Cognitive Impairment in Patients with Severe COPD: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
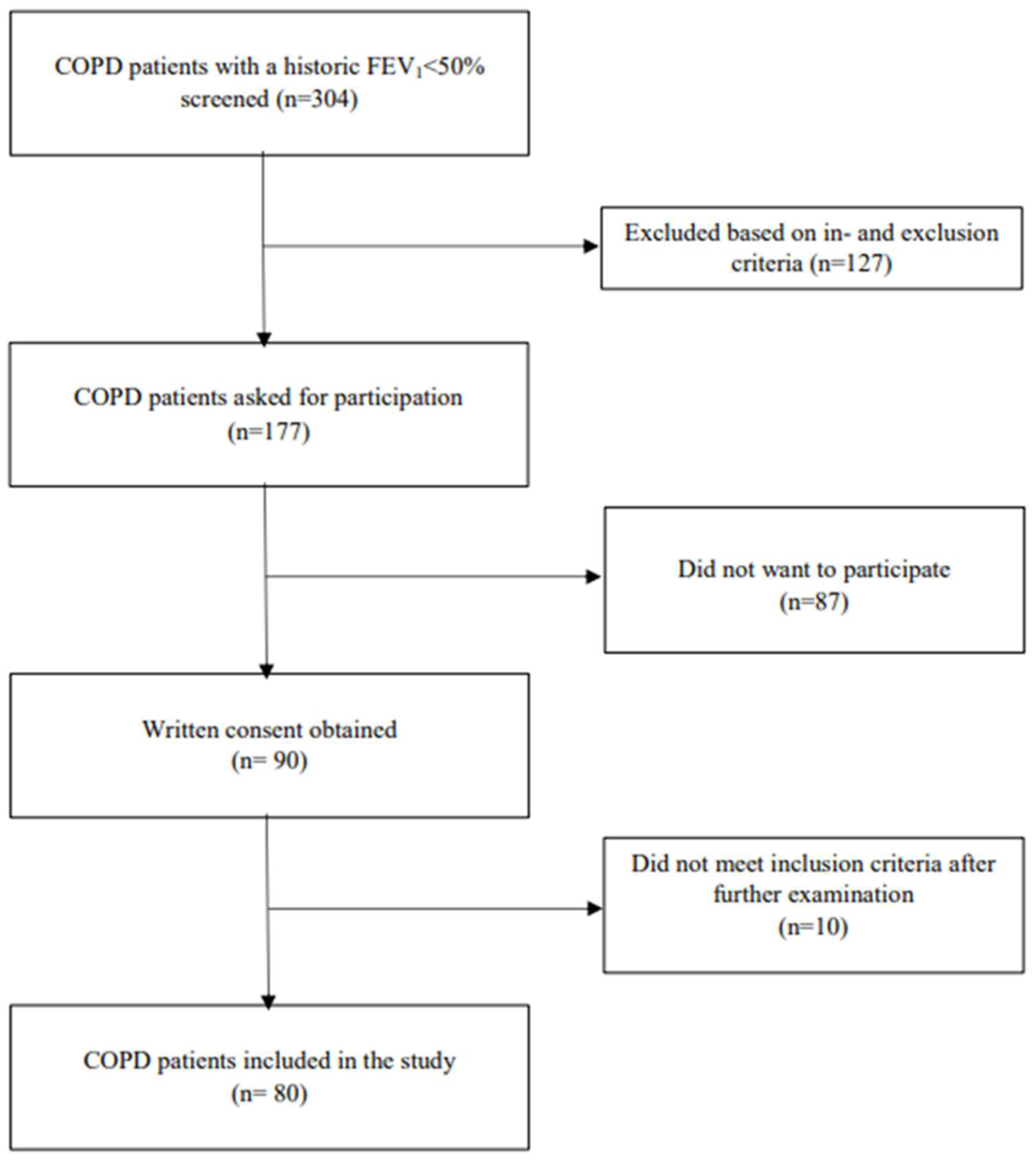
2.1. Study Population
2.2. Data Collection
2.3. Questionnaires
2.4. Clinical Assessment
2.5. Cognitive Assessment
2.6. Statistics
3. Results
3.1. Participant Characteristics
3.2. Cognitive Impairment
3.3. Characteristics of Patients with a MoCA Score < 26 and ≥26
3.4. Non-Completion of Cognitive Tests
3.5. Supplementary Subgroup Analysis
3.6. Missing Data
4. Discussion
4.1. Cognitive Impairment
4.2. Characteristics of Patients with Cognitive Impairment
4.3. Assessment of Cognitive Function
4.4. Strengths and Limitations
4.5. Implication to Clinical Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHI | Apnea Hypopnea Index |
| ANOVA | Analyses of variance |
| ATC | Anatomical Therapeutic Chemical |
| BMI | Body Mass Index |
| CAT | COPD Assessment Test |
| CI | Cognitive Impairment |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRM | CardioRespiratory Monitor |
| CRT | Continuous Reaction Time test |
| FEV1 | Forced expiratory volume in 1 s |
| FVC | Forced vital capacity |
| GOLD | Global initiative for chronic Obstructive Lung Disease |
| HADS | Hospital Anxiety and Depression Scale |
| IQR | Interquartile Range |
| mMRC | Modified Medical Research Council breathlessness |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| OPEN | Open Patient data Explorative Network |
| OSA | Obstructive Sleep Apnea |
| PaCO2 | Partial pressure of carbon dioxide |
| PaO2 | Partial pressure of oxygen |
| SaO2 | Arterial oxygen saturation |
| SD | Standard deviation |
| T90 | Percentage of sleep with oxygen saturation under 90% |
| 6MWT | 6-min walk test |
References
- Morley, J.E. Chronic obstructive pulmonary disease: A disease of older persons. J. Am. Med. Dir. Assoc. 2014, 15, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.W.; Getov, S.V.; Jones, P.W. Cognitive function in COPD. Eur. Respir. J. 2010, 35, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, D.R.; Lavoie, K.L. Recognition, diagnosis, and treatment of cognitive and psychiatric disorders in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Li, P.; Gong, T.; Wu, M.; Fu, J.; Nie, M.; Dong, Y.; Hu, K. Severe obstructive sleep apnea in patients with chronic obstructive pulmonary disease is associated with an increased prevalence of mild cognitive impairment. Sleep Med. 2020, 75, 522–530. [Google Scholar] [CrossRef]
- Klein, M.; Gauggel, S.; Sachs, G.; Pohl, W. Impact of chronic obstructive pulmonary disease (COPD) on attention functions. Respir. Med. 2010, 104, 52–60. [Google Scholar] [CrossRef]
- Schou, L.; Østergaard, B.; Rasmussen, L.S.; Rydahl-Hansen, S.; Phanareth, K. Cognitive dysfunction in patients with chronic obstructive pulmonary disease—A systematic review. Respir. Med. 2012, 106, 1071–1081. [Google Scholar]
