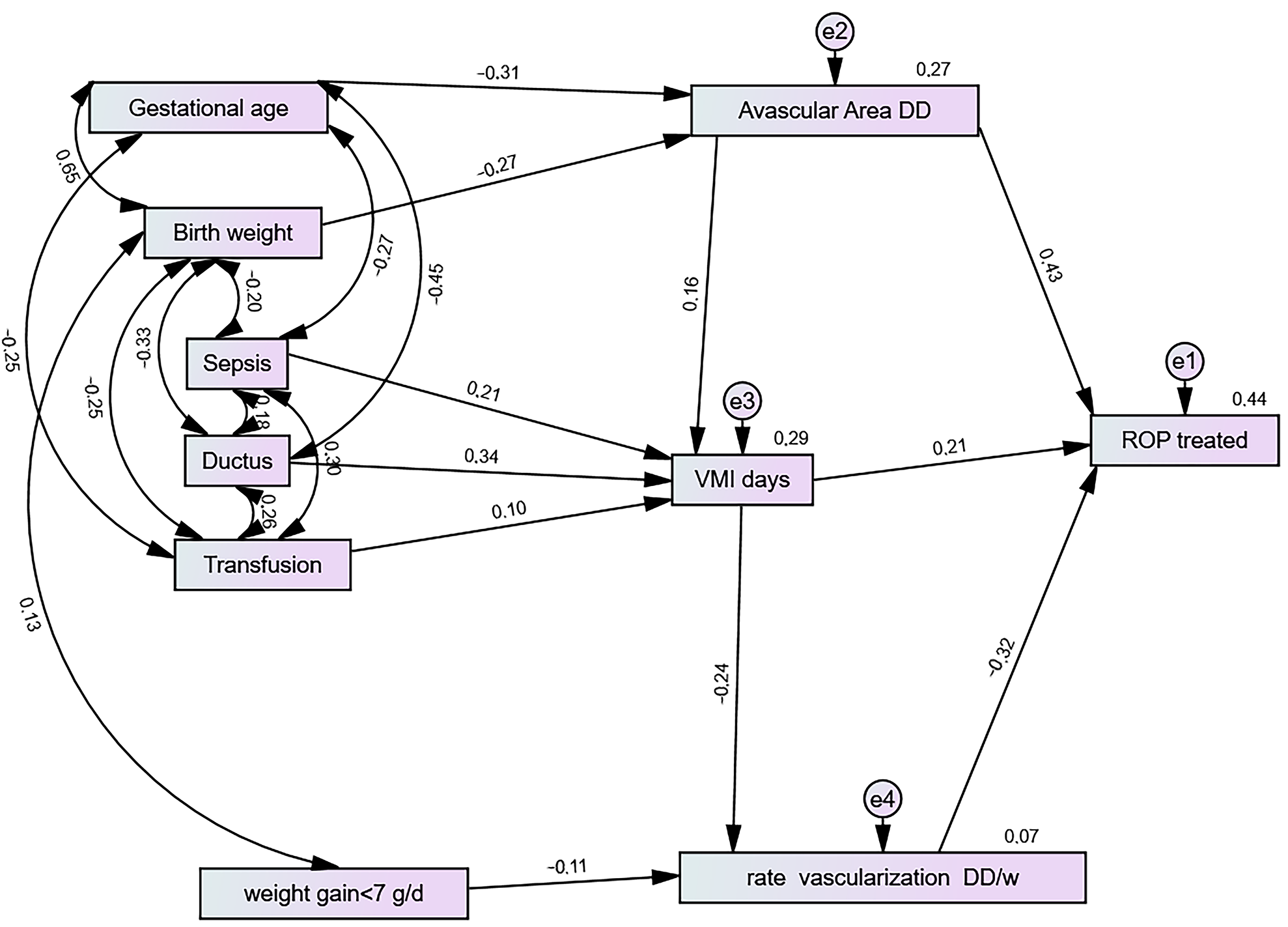
3.5. Structural Equation and Risk Factors for ROP Treatment
3.6. Avascular Area (DD)
3.7. Vascularisation Rate (DD/w)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Good, W.V. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. 2004, 102, 233–248. [Google Scholar] [PubMed]
- Hutchinson, A.K.; Melia, M.; Yang, M.B.; VanderVeen, D.K.; Wilson, L.B.; Lambert, S.R. Clinical models and algorithms for the prediction of retinopathy of prematurity: A report by the American Academy of Ophthalmology. Ophthalmology 2016, 123, 804–816. [Google Scholar] [CrossRef]
- Wu, R.; Chen, H.; Bai, Y.; Zhang, Y.; Feng, S.; Lu, X. Prediction models for retinopathy of prematurity occurrence based on artificial neural network. BMC Ophthalmol. 2024, 24, 323. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Anderson, J.E.; Coyner, A.S.; Ostmo, S.; Sonmez, K.; Erdogmus, D.; Jordan, B.K.; McEvoy, C.T.; Dukhovny, D.; Schelonka, R.L.; et al. Quantification of early neonatal oxygen exposure as a risk factor. Retinopathy of prematurity requiring treatment. Ophthalmol. Sci. 2021, 1, 100070. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.; Härtel, C.; Klingenberg, C.; Herting, E.; Fortmann, M.I.; Speer, C.P.; Stensvold, H.J.; Huncikova, Z.; Rønnestad, A.E.; Nentwich, M.M.; et al. Neonatal sepsis episodes and retinopathy of prematurity in very preterm infants. JAMA Netw. Open 2024, 7, e2423933. [Google Scholar] [CrossRef] [PubMed]
- Protsyk, O.; García-Serrano, J.L. Mechanical Ventilation, Retinal avascularity and rate of vascularisation: A triad of predictors for retinopathy of prematurity treatment. J. Pers. Med. 2024, 14, 379. [Google Scholar] [CrossRef]
- Padhi, T.R.; Bhusal, U.; Padhy, S.K.; Patel, A.; Kelgaonker, A.; Khalsa, A.; Das, T.; Kapil, V.; Shah, M.; Sugumar, S.; et al. The retinal vascular growth rate in babies with retinopathy of prematurity could indicate treatment need. Indian J. Ophthalmol. 2022, 70, 1270–1277. [Google Scholar] [CrossRef]
- Kim, S.J.; Port, A.D.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [CrossRef]
- Matsumoto, T.; Saito, Y.; Itokawa, T.; Shiba, T.; Oba, M.S.; Takahashi, H.; Hori, Y. Retina VEGF levels correlate with ocular circulation measured by a laser speckle-micro system in an oxygen-induced retinopathy rat model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1981–1990. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, Y.; Su, L.; Chen, X.; Shao, X.; Ea, V.; Shang, Z.; Zhang, X.; Barnstable, C.J.; Li, X.; et al. Novel regulators of retina neovascularization: A proteomics approach. J. Proteome Res. 2022, 21, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E. Pathophysiology of retinopathy of prematurity. Annu. Rev. Vis. 2023, 9, 39–70. [Google Scholar] [CrossRef]
