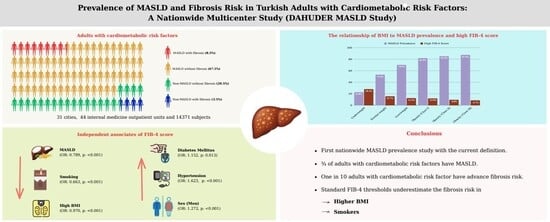
Prevalence of MASLD and Fibrosis Risk in Turkish Adults with Cardiometabolic Risk Factors: A Nationwide Multicenter Study (DAHUDER MASLD Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data Collection and Anthropometric Measurements
2.4. Laboratory Measurements
2.5. Definition of Cardiometabolic Risk Factors and Metabolic Disorders
2.6. Hepatic Steatosis and Fibrosis Assessment
2.7. Statistical Analysis
3. Results
3.1. The Characteristics of Subjects According to the Diagnosis of MASLD
3.2. The Characteristics of Subjects According to the FIB-4 Score
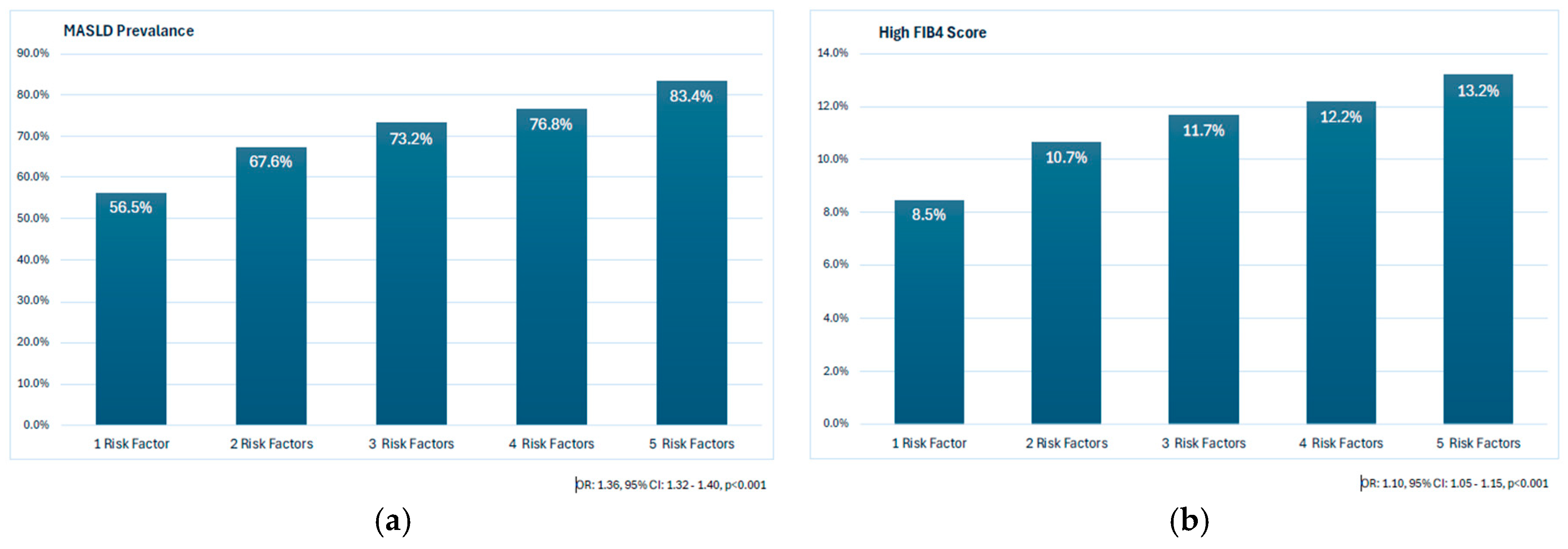
3.3. The Prevalence of MASLD and High FIB-4 Scores According to CMRFs
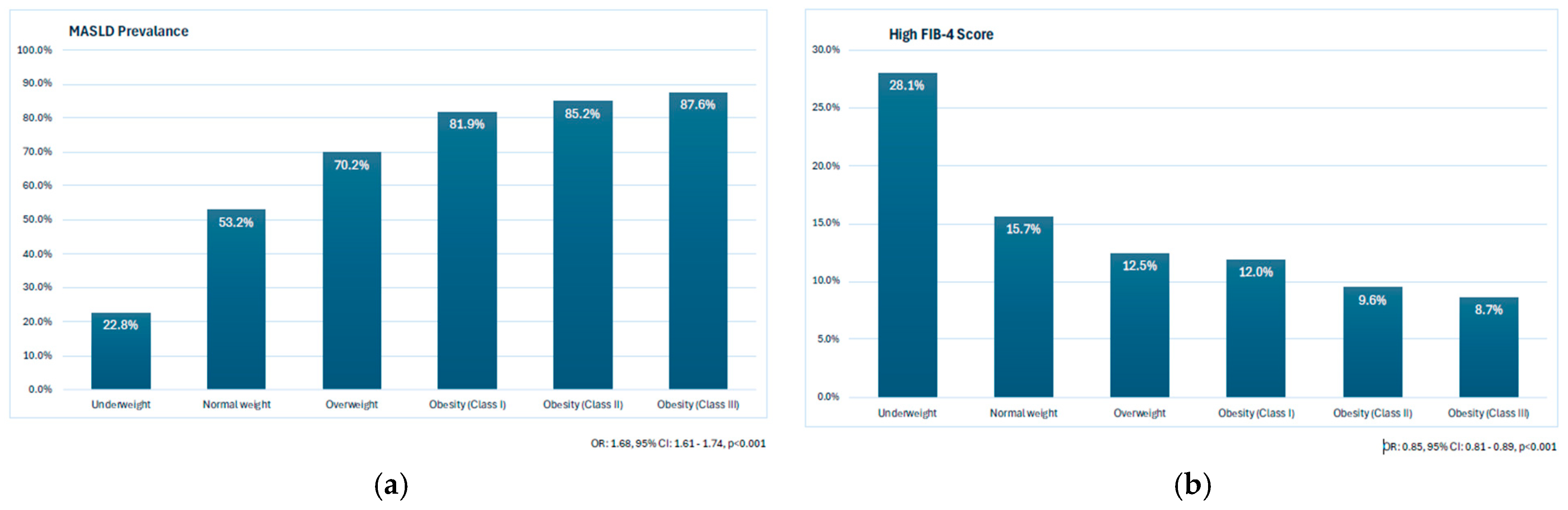
3.4. The Prevalence of MASLD and High FIB-4 Scores According to BMI
3.5. The Independent Determinants of High FIB-4 Scores
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
5.1. Strengths and Limitations
5.2. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 694–701. [Google Scholar] [CrossRef]
- Kirik, A.; Dogru, T.; Yanik, B.; Sen, H.; Eroglu, M.; Baykan, O.; Bozyel, E.A.; Ergene, A.; Selcuk, E.; Tasci, I.; et al. The relationship of circulating MOTS-c level with liver fibrosis and metabolic components in patients with metabolic dysfunction-associated fatty liver disease. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8074–8080. [Google Scholar]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar]
- Oral, A.; Solmaz, I.; Koca, N.; Topaloglu, U.S.; Demir, I.; Dundar, A.; Kirik, A.; Basci, O.K.; Sen, H.; Binnetoglu, E.; et al. Obesity-Related Disorders in Türkiye: A Multi Center, Retrospective, Cross-Sectional Analysis from the OBREDI-TR Study. J. Clin. Med. 2025, 14, 2680. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Lim, W.H.; Lim, G.E.H.; Tan, D.J.H.; Syn, N.; Muthiah, M.D.; Huang, D.Q.; Loomba, R. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 931–939.e5. [Google Scholar] [CrossRef] [PubMed]