- Tudorache, E.; Fildan, A.P.; Frandes, M.; Dantes, E.; Tofolean, D.E. Aging and extrapulmonary effects of chronic obstructive pulmonary disease. Clin. Interv. Aging 2017, 12, 1281–1287. [Google Scholar] [CrossRef]
- Au, B.; Dale-McGrath, S.; Tierney, M.C. Sex differences in the prevalence and incidence of mild cognitive impairment: A meta-analysis. Ageing Res. Rev. 2017, 35, 176–199. [Google Scholar] [CrossRef]
- Tervo, S.; Kivipelto, M.; Hänninen, T.; Vanhanen, M.; Hallikainen, M.; Mannermaa, A.; Soininen, H. Incidence and risk factors for mild cognitive impairment: A population-based three-year follow-up study of cognitively healthy elderly subjects. Dement. Geriatr. Cogn. Disord. 2004, 17, 196–203. [Google Scholar]
- Raffaele, A.I.; Andrea, C.; Claudio, P.; Luigi, T.; Domenico, A.; Aldo, S.; Orsola, I.; Franco, R. Drawing impairment predicts mortality in severe COPD. Chest 2006, 130, 1687–1694. [Google Scholar] [CrossRef]
- Grant, I.; Heaton, R.K.; McSweeny, A.J.; Adams, K.M.; Timms, R.M. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch. Intern. Med. 1982, 142, 1470–1476. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, X.; Shi, X.; Zheng, Z.; Zhang, A.; Guo, J.; Fang, Y. Chronic Obstructive Pulmonary Disease as a Risk Factor for Cognitive Dysfunction: A Meta-Analysis of Current Studies. J. Alzheimer’s Dis. 2016, 52, 101–111. [Google Scholar] [CrossRef]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr. Pol. 2016, 50, 1039–1052. [Google Scholar] [PubMed]
- Land, M.; Horwood, J. Which parts of the road guide steering? Nature 1995, 377, 339–340. [Google Scholar] [CrossRef]
- Lauridsen, M.M.; Thiele, M.; Kimer, N.; Vilstrup, H. The continuous reaction times method for diagnosing, grading, and monitoring minimal/covert hepatic encephalopathy. Metab. Brain Dis. 2013, 28, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, M.M.; Grønbæk, H.; Næser, E.B.; Leth, S.T.; Vilstrup, H. Gender and age effects on the continuous reaction times method in volunteers and patients with cirrhosis. Metab. Brain Dis. 2012, 27, 559–565. [Google Scholar] [CrossRef]
- Boelsbjerg, H.B.; Kurita, G.P.; Sjøgren, P.; Hansen, N.V. Combining subjective and objective appraisals of cognitive dysfunction in patients with cancer: A deeper understanding of meaning and impact on suffering? Support. Care Cancer 2022, 30, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Cairns, A.; Wickwire, E.; Schaefer, E.; Nyanjom, D. A pilot validation study for the NOX T3(TM) portable monitor for the detection of OSA. Sleep Breath. 2014, 18, 609–614. [Google Scholar] [CrossRef]