- Nayeri, U.A.; Buhimschi, C.S.; Zhao, G.; Buhimschi, I.A.; Bhandari, V. Components of the antepartum, intrapartum, and postpartum exposome impact on distinct short-term adverse neonatal outcomes of premature infants: A prospective cohort study. PLoS ONE 2018, 13, e0207298. [Google Scholar] [CrossRef]
- Calzi, S.L.; Shaw, L.C.; Moldovan, L.; Shelley, W.C.; Qi, X.; Racette, L.; Quigley, J.L.; Fortmann, S.D.; Boulton, M.E.; Yoder, M.C.; et al. Progenitor cell combination normalizes retinal vascular development in the oxygen-induced retinopathy (OIR) model. JCI Insight 2019, 4, e129224. [Google Scholar] [CrossRef] [PubMed]
- Fierson, W.M. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2018, 143, e20183061. [Google Scholar] [CrossRef] [PubMed]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Wang, S.K.; Korot, E.; Zaidi, M.; Ji, M.H.; Al-Moujahed, A.; Callaway, N.F.; Kumm, J.; Moshfeghi, D.M. Modeling absolute zone size in retinopathy of prematurity in relation to axial length. Sci. Rep. 2022, 12, 4717. [Google Scholar] [CrossRef]
- Oliveira, A.A.; Almeida, M.F.; Silva, Z.P.D.; Assunção, P.L.; Silva, A.M.R.; Santos, H.G.D.; Alencar, G.P. Factors associated with preterm birth; from logistic regression to structural equation modeling. Cad. Saude Publica 2019, 35, e00211917. [Google Scholar] [CrossRef]
- Bentler, P.M.; Stein, J.A. Structural equation model in medical research. Stat. Methods Med. Res. 1992, 1, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Y. RMSA, CFI, and TLI in structural equation modeling with ordered categorical data: The story they tell depends on the estimation method used. Behav. Res. Methods 2019, 51, 409–428. [Google Scholar] [CrossRef]
- Peugh, J.; Fedon, D.F. “How well does your structural equation model fit your data?”: Is Marcoulides and Yuan’s Equivalence Test the answer? CBE Life Sci. Educ. 2020, 19, es5. [Google Scholar] [CrossRef] [PubMed]
- Seyghalani Talab, F.; Ahadinezhad, B.; Khosravizadeh, O.; Amerzadeh, M. A Model of the organizational resilience of hospitals in emergencies and disasters. BMC Emerg. Med. 2024, 24, 105. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, D.B.; Palmer, E.A.; Plotsky, D.F.; Metz, H.S.; Flynn, J.T.; Tung, B.; Hardy, R.J. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1993, 100, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Robles-Holmes, H.; Coyner, A.S.; Campbell, J.P.; Nudleman, E. Imaging features associated with persistent avascular retina in retinopathy of prematurity. Ophthalmology 2024, 131, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Yehiam, S.Z.; Simkin, S.K.; Al-Taie, R.; Wong, M.; Battin, M.; Dai, S. Retina vascularisation in retinopathy of prematurity: It is the consequence of changing oxygen saturation. Front. Pediatr. 2023, 11, 1203068. [Google Scholar] [CrossRef]