- Mignot, V.; Chirica, C.; Tron, L.; Borowik, A.; Borel, A.L.; Rostaing, L.; Bouillet, L.; Decaens, T.; Guergour, D.; Costentin, C.E. Early screening for chronic liver disease: Impact of a FIB-4 first integrated care pathway to identify patients with significant fibrosis. Sci. Rep. 2024, 14, 20720. [Google Scholar] [CrossRef]
- Phoolchund, A.G.S.; Khakoo, S.I. MASLD and the Development of HCC: Pathogenesis and Therapeutic Challenges. Cancers 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.G.; Rau, M.; Geier, A. Screening for nonalcoholic fatty liver disease-when, who and how? World J. Gastroenterol. 2021, 27, 5803–5821. [Google Scholar] [CrossRef]
- Loomba, R.; Adams, L.A. Advances in non-invasive assessment of hepatic fibrosis. Gut 2020, 69, 1343–1352. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef]
- Şahintürk, Y.; Köker, G.; Koca, N.; Sümbül, H.E.; Demir, İ.; Keskin, H.; Yaylacı, S.; Solmaz, İ.; Açmaz, B.; Yıldız, H.; et al. Metabolic Dysfunction-Associated Fatty Liver Disease and Fibrosis Status in Patients with Type 2 Diabetes Treated at Internal Medicine Clinics: Türkiye DAHUDER Awareness of Fatty Liver Disease (TR-DAFLD) Study. Turk. J. Gastroenterol. 2024, 35, 643–650. [Google Scholar]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2024, 29, 101133. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Zelber-Sagi, S.; Lazarus, J.V.; Wong, V.W.-S.; Yilmaz, Y.; Duseja, A.; Eguchi, Y.; Castera, L.; Pessoa, M.G.; Oliveira, C.P.; et al. Global Consensus Recommendations for Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Gastroenterology 2025, 169, 1017–1032.e2. [Google Scholar] [CrossRef]
- Eslam, M.; Fan, J.-G.; Yu, M.-L.; Wong, V.W.-S.; Cua, I.H.; Liu, C.-J.; Tanwandee, T.; Gani, R.; Seto, W.-K.; Alam, S.; et al. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease. Hepatol. Int. 2025, 19, 261–301. [Google Scholar] [CrossRef]
- Clusmann, J.; Balaguer-Montero, M.; Bassegoda, O.; Schneider, C.V.; Seraphin, T.; Paintsil, E.; Luedde, T.; Lopez, R.P.; Calderaro, J.; Gilbert, S.; et al. The barriers for uptake of artificial intelligence in hepatology and how to overcome them. J. Hepatol. 2025. [Google Scholar] [CrossRef]
