Uveitis in Longstanding Axial Spondyloarthritis and Its Association with Biologic Therapy Initiation: Data from the REGISPON-3 Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Variables
- -
- Sociodemographic variables: age, sex, education, and smoking status were collected.
- -
- Disease-related variables: disease duration (time since the onset of symptoms and the study visit), diagnosis delay (time between the onset of symptoms and the diagnosis), any episode of peripheral symptoms (i.e., arthritis, enthesitis and dactylitis), diagnosis of extra-musculoskeletal manifestations (i.e., AAU, IBD, or psoriasis), HLA-B27 status and c-reactive protein (mg/L) were collected.
- -
- Patient Reported Outcomes (PROs): Disease activity was assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [15] and the Ankylosing Spondylitis Disease Activity Score (ASDAS) [16]. Functional status was evaluated with the Bath Ankylosing Spondylitis Functional Index (BASFI) [17].
- -
- Treatment information: Data were collected on the use of non-steroidal anti-inflammatory drugs (NSAIDs), conventional synthetic DMARDs (csDMARDs), biologic DMARDs (bDMARDs), and targeted synthetic DMARDs (tsDMARDs) across the patients’ full history since disease initiation. Information on treatment initiation and discontinuation dates, types of ts/bDMARDs prescribed, and treatment duration were also obtained from clinical records and prescription data.