- Ortega-Molina, J.M.; de Solans-Perez Larraya, A.; Salgado-Miranda, A.; Jerez-Calero, A.; Uberos-Fernandez, J.; Gonzalez Ramirez, A.R.; García-Serrano, J.L. Risk factors of retinopathy of prematurity associated with delayed retinal vascular development. Iran J. Pediatr. 2017, 27, e7642. [Google Scholar] [CrossRef]
- Solans Pérez de Larraya, A.M.; Ortega-Molina, J.M.; Fernández, J.U.; Escudero-Gómez, J.; Salgado-Miranda, A.D.; Chaves-Samaniego, M.J.; Serrano, J.L.G. Retinal vascular speed < 0.5 disc diameter per week as an early sign of retinopathy of prematurity requiring treatment. Eur. J. Ophthalmol. 2018, 28, 441–445. [Google Scholar]
- Zhang, X.; Peng, J.; Yang, Y.; Liu, Y.; Zhang, W.; Gu, V.Y.; Liu, H.; Xiao, H.; Yin, J.; Xu, Y.; et al. Vascular development analysis: Tertiary anti-vascular endothelial growth factor therapy second reactivation of premature birth retinopathy. Front. Med. 2024, 11, 1421894. [Google Scholar] [CrossRef] [PubMed]
- Trzcionkowska, K.; Groenendaal, F.; Andriessen, P.; Dijk, P.H.; van den Dungen, F.A.; van Hillegersberg, J.L.; Koole, S.; Kornelisse, R.F.; van Westering-Kroon, E.; von Lindern, J.S.; et al. Risk factors for retinopathy of prematurity in the Netherlands: A comparison of two cohorts. Neonatology 2021, 118, 462–469. [Google Scholar] [CrossRef]
- De Las Rivas Ramírez, N.; Luque-Aranda, G.; Rius-Díaz, F.; Pérez-Frías, F.J.; Sánchez-Tamayo, T. Risk factors associated with retinopathy of premature development and progression. Sci. Rep. 2022, 12, 21977. [Google Scholar] [CrossRef]
- Golubinskaya, V.; Nilsson, H.; Rydbeck, H.; Hellström, W.; Hellgren, G.; Hellström, A.; Sävman, K.; Mallard, C. Cytokine and growth factor correlation networks associated with morbidities in extremely preterm infants. BMC Pediatr. 2024, 24, 723. [Google Scholar] [CrossRef]
- Srejovic, J.V.; Muric, M.D.; Jakovljevic, V.L.; Srejovic, I.M.; Sreckovic, S.B.; Petrovic, N.T.; Todorovic, D.Z.; Bolevich, S.B.; Vulovic, T.S.S. Molecular and cellular mechanisms involved in the pathophysiology of retinal vascular disease-interplay between inflammation and oxidative stress. Int. J. Mol. Sci. 2024, 25, 11850. [Google Scholar] [CrossRef] [PubMed]
- Moir, J.T.; Hyman, M.J.; Skondra, D.; Rodriguez, S.H. Risk factors for severe retinopathy of prematurity stratified by birth weight and gestational age in privately insured infants. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2024, 28, 104049. [Google Scholar] [CrossRef]