- Lewis, C.A.; Fergusson, W.; Eaton, T.; Zeng, I.; Kolbe, J. Isolated nocturnal desaturation in COPD: Prevalence and impact on quality of life and sleep. Thorax 2009, 64, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Eshkoor, S.A.; Hamid, T.A.; Mun, C.Y.; Ng, C.K. Mild cognitive impairment and its management in older people. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Orgeta, V.; Leung, P.; del-Pino-Casado, R.; Qazi, A.; Orrell, M.; Spector, A.E.; Methley, A.M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2022, 4, Cd009125. [Google Scholar]
- Yin, M.; Wang, H.; Hu, X.; Li, X.; Fei, G.; Yu, Y. Patterns of brain structural alteration in COPD with different levels of pulmonary function impairment and its association with cognitive deficits. BMC Pulm. Med. 2019, 19, 203. [Google Scholar] [CrossRef]
- Wong, G.K.C.; Mak, J.S.Y.; Wong, A.; Zheng, V.Z.Y.; Poon, W.S.; Abrigo, J.; Mok, V.C.T. Minimum Clinically Important Difference of Montreal Cognitive Assessment in aneurysmal subarachnoid hemorrhage patients. J. Clin. Neurosci. 2017, 46, 41–44. [Google Scholar] [CrossRef]
- Hansen, K.K.; Hilberg, O.; Jensen, H.I.; Løkke, A.; Farver-Vestergaard, I. The Association Between Cognitive Functions and Psychological Factors in Patients with Severe COPD. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2065–2078. [Google Scholar] [CrossRef]
- Hazra, A. Using the confidence interval confidently. J. Thorac. Dis. 2017, 9, 4125–4130. [Google Scholar] [CrossRef]
- Ward, A.; Arrighi, H.M.; Michels, S.; Cedarbaum, J.M. Mild cognitive impairment: Disparity of incidence and prevalence estimates. Alzheimer’s Dement. 2012, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Dulohery, M.M.; Schroeder, D.R.; Benzo, R.P. Cognitive function and living situation in COPD: Is there a relationship with self-management and quality of life? Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1883–1889. [Google Scholar] [CrossRef]
- Disler, R.T.; Spiliopoulos, N.; Inglis, S.C.; Currow, D.C.; Davidson, P.M. Cognitive screening in chronic obstructive pulmonary disease: Patient’s perspectives. Disabil. Rehabil. 2020, 42, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Andrianopoulos, V.; Vogiatzis, I.; Gloeckl, R.; Bals, R.; Koczulla, R.A.; Kenn, K. Cerebral oxygen availability during exercise in COPD patients with cognitive impairment. Respir. Physiol. Neurobiol. 2018, 254, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Andrianopoulos, V.; Gloeckl, R.; Schneeberger, T.; Jarosch, I.; Vogiatzis, I.; Hume, E.; Koczulla, R.A.; Kenn, K. Benefits of pulmonary rehabilitation in COPD patients with mild cognitive impairment—A pilot study. Respir. Med. 2021, 185, 106478. [Google Scholar] [CrossRef]
- Qian, H.; Lin, H.; Li, Y. Assessment of cognition and associated factors in patients with stable chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua Jiehe He Huxi Zazhi = Chin. J. Tuberc. Respir. Dis. 2014, 37, 769–773. (In Chinese) [Google Scholar]
- Dag, E.; Bulcun, E.; Turkel, Y.; Ekici, A.; Ekici, M. Factors Influencing Cognitive Function in Subjects With COPD. Respir. Care 2016, 61, 1044–1050. [Google Scholar] [CrossRef]