- Gao, B.; Duan, W. The current status and future directions of artificial intelligence in the prediction, diagnosis, and treatment of liver diseases. Digit Health. 2025, 11, 20552076251325418. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Yilmaz, N.; Ates, F.; Karakaya, F.; Gokcan, H.; Kaya, E.; Adali, G.; Kartal, A.C.; Sen, I.; Ahishali, E.; et al. The Prevalence of Metabolic Associated Fatty Liver Disease in The Turkish Population: A Multicenter Study. Hepatol. Forum 2021, 2, 37–42. [Google Scholar] [CrossRef]
- Degertekin, B.; Tozun, N.; Demir, F.; Soylemez, G.; Parkan, S.; Gurtay, E.; Mutlu, D.; Toraman, M.; Seymenoglu, T.H. The Changing Prevalence of Non-Alcoholic Fatty Liver Disease (NAFLD) in Turkey in the Last Decade. Turk. J. Gastroenterol. 2021, 32, 302–312. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, I.R.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S27–S49. [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018, Erratum in Eur. Heart J. 2025, 46, 1300. [Google Scholar] [CrossRef]
- Zeitouni, M.; Sabouret, P.; Kerneis, M.; Silvain, J.; Collet, J.-P.; Bruckert, E.; Montalescot, G. 2019 ESC/EAS Guidelines for management of dyslipidaemia: Strengths and limitations. Eur. Heart J.—Cardiovasc. Pharmacother. 2021, 7, 324–333. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yang, T.; Yin, J.; Li, J.; Wang, Q. The influence of different combinations of cardiometabolic risk factors on the prevalence of MASLD and risk of advanced fibrosis deserves attention. J. Hepatol. 2024, 80, e82–e85. [Google Scholar] [CrossRef]
- Sezgin, O.; Akpinar, H.; Ozer, B.; Toruner, M.; Bal, K.; Bor, S. The Abdominal Ultrasonography Results of Cappadocia Cohort Study of Turkey Reveals High Prevalence of Fatty Liver. Turk. J. Gastroenterol. 2023, 34, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.; Owrangi, S.; Yilmaz, Y.; El-Kassas, M.; Alswat, K.; Alqahtani, S.A. Prevalence of metabolic dysfunction-associated steatotic liver disease in the Middle East and North Africa. Liver Int. 2024, 44, 1061–1070. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef]