2.3. Outcomes
2.4. Statistical Analysis
3. Results
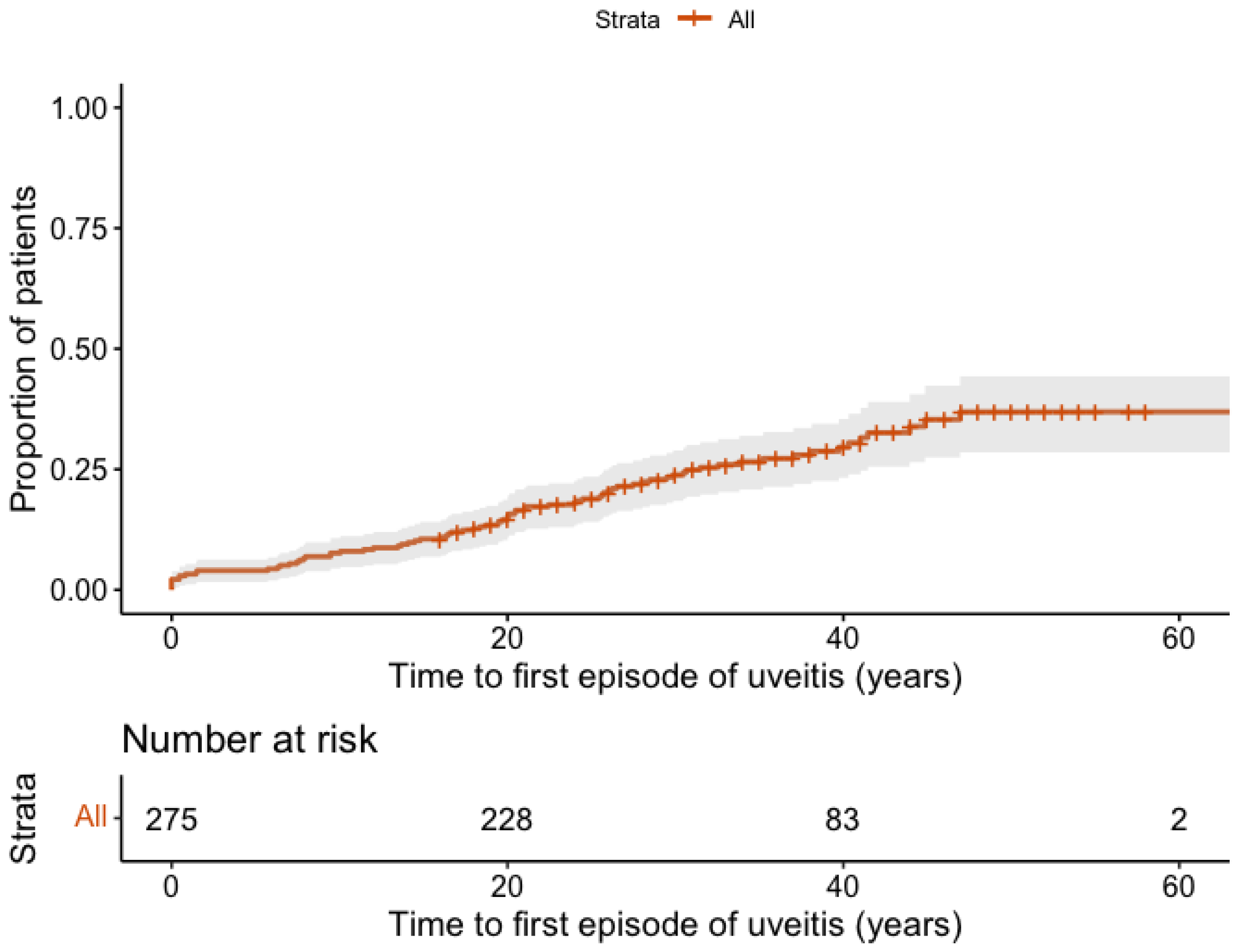
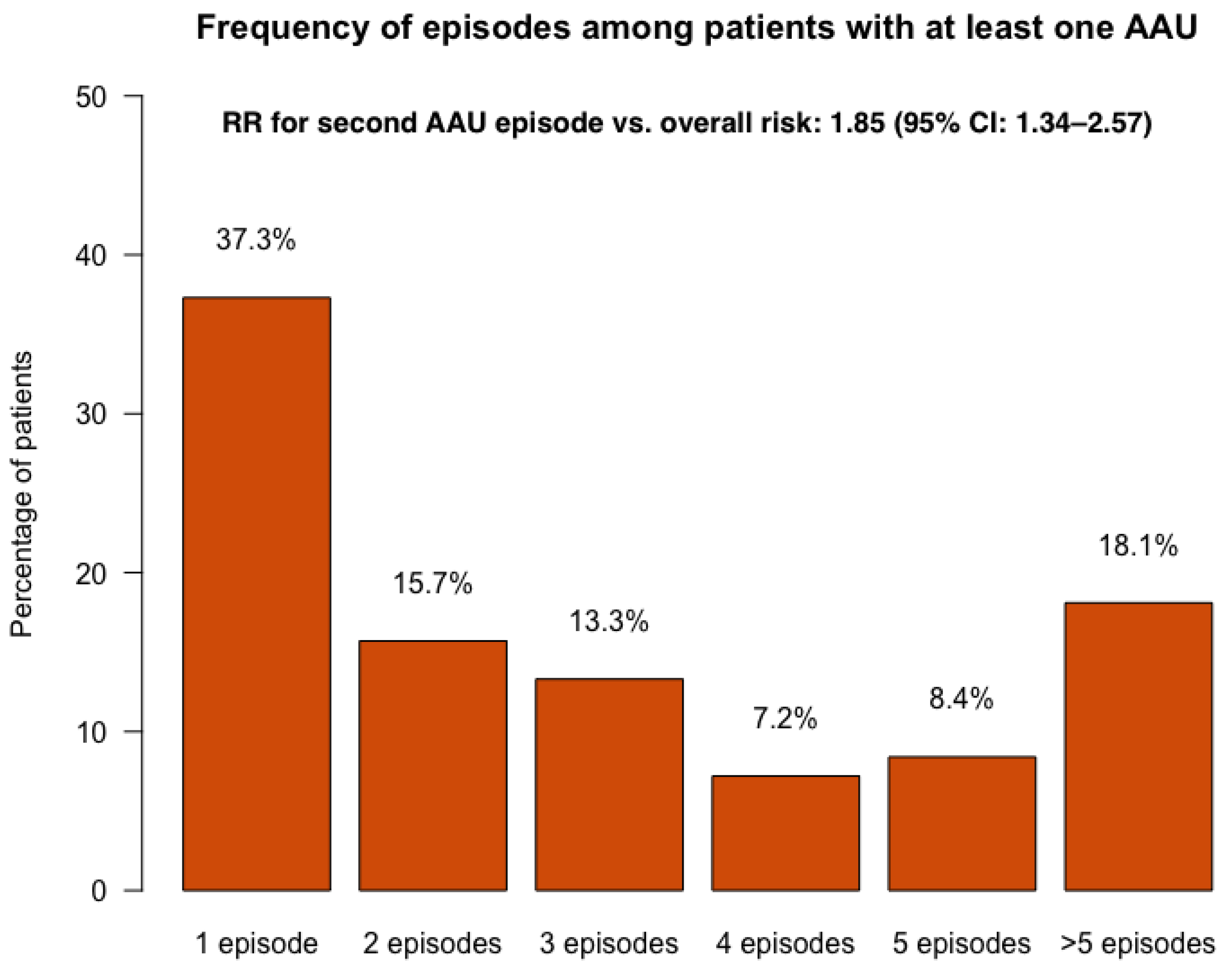
3.1. Prevalence and Incidence of AAU
3.2. Baseline Predictive Factors of AAU
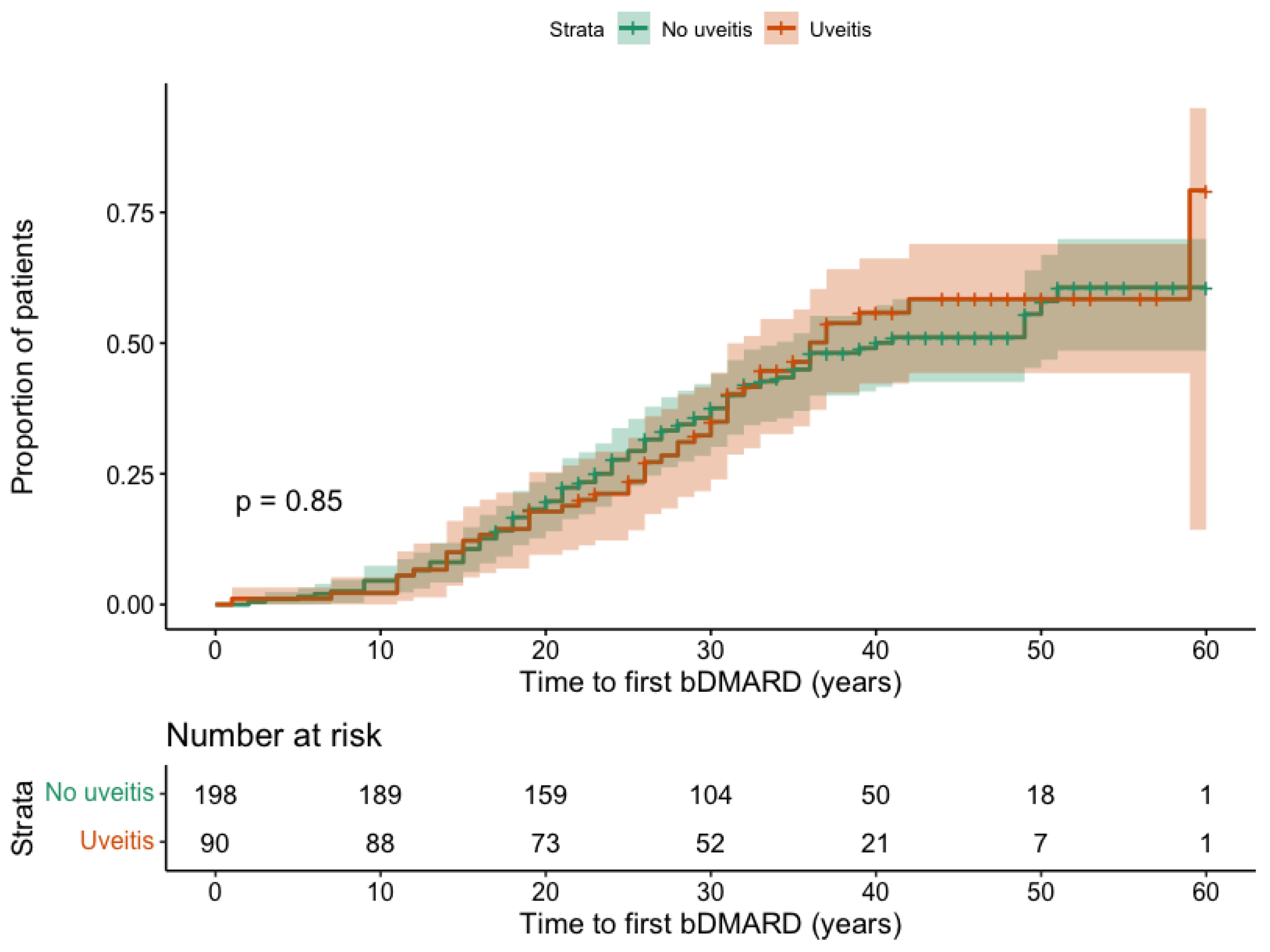
3.3. Influence of AAU on the bDMARD Initiation
3.4. Influence of AAU on the bDMARD Retention Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef]
- Bacchiega, A.B.S.; Balbi, G.G.M.; Ochtrop, M.L.G.; de Andrade, F.A.; Levy, R.A.; Baraliakos, X. Ocular involvement in patients with spondyloarthritis. Rheumatology 2017, 56, 2060–2067. [Google Scholar] [CrossRef]
- Frantz, C.; Portier, A.; Etcheto, A.; Monnet, D.; Brezin, A.; Roure, F.; Elhai, M.; Burki, V.; Fabreguet, I.; Koumakis, E.; et al. Acute anterior uveitis in spondyloarthritis: A monocentric study of 301 patients. Clin. Exp. Rheumatol. 2019, 37, 26–31. [Google Scholar] [PubMed]
- Zeboulon, N.; Dougados, M.; Gossec, L. Prevalence and characteristics of uveitis in the spondyloarthropathies: A systematic literature review. Ann. Rheum. Dis. 2008, 67, 955–959. [Google Scholar] [CrossRef]
- López-Medina, C.; Molto, A.; Sieper, J.; Duruöz, T.; Kiltz, U.; Elzorkany, B.; Hajjaj-Hassouni, N.; Burgos-Vargas, R.; Maldonado-Cocco, J.; Ziade, N.; et al. Prevalence and distribution of peripheral musculoskeletal manifestations in spondyloarthritis including psoriatic arthritis: Results of the worldwide, cross-sectional ASAS-PerSpA study. RMD Open 2021, 7, e001450. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, C.; van Onna, M.; Boonen, A.; van Tubergen, A. Global prevalence of spondyloarthritis: A systematic review and meta-regression analysis. Arthritis Care Res. 2016, 68, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, I.; Ladehesa-Pineda, M.L.; Puche-Larrubia, M.Á.; Ortega-Castro, R.; Font-Ugalde, P.; Pérez-Guijo, V.; Escudero-Contreras, A.; Diaz-Villalón, G.; López-Medina, C.; Collantes-Estévez, E. Uveitis as the first symptom in spondyloarthritis and its association with the evolution of the disease. Results from the REGISPONSER registry. Jt. Bone Spine 2021, 88, 105136. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Asquith, M. The microbiome and HLA-B27-associated acute anterior uveitis. Nat. Rev. Rheumatol. 2018, 14, 704–713. [Google Scholar] [CrossRef]
- Wendling, D.; Prati, C.; Lequerré, T.; Miceli, C.; Dougados, M.; Molto, A.; Guillot, X. Uveitis occurrence in early inflammatory back pain. Five years data from the prospective French nationwide DESIR cohort. Jt. Bone Spine 2021, 88, 105100. [Google Scholar] [CrossRef]
- Maldonado-Ficco, H.; López-Medina, C.; Perez-Alamino, R.; Waimann, C.A.; Maldonado-Cocco, J.A.; Moltó, A.; Dougados, M.; Landewé, R.B.M.; van der Heijde, D.; Bosch, F.v.D. Prevalence and incidence of uveitis in patients with spondyloarthritis: The impact of the biologics era. Data from the international ASAS-COMOSPA study. Rheumatology 2025, 64, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Collantes, E.; Zarco, P.; Munoz, E.; Juanola, X.; Mulero, J.; Fernandez-Sueiro, J.L.; Torre-Alonso, J.C.; Gratacos, J.; Gonzalez, C.; Batlle, E.; et al. Disease pattern of spondyloarthropathies in Spain: Description of the first national registry (REGISPONSER) extended report. Rheumatology 2007, 46, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Puche-Larrubia, M.Á.; Ladehesa-Pineda, L.; Ábalos-Aguilera, M.C.; Ruiz-Vílchez, D.; Berbel-Arcobé, L.; Juanola-Roura, X.; Arévalo-Salaet, M.; Moreno, M.; Almodóvar-González, R.; Pijoan-Moratalla, C.; et al. Seventeen-year reassessment of diagnostic transitions, biologic therapy initiation and mortality in spondyloarthritis: Results from the REGISPON-3 study. RMD Open 2025, 11. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; Van Der Linden, S.; Juhlin, R.; Huitfeldt, B.; Amor, B.; Calin, A.; Cats, A.; Dijkmans, B.; Olivieri, I.; Pasero, G.; et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondyloarthropathy. Arthritis Rheum. 1991, 34, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Lukas, C.; Landewé, R.; Sieper, J.; Dougados, M.; Davis, J.; Braun, J.; van der Linden, S.; van der Heijde, D. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2009, 68, 18–24. [Google Scholar] [CrossRef]