- Torres-Sánchez, I.; Rodríguez-Alzueta, E.; Cabrera-Martos, I.; López-Torres, I.; Moreno-Ramírez, M.P.; Valenza, M.C. Cognitive impairment in COPD: A systematic review. J. Bras. Pneumol. 2015, 41, 182–190. [Google Scholar] [CrossRef]
- Pallesen, A.V.J.; Mierau, J.O.; Christensen, F.K.; Mortensen, L.H. Educational and income inequalities across diseases in Denmark: A register-based cohort study. Lancet Public Health 2024, 9, e916–e924. [Google Scholar]
| Patients with COPD (n = 80) | Controls (n = 22) | p | |
|---|---|---|---|
| Age (yrs), mean (SD) | 64 (7) | 61 (9) | 0.13 |
| Sex (male), n (%) | 42 (53) | 7 (32) | 0.09 |
| Cohabitation, n (%) | |||
| Living alone | 27 (34) | 1 (5) | <0.01 |
| Living with partner | 53 (66) | 21 (95) | |
| Education level, n (%) | |||
| None | 17 (21) | 4 (18) | <0.01 |
| Short (2–3 yrs) | 46 (58) | 6 (27) | |
| Moderate/Long (3–6 yrs) | 17 (21) | 12 (55) | |
| Employment status, n (%) | |||
| Not working | 21 (26) | 0 (0) | <0.01 |
| Working | 22 (28) | 12 (55) | |
| Pensioner | 37 (46) | 10 (45) | |
| Smoking, n (%) | |||
| Smoker | 30 (37) | 1 (5) | 0.00 |
| Former | 50 (63) | 9 (41) | |
| Never | 0 (0) | 12 (54) | |
| Body mass index (kg/m2), mean (SD) | 25 (5) | 25 (5) | 0.79 |
| Blood pressure (mmHg), mean (SD) | |||
| Systolic | 138 (15) | 137 (11) | 0.82 |
| Diastolic | 83 (10) | 84 (11) | 0.69 |
| Pulse (beats/min), mean (SD) | 83 (15) | 70 (11) | 0.00 |
| Saturation (percent), mean (SD) | 96 (2) | 98 (1) | 0.00 |
| Temperature (Celsius), mean (SD) | 36.4 (0.4) | 36.3 (0.6) | 0.54 |
| mMRC (0–4), median (IQR) | 2 (2–3) | 0 (0–0) | 0.00 |
| Exacerbations < 1 year, n (%) | |||
| None | 29 (36) | - | |
| 1 | 24 (30) | - | |
| 2 | 13 (16) | - | |
| >2 | 14 (18) | - | |
| CAT score (0–40), mean (SD) | 18 (7) | - | |
| Lung function, mean (SD) | |||
| FEV1, % of predicted | 35 (8) | 99 (11) | 0.00 |
| FEV1/FVC | 41.54 (9.03) | 80.40 (6.65) | 0.00 |
| Arterial blood test, mean (SD) | n = 68 | n = 3 | |
| pH | 7.43 (0.02) | 7.42 (0.02) | 0.29 |
| PaCO2 (kPa) | 5.15 (0.55) | 5.07 (0.06) | 0.26 |
| PaO2 (kPa) | 9.83 (1.25) | 13.17 (0.61) | <0.01 |
| Comorbidity (ICD-10), n (%) | |||
| 0 comorbidity | 11 (14) | 12 (55) | 0.00 |
| 1–2 comorbidities | 30 (37) | 6 (27) | |
| >3 comorbidities | 39 (49) | 4 (18) | |
| Charlson Comorbidity Index Score, median (IQR) | 1 (1–2) | 0 (0–0) | 0.00 |
| HADS—Anxiety (0–21), mean (SD) | 5 (4) | 2 (2) | 0.00 |
| HADS—Depression (0–21), mean (SD) | 3 (3) | 1 (1) | 0.00 |
| Medicine (ATC system) | |||
| N–central nervous system | 24 (30) | 1 (5) | 0.01 |
| N02A–opioid | 6 (8) | 0 (0) | 0.34 |
| N03A–antiepileptic | 4 (5) | 0 (0) | 0.58 |
| N05A–antipsychotic | 2 (3) | 0 (0) | 1.00 |
| N06A–antidepressant | 9 (11) | 1 (5) | 0.69 |
| 6-min walk (meter), mean (SD) | 382 (98) * | 580 (61) | 0.00 |