- Fu, H.; Yu, H.; Zhao, Y.; Chen, J.; Liu, Z. Association between hypertension and the prevalence of liver steatosis and fibrosis. BMC Endocr. Disord. 2023, 23, 85. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease-related risk of cardiovascular disease and other cardiac complications. Diabetes, Obes. Metab. 2022, 24, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Somnay, K.; Wadgaonkar, P.; Sridhar, N.; Roshni, P.; Rao, N.; Wadgaonkar, R. Liver Fibrosis Leading to Cirrhosis: Basic Mechanisms and Clinical Perspectives. Biomedicines 2024, 12, 2229. [Google Scholar] [CrossRef]
- Ochoa-Allemant, P.; Hubbard, R.A.; Kaplan, D.E.; Serper, M. Adverse Liver Outcomes, Cardiovascular Events, and Mortality in Steatotic Liver Disease. JAMA Intern. Med. 2025, 185, 986. [Google Scholar] [CrossRef]
- Schreiner, A.D.; Moran, W.P.; Zhang, J.; Livingston, S.; Marsden, J.; Mauldin, P.D.; Koch, D.; Gebregziabher, M. The Association of Fibrosis-4 Index Scores with Severe Liver Outcomes in Primary Care. J. Gen. Intern. Med. 2022, 37, 3266–3274. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Julián, M.T.; Arteaga, I.; Torán-Monserrat, P.; Pera, G.; de Oca, A.P.-M.; Ruiz-Rojano, I.; Casademunt-Gras, E.; Chacón, C.; Alonso, N. The Link between Abdominal Obesity Indices and the Progression of Liver Fibrosis: Insights from a Population-Based Study. Nutrients 2024, 16, 1586. [Google Scholar] [CrossRef]
- Purdy, J.C.; Shatzel, J.J. The hematologic consequences of obesity. Eur. J. Haematol. 2021, 106, 306–319. [Google Scholar] [CrossRef]
- Akhavan Rezayat, A.; Dadgar Moghadam, M.; Ghasemi Nour, M.; Shirazinia, M.; Ghodsi, H.; Rouhbakhsh Zahmatkesh, M.R.; Tavakolizadeh Noghabi, M.; Hoseini, B.; Akhavan Rezayat, K. Association between smoking and nonalcoholic fatty liver disease: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 2050312117745223. [Google Scholar] [CrossRef]