| Risk Factor | p Value | Odds Ratio (95% CI) | R2 Nagelkerke |
|---|---|---|---|
| Each additional diameter of avascular area (DD) | 0.000 | 2.32 (1.89–2.9) | 38.6% |
| Retinal vascularisation rate, <0.5 vs. ≥0.5 DD/w | 0.000 | 21.05 (9.6–48) | 38.1% |
| Duration of invasive respiratory support (days) | 0.000 | 1.075(1.05–1.1) | 23.0% |
| Birth weight (g)/100 | 0.000 | 0.65 (0.56–0.76) | 19.7% |
| Apnoea | 0.000 | 9.7 (4.7–19.7) | 19.1% |
| Gestational age (weeks) | 0.000 | 0.62 (0.53–0.73) | 18.3% |
| Number of associated comorbidities | 0.000 | 1.23 (1.12–1.34) | 10.9% |
| Hydrocephalus shunt | 0.000 | 13.8 (3.5–54) | 8.3% |
| Indomethacin | 0.000 | 4.3 (2.1–8.6) | 8.0% |
| Patent ductus arteriosus | 0.000 | 3.3 (1.17–10.1) | 7.4% |
| Hyaline membrane ≥ 3 | 0.000 | 3.1 (1.7–5.7) | 7.1% |
| Brain haemorrhage (yes/no) | 0.000 | 3.6 (1.8–7.2) | 6.6% |
| Transfusion | 0.001 | 2.2 (1.32–3.07) | 6.0% |
| Sepsis | 0.001 | 2.6 (1.4–4.7) | 5.4% |
| High-flow nasal cannula (days) | 0.002 | 1.03 (1.01–1.04) | 4.9% |
| Weight gain, <7 g/day, 4th postnatal week | 0.005 | 2.7 (1.3–5.5) | 3.7% |
| Surfactant treatment | 0.024 | 1.96 (1.09–3.5) | 2.6% |
| Risk Factors | p Value | Odds Ratio (95% CI) |
|---|---|---|
| Duration of invasive respiratory support, days | 0.001 | 1.05 (1.02–1.08) |
| Each additional diameter of avascular area (DD) | 0.000 | 2.3 (1.7–3.0) |
| Retinal vascularization rate, <0.5 vs. ≥0.5 DD/week | 0.000 | 20.2 (7.3–55.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Serrano, J.L.; Protsyk, O.; Domech-Serrano, T.; Uberos Fernández, J. Structural Equation Modelling of Retinopathy of Prematurity Treatment Integrating Both Physical and Clinical Effects. J. Clin. Med. 2025, 14, 297. https://doi.org/10.3390/jcm14020297
García-Serrano JL, Protsyk O, Domech-Serrano T, Uberos Fernández J. Structural Equation Modelling of Retinopathy of Prematurity Treatment Integrating Both Physical and Clinical Effects. Journal of Clinical Medicine. 2025; 14(2):297. https://doi.org/10.3390/jcm14020297
Chicago/Turabian StyleGarcía-Serrano, José Luis, Olena Protsyk, Teresa Domech-Serrano, and José Uberos Fernández. 2025. "Structural Equation Modelling of Retinopathy of Prematurity Treatment Integrating Both Physical and Clinical Effects" Journal of Clinical Medicine 14, no. 2: 297. https://doi.org/10.3390/jcm14020297
APA StyleGarcía-Serrano, J. L., Protsyk, O., Domech-Serrano, T., & Uberos Fernández, J. (2025). Structural Equation Modelling of Retinopathy of Prematurity Treatment Integrating Both Physical and Clinical Effects. Journal of Clinical Medicine, 14(2), 297. https://doi.org/10.3390/jcm14020297