| Diagnosed with OSA and/or ND, n (%) | 56 (70) | 6 (27) | 0.00 |
| AHI (numbers per hour), median (IQR) | 8 (5–13) | 9 (3–14) | 0.72 |
| T90 (percentage), median (IQR) | 24 (7–69) | 1 (0–2) | 0.00 |
| Patients with COPD (n = 80) | Controls (n = 22) | Differences (95% CI) | p | |
|---|---|---|---|---|
| MoCA score, mean (95% CI) | 25.95 (25.45–26.45) | 26.82 (25.91–27.72) | 0.87 (−0.15–1.88) | 0.09 |
| <26, n (%) | 32 (40) | 6 (27) | 0.27 | |
| 26–30 (normal), n (%) | 48 (60) | 16 (73) | ||
| Specific domains from MoCA, median (95%CI) | ||||
| Visuospatial (0–4) | 2 (1–2) | 2 (2–2) | 0.02 | |
| Executive function (0–4) | 3 (3–3) | 4 (3–4) | 0.07 | |
| Attention (0–6) | 6 (6–6) | 6 (6–6) | 0.32 | |
| Language (0–5) | 5 (5–5) | 5 (5–5) | 0.16 | |
| Short-term memory (0–5) | 3 (3–4) | 4 (3–4) | 0.88 | |
| CRT index, mean (95% CI) | 2.039 (1.884–2.194) | 2.306 (2.111–2.500) | 0.267 (0.023–0.511) | 0.03 |
| ≤1.900, n (%) | 38 (48) | 4 (18) | 0.01 | |
| >1.900, n (%) | 42 (52) | 18 (82) | ||
| Driving time, n (%) | n = 79 | n = 22 | ||
| <20 min | 19 (24) | 1 (5) | 0.04 | |
| 20 min | 60 (76) | 21 (95) | ||
| SD from center of the road, median (95% CI) | 0.365 (0.334–0.455) | 0.309 (0.252–0.452) | 0.056 (0.002–0.11) | 0.04 |
| Average response time (sec), median (95% CI) | 2.63 (2.33–2.99) | 2.6 (2.41–3.09) | 0.03 (−0.49–0.56) | 0.72 |
| Patients with CI (n = 32) | Patients without CI (n = 48) | p | |
|---|---|---|---|
| Age (years), mean (SD) | 67 (8) | 62 (6) | <0.01 |
| Sex (male), mean (SD) | 15 (47) | 27 (56) | 0.41 |
| Cohabitation, n (%) | |||
| Living alone | 9 (28) | 18 (38) | 0.39 |
| Living with partner | 23 (72) | 30 (62) | |
| Education level, n (%) | |||
| None | 9 (28) | 8 (17) | 0.37 |
| Short (2–3 yrs) | 18 (56) | 28 (58) | |
| Moderate/long (3–6 yrs) | 5 (16) | 12 (25) | |
| Employment status, n (%) | |||
| Not working | 7 (22) | 14 (29) | <0.01 |
| Working | 3 (9) | 19 (40) | |
| Pensioner | 22 (69) | 15 (31) | |
| Smoking, n (%) | |||
| Smoker | 12 (38) | 18 (38) | 1.00 |
| Former | 20 (62) | 30 (62) | |
| Never | 0 (0) | 0 (0) | |
| Body mass index (kg/m2), mean (SD) | 25 (5) | 25 (5) | 0.52 |
| Blood pressure (mmHg), mean (SD) | |||
| Systolic | 142 (14) | 135 (15) | 0.03 |
| Diastolic | 81 (11) | 84 (10) | 0.30 |
| Pulse (beats/min), mean (SD) | 81 (18) | 84 (13) | 0.41 |
| Saturation (%), mean (SD) | 95 (2) | 96 (2) | 0.69 |
| Temperature (Celsius), mean (SD) | 36.4 (0.4) | 36.4 (0.5) | 0.98 |
| mMRC (0–4), median (IQR) | 3 (2–3) | 2 (1–3) | 0.02 |
| Exacerbations < 1 year, n (%) | |||
| None | 12 (38) | 17 (36) | 0.45 |
| 1 | 8 (25) | 16 (33) | |
| 2 | 4 (12) | 9 (19) | |
| >2 | 8 (25) | 6 (12) | |
| CAT score (0–40), mean (SD) | 19 (6) | 16 (7) | <0.05 |
| FEV1 (% of predicted), mean (SD) | 36 (8) | 34 (9) | 0.27 |
| PaCO2 (kPa), mean (SD) | 5.03 (0.54) (n = 27) | 5.23 (0.56) (n = 41) | 0.15 |