- Ghahremanfard, F.; Semnani, V.; Ghorbani, R.; Malek, F.; Behzadfar, A.; Zahmatkesh, M. Effects of cigarette smoking on morphological features of platelets in healthy men. Saudi Med. J. 2015, 36, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Eren, F.; Kaya, E.; Yilmaz, Y. Accuracy of Fibrosis-4 index and non-alcoholic fatty liver disease fibrosis scores in metabolic (dysfunction) associated fatty liver disease according to body mass index: Failure in the prediction of advanced fibrosis in lean and morbidly obese individuals. Eur. J. Gastroenterol. Hepatol. 2022, 34, 98–103. [Google Scholar] [CrossRef]
- Bozic, D.; Podrug, K.; Mikolasevic, I.; Grgurevic, I. Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal. Diagnostics 2022, 12, 2287. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Kojima, S.-I.; Watanabe, N.; Numata, M.; Ogawa, T.; Matsuzaki, S. Increase in the prevalence of fatty liver in Japan over the past 12 years: Analysis of clinical background. J. Gastroenterol. 2003, 38, 954–961. [Google Scholar] [CrossRef]
- Milani, I.; Chinucci, M.; Leonetti, F.; Capoccia, D. MASLD: Prevalence, Mechanisms, and Sex-Based Therapies in Postmenopausal Women. Biomedicines 2025, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71.e15. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Taub, R.; Neff, G.W.; Lucas, K.J.; Labriola, D.; Moussa, S.E.; Alkhouri, N.; Bashir, M.R. Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
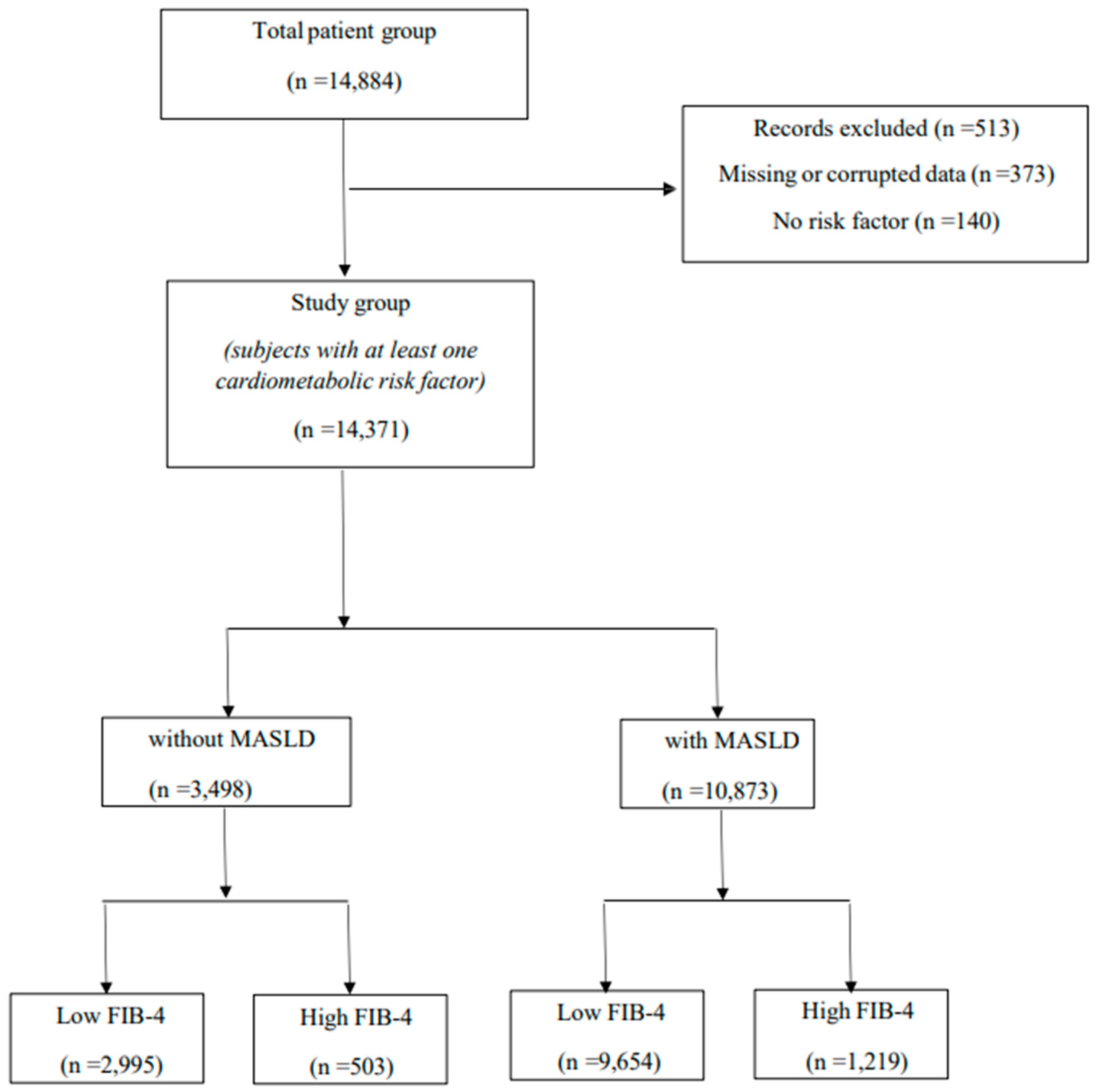

| Variables | Total Population n = 14,371 | With MASLD n = 10,873 (75.7%) | Without MASLD n = 3498 (24.3%) | p Values |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age (year) * | 51.3 ± 14.4 | 51.4 ± 16.42 | 51.2 ± 13.6 | 0.442 |
| Sex (women) ** | 8827 (61.4) | 6503 (59.8) | 2324 (66.4) | <0.001 |
| BMI (kg/m2) * | 31.4 ± 6.0 | 32.2 ± 10.0 | 29.1 ± 5.6 | <0.001 |
| Smoking ** | 3996 (27.8) | 3090 (28.4) | 906 (25.9) | 0.004 |
| Exercise ** | 2804 (19.5) | 1902 (17.5) | 902 (25.8) | <0.001 |