- Calin, A.; Garrett, S.; Whitelock, H.; Kennedy, L.G.; O’Hea, J.; Mallorie, P.; Jenkinson, T. A new approach to defining functional ability in ankylosing spondylitis: The development of the Bath Ankylosing Spondylitis Functional Index. J. Rheumatol. 1994, 21, 2281–2285. [Google Scholar]
- Stolwijk, C.; van Tubergen, A.; Castillo-Ortiz, J.D.; Boonen, A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 65–73. [Google Scholar] [CrossRef]
- Canouï-Poitrine, F.; Lekpa, F.K.; Farrenq, V.; Boissinot, V.; Hacquard-Bouder, C.; Comet, D.; Bastuji-Garin, S.; Thibout, E.; Claudepierre, P. Prevalence and factors associated with uveitis in spondyloarthritis patients in France: Results from an observational survey. Arthritis Care Res. 2012, 64, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Llop, M.; Gratacós, J.; Moreno, M.; Salaet, M.A.; Calvet, J.; Berenguer-Llergo, A.; Dougados, M.; Molto, A.; López-Medina, C. Sex differential impact of comorbidities in spondyloarthritis: Data from COMOSPA study. RMD Open 2024, 10, e003776. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Humira: EPAR—Product Information [Internet]; EMA: Amsterdam, The Netherlands, 2023; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/humira (accessed on 17 July 2025).