| PaO2 (kPa), mean (SD) | 9.79 (1.28) (n = 27) | 9.86 (1.25) (n = 41) | 0.83 |
| Number of comorbidities, n (%) | |||
| 0 comorbidity | 2 (6) | 9 (19) | 0.28 |
| 1–2 comorbidities | 13 (41) | 17 (35) | |
| >3 comorbidities | 17 (53) | 22 (46) | |
| 6-min walk (meter), mean (SD) | 355 (102) (n = 30) | 400 (92) (n = 45) | >0.05 |
| Diagnosed with OSA and/or ND, n (%) | 20 (63) | 36 (75) | 0.23 |
| AHI (numbers per hour), median (IQR) | 8 (5–15) | 8 (5–12) | 0.64 |
| T90 (percentage), median (IQR) | 22 (1–44) | 25 (10–79) | 0.15 |
| CRT index, mean (SD) | 1.947 (0.714) | 2.1 (0.684) | 0.34 |
| Low (<1.9), n (%) | 17 (53) | 21 (44) | 0.41 |
| Normal (≥1.9), n (%) | 15 (47) | 27 (56) | |
| SD from center of the road, median (IQR) | 0.509 (0.317–4.523) (n = 31) | 0.339 (0.289–0.822) | 0.04 |
| Average time response (sec.), median (IQR) | 3.71 (2.21–4.75) (n = 31) | 2.39 (2.03–3.06) | <0.01 |
| Driving Time (Minutes) | <10 (n = 13) | 10–19.99 (n = 6) | 20 (n = 60) | p |
|---|---|---|---|---|
| Age (yrs), mean (SD) | 69 (5) | 67 (7) | 62 (7) | <0.01 |
| Sex (male), n (%) | 3 (23) | 3 (50) | 35 (58) | 0.07 |
| 6-min walk (meter), mean (SD) | 321 (113) * | 390 (110) | 397 (89) ** | 0.047 |
| MoCA score, mean (SD) | 25 (3) | 27 (2) | 26 (2) | 0.04 |
| Low (<26), n (%) | 8 (62) | 1 (17) | 22 (37) | 0.13 |
| Normal (26–30), n (%) | 5 (38) | 5 (83) | 38 (63) | |
| CRT index, mean (SD) | 1.883 (0.528) | 1.995 (0.450) | 2.074 (0.753) | 0.67 |
| Low (<1.9), n (%) | 4 (31) | 3 (50) | 31 (52) | 0.39 |
| Normal (≥1.9), n (%) | 9 (69) | 3 (50) | 29 (48) | |
| SD from center of the road, median (IQR) | 7.305 (5.791–10.332) | 3.662 (2.963–4.523) | 0.335 (0.289–0.432) | <0.001 |
| Average response time (seconds), median (IQR) | 3.94 (2.76–5.7) | 1.99 (1.49–4.75) | 2.52 (2.19–3.32) | 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, K.K.; Farver-Vestergaard, I.; Jensen, H.I.; Løkke, A.; Hilberg, O. Cognitive Impairment in Patients with Severe COPD: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 7122. https://doi.org/10.3390/jcm14197122
Hansen KK, Farver-Vestergaard I, Jensen HI, Løkke A, Hilberg O. Cognitive Impairment in Patients with Severe COPD: A Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(19):7122. https://doi.org/10.3390/jcm14197122
Chicago/Turabian StyleHansen, Kristina Kock, Ingeborg Farver-Vestergaard, Hanne Irene Jensen, Anders Løkke, and Ole Hilberg. 2025. "Cognitive Impairment in Patients with Severe COPD: A Cross-Sectional Study" Journal of Clinical Medicine 14, no. 19: 7122. https://doi.org/10.3390/jcm14197122
APA StyleHansen, K. K., Farver-Vestergaard, I., Jensen, H. I., Løkke, A., & Hilberg, O. (2025). Cognitive Impairment in Patients with Severe COPD: A Cross-Sectional Study. Journal of Clinical Medicine, 14(19), 7122. https://doi.org/10.3390/jcm14197122