| Comorbid diseases | ||||
| T2DM ** | 6181 (43.0) | 4919 (45.2) | 1262 (36.1) | <0.001 |
| Hypertension ** | 6104 (42.5) | 4765 (43.8) | 1339 (38.3) | <0.001 |
| Dyslipidemia ** | 6403 (44.6) | 5183 (47.7) | 1220 (34.9) | <0.001 |
| MetS ** | 11,544 (80.3) | 9062 (83.3) | 2482 (71.0) | <0.001 |
| Obesity ** | 7822 (54.4) | 6544 (60.2) | 1278 (36.5) | <0.001 |
| Laboratory and markers | ||||
| Platelet (×103) * | 276.0 ± 77.0 | 277.5 ± 77.8 | 270.1 ± 73.3 | <0.001 |
| AST (IU/L) * | 24.2 ± 24.5 | 24.4 ± 22.6 | 23.6 ± 30.9 | 0.126 |
| ALT (IU/L) * | 28.7 ± 32.7 | 28.9 ± 30.8 | 27.5 ± 39.5 | 0.032 |
| FIB-4 score * | 0.99 ± 1.5 | 0.96 ± 1.6 | 1.1 ± 1.1 | 0.001 |
| High FIB-4 score ** | 1722 (12.0) | 1219 (11.2) | 503 (14.4) | <0.001 |
| Variables | Total Population n = 14,371 | Low FIB-4 Score n = 12,649 (88.0%) | High FIB-4 Score n = 1722 (12.0%) | p Values |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age (year) * | 51.3 ± 14.4 | 50.1 ± 14.4 | 60.1 ± 10.9 | <0.001 |
| Sex (women) ** | 8827 (61.4) | 7834 (62.0) | 984 (57.1) | <0.001 |
| Smoking ** | 3996 (27.8) | 3627 (28.7) | 369 (21.4) | <0.001 |
| Exercise ** | 2804 (19.5) | 2527 (20.0) | 277 (16.1) | <0.001 |
| BMI (kg/m2) * | 31.4 ± 6.0 | 31.6 ± 6.0 | 30.4 ± 5.8 | <0.001 |
| Comorbid diseases | ||||
| T2DM ** | 6181 (43.0) | 5337 (42.2) | 844 (49.0) | <0.001 |
| Hypertension ** | 6104 (42.5) | 5183 (41.0) | 921 (53.5) | <0.001 |
| Dyslipidemia ** | 6403 (44.6) | 5598 (44.3) | 805 (46.7) | 0.051 |
| MetS ** | 11,544 (80.3) | 10,104 (79.9) | 1440 (83.6) | <0.001 |
| Obesity ** | 7822 (54.4) | 6974 (55.1) | 848 (49.2) | <0.001 |
| MASLD ** | 10,873 (75.7) | 9654 (76.3) | 1219 (70.8) | <0.001 |
| Laboratory and markers | ||||
| Platelet (×103) * | 276.0 ± 77.0 | 286.8 ± 72.7 | 196.5 ± 59.7 | <0.001 |
| AST (IU/L) * | 24.2 ± 24.5 | 21.5 ± 10.3 | 44.1 ± 61.3 | <0.001 |
| ALT (IU/L) * | 28.7 ± 32.7 | 27.1 ± 24.5 | 39.9 ± 66.2 | <0.001 |
| FIB-4 score * | 0.99 ± 1.5 | 0.79 ± 0.34 | 2.42 ± 3.82 | <0.001 |