- Roche, D.; Badard, M.; Boyer, L.; Lafforgue, P.; Pham, T. Incidence of anterior uveitis in patients with axial spondyloarthritis treated with anti-TNF or anti-IL17A: A systematic review, a pairwise and network meta-analysis of randomized controlled trials. Arthritis Res. Ther. 2021, 23, 192. [Google Scholar] [CrossRef] [PubMed]
- Bechman, K.; Yang, Z.; Adas, M.; Nagra, D.; Suğuzlar, A.; Russell, M.D.; Wilson, N.; Steer, S.; Norton, S.; Galloway, J. Incidence of uveitis in patients with axial spondylarthritis treated with biologics or targeted synthetics: A systematic review and network meta-analysis. Arthritis Rheumatol. 2024, 76, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xie, Q.; He, X.; Lu, Y.; Li, M.; Shuai, S. Risk of new-onset and recurrent uveitis with different biologics for ankylosing spondylitis: A network meta-analysis. Front. Immunol. 2025, 16, 1556313. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.C.; Lee, H.S.; Yang, J.; Park, M.-C. Comparative risk of incident and recurrent acute anterior uveitis across different biological agents in patients with ankylosing spondylitis. Rheumatology 2025, 64, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Rudwaleit, M.; van Gaalen, F.A.; Haroon, N.; Gensler, L.S.; Fleurinck, C.; Marten, A.; Massow, U.; de Peyrecave, N.; Vaux, T.; et al. Low uveitis rates in patients with axial spondyloarthritis treated with bimekizumab: Pooled results from the phase 2b/3 trials. Ann. Rheum. Dis. 2024, 83, 1722–1730. [Google Scholar] [CrossRef]
- Poddubnyy, D.; Parikh, B.; Elewaut, D.; Navarro-Compán, V.; Siebert, S.; Paley, M.; Coombs, D.; Lagunes, I.; Biljan, A.; Nakasato, P.; et al. Development of extramusculoskeletal manifestations in upadactinib-treated patients with psoriatic arthritis or axial spondyloarthritis. Arthritis Rheumatol. 2025, 77, 536–546. [Google Scholar] [CrossRef]

| Total = 299 N (%) or Mean (SD) | |
|---|---|
| Sex (female), n (%) | 87 (20.1%) |
| Age, mean (SD) | 60.6 (10.2) |
| Ever smoker, n (%) | 183 (61.2%) |
| Disease duration, mean (SD) | 36.7 (10.4) |
| Diagnosis delay, mean (SD) | 7.3 (8.3) |
| HLA-B27, n (%) | 261 (90.3%) |
| Inflammatory back pain, n (%) | 291 (97.9%) |
| Peripheral synovitis, n (%) | 96 (32.7%) |
| Enthesitis, n (%) | 75 (25.3%) |
| Dactylitis, n (%) | 15 (5.1%) |
| Acute anterior uveitis, n (%) | 100 (33.4%) |
| Psoriasis, n (%) | 28 (9.6%) |
| IBD, n (%) | 24 (8.0%) |
| Sacroiliitis, n (%) | 268 (91.8%) |
| BASDAI (0–10), mean (SD) | 3.6 (2.3) |
| CRP mg/L, mean (SD) | 5.0 (9.7) |
| ASDAS, mean (SD) | 2.5 (1.3) |
| BASFI (0–100), mean (SD) | 36.8 (23.1) |
| mSASSS, mean (SD) | 21.5 (22.5) |
| ts/bDMARD use ever, n (%) | 147 (49.2%) |
| csDMARD use ever, n (%) | 112 (37.5%) |
| Univariable Cox Regression HR (95%CI) | p-Value | Multivariable Cox Regression * HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Sex (female) | 1.53 (0.96–2.43) | 0.074 | 1.65 (1.02–2.66) | 0.042 |
| Diagnosis delay | 0.99 (0.96–1.02) | 0.350 | ||
| HLA-B27 | 1.34 (0.58–3.10) | 0.487 | ||
| Axial pain | 0.52 (0.13–2.14) | 0.368 | ||
| Buttock pain | 0.79 (0.49–1.29) | 0.355 | ||
| Synovitis | 0.76 (0.46–1.28) | 0.304 | ||
| Enthesitis | 1.43 (0.91–2.26) | 0.125 | 1.64 (1.03–2.61) | 0.038 |
| Psoriasis | 1.64 (0.66–4.06) | 0.285 | 2.29 (0.90–5.82) | 0.083 |
| IBD | 1.26 (0.39–4.01) | 0.697 | ||
| Family history of SpA | 1.22 (0.90–1.66) | 0.201 | 1.18 (0.88–1.63) | 0.249 |
| Sacroiliitis | 2.15 (0.53–8.77) | 0.286 | 2.80 (0.68–11.5) | 0.154 |
| BASRI total | 0.98 (0.92–1.04) | 0.527 | ||
| CRP | 0.99 (0.97–1.01) | 0.439 | ||
| ASDAS | 0.88 (0.70–1.12) | 0.306 | ||
| bDMARD at baseline | 1.26 (0.65–2.45) | 0.500 | ||
| csDMARD at baseline | 1.29 (0.75–2.22) | 0.351 |
| Overall Population N = 139 | Patients with a History of AAU N = 46 | Patients Without a History of AAU N = 93 | p-Value | |
|---|---|---|---|---|
| TNF inhibitors | 133 (95.7%) | 45 (97.8%) | 88 (94.6%) | 0.664 |
| - Adalimumab | 72 (51.8%) | 33 (71.7%) | 39 (41.9%) | <0.001 |
| - Etanercept | 61 (43.9%) | 18 (39.1%) | 43 (46.3%) | 0.427 |
| - Infliximab | 55 (39.6%) | 19 (41.3%) | 36 (38.7%) | 0.769 |
| - Certolizumab | 9 (6.5%) | 5 (10.9%) | 4 (4.3%) | 0.157 |
| - Golimumab | 29 (20.9%) | 11 (23.9%) | 18 (19.4%) | 0.658 |
| IL-17A inhibitors | 21 (15.1%) | 8 (17.4%) | 13 (14.0%) | 0.597 |
| - Secukinumab | 20 (14.4%) | 8 (17.4%) | 12 (12.9%) | 0.478 |
| - Ixekizumab | 4 (2.9%) | 1 (2.2%) | 3 (3.2%) | 1.000 |
| - Bimekizumab | 0 | 0 | 0 | - |
| JAK inhibitors | 2 (1.4%) | 1 (2.2%) | 1 (1.0%) | 1.000 |
| - Tofacitinib | 0 | 0 | 0 | - |
| - Upadacitinib | 2 (1.4%) | 1 (2.2%) | 1 (1.0%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-León, A.M.; Ladehesa-Pineda, M.L.; Puche-Larrubia, M.Á.; Ábalos-Aguilera, M.C.; Ruiz-Vilchez, D.; Escudero-Contreras, A.; Collantes-Estévez, E.; Collantes-Sánchez, C.M.; López-Medina, C.; REGISPON-3 Study Group. Uveitis in Longstanding Axial Spondyloarthritis and Its Association with Biologic Therapy Initiation: Data from the REGISPON-3 Cohort. J. Clin. Med. 2025, 14, 7128. https://doi.org/10.3390/jcm14197128
Sánchez-León AM, Ladehesa-Pineda ML, Puche-Larrubia MÁ, Ábalos-Aguilera MC, Ruiz-Vilchez D, Escudero-Contreras A, Collantes-Estévez E, Collantes-Sánchez CM, López-Medina C, REGISPON-3 Study Group. Uveitis in Longstanding Axial Spondyloarthritis and Its Association with Biologic Therapy Initiation: Data from the REGISPON-3 Cohort. Journal of Clinical Medicine. 2025; 14(19):7128. https://doi.org/10.3390/jcm14197128
Chicago/Turabian StyleSánchez-León, Ana María, María Lourdes Ladehesa-Pineda, María Ángeles Puche-Larrubia, María Carmen Ábalos-Aguilera, Desirée Ruiz-Vilchez, Alejandro Escudero-Contreras, Eduardo Collantes-Estévez, Carlos M. Collantes-Sánchez, Clementina López-Medina, and REGISPON-3 Study Group. 2025. "Uveitis in Longstanding Axial Spondyloarthritis and Its Association with Biologic Therapy Initiation: Data from the REGISPON-3 Cohort" Journal of Clinical Medicine 14, no. 19: 7128. https://doi.org/10.3390/jcm14197128
APA StyleSánchez-León, A. M., Ladehesa-Pineda, M. L., Puche-Larrubia, M. Á., Ábalos-Aguilera, M. C., Ruiz-Vilchez, D., Escudero-Contreras, A., Collantes-Estévez, E., Collantes-Sánchez, C. M., López-Medina, C., & REGISPON-3 Study Group. (2025). Uveitis in Longstanding Axial Spondyloarthritis and Its Association with Biologic Therapy Initiation: Data from the REGISPON-3 Cohort. Journal of Clinical Medicine, 14(19), 7128. https://doi.org/10.3390/jcm14197128