| Group | Total (n) | MASLD (n) | Prevalence (%) |
|---|---|---|---|
| DM (+)/HT (+) | 3730 | 3017 | 80.9% |
| DM (+)/HT (−) | 2451 | 1902 | 77.6% |
| DM (−)/HT (+) | 2374 | 1748 | 73.6% |
| DM (−)/HT (−) | 5816 | 4206 | 72.3% |
| Score | n | % |
|---|---|---|
| FIB-4 (age-adjusted) | 1778 | 12.4 |
| FIB-4 ≥ 2.67 | 330 | 2.3 |
| APRI ≥ 0.7 | 375 | 2.6 |
| APRI ≥ 1.0 | 207 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirik, A.; Sumbul, H.E.; Koca, N.; Paşalı Kilit, T.; Demiral Sezer, S.; Binnetoglu, E.; Araç, E.; Solmaz, İ.; Şen, H.; Demirci, İ.; et al. Prevalence of MASLD and Fibrosis Risk in Turkish Adults with Cardiometabolic Risk Factors: A Nationwide Multicenter Study (DAHUDER MASLD Study). J. Clin. Med. 2025, 14, 7098. https://doi.org/10.3390/jcm14197098
Kirik A, Sumbul HE, Koca N, Paşalı Kilit T, Demiral Sezer S, Binnetoglu E, Araç E, Solmaz İ, Şen H, Demirci İ, et al. Prevalence of MASLD and Fibrosis Risk in Turkish Adults with Cardiometabolic Risk Factors: A Nationwide Multicenter Study (DAHUDER MASLD Study). Journal of Clinical Medicine. 2025; 14(19):7098. https://doi.org/10.3390/jcm14197098
Chicago/Turabian StyleKirik, Ali, Hilmi Erdem Sumbul, Nizameddin Koca, Türkan Paşalı Kilit, Sibel Demiral Sezer, Emine Binnetoglu, Eşref Araç, İhsan Solmaz, Hacer Şen, İbrahim Demirci, and et al. 2025. "Prevalence of MASLD and Fibrosis Risk in Turkish Adults with Cardiometabolic Risk Factors: A Nationwide Multicenter Study (DAHUDER MASLD Study)" Journal of Clinical Medicine 14, no. 19: 7098. https://doi.org/10.3390/jcm14197098
APA StyleKirik, A., Sumbul, H. E., Koca, N., Paşalı Kilit, T., Demiral Sezer, S., Binnetoglu, E., Araç, E., Solmaz, İ., Şen, H., Demirci, İ., Abaylı, B., Akan, H., Akkuş, C., Aksakal, B., Aktas, G., Alakuş, Ö. F., Atak Tel, B. M., Aydın, A., Bahçebaşı, S., ... Doğru, T. (2025). Prevalence of MASLD and Fibrosis Risk in Turkish Adults with Cardiometabolic Risk Factors: A Nationwide Multicenter Study (DAHUDER MASLD Study). Journal of Clinical Medicine, 14(19), 7098. https://doi.org/10.3390/jcm14197098