Retinal Vascular Density and Vessel Diameter in Sturge–Weber Syndrome Assessed by OCT-Angiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Ophthalmological Examinations
2.2. Imaging Device and Settings
2.3. Image Processing and Analysis
2.4. Statistical Analysis
2.5. Limitations of Current OCTA Technology
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggieri, M.; Polizzi, A.; Marceca, G.P.; Catanzaro, S.; Praticò, A.D.; Di Rocco, C. Introduction to phacomatoses (neurocutaneous disorders) in childhood. Childs Nerv. Syst. 2020, 36, 2229–2268. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W. Neuro-ophthalmic features of the neurocutaneous syndromes. Int. Ophthalmol. Clin. 2012, 52, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Behrman, R.E.; Vaughan, V.C. Libro di testo di pediatria Nelson. J. Dev. Behav. Pediatr. 1988, 9, 239–243. [Google Scholar]
- Çelebí, S.; Alagöz, G.; Aykan, Ü. Ocular findings in Sturge-Weber syndrome. Eur. J. Ophthalmol. 2000, 10, 239–243. [Google Scholar] [CrossRef]
- Roach, E.S. Neurocutaneous syndromes. Pediatr. Clin. N. Am. 1992, 39, 591–620. [Google Scholar] [CrossRef]
- Lew, S.M.; Menezes, A.H. Sturge-Weber Syndrome: A Review. Pediatr. Neurol. 1995, 12, 244–252. [Google Scholar]
- Byard, R.W. Sturge-Weber syndrome: A case report with particular reference to the characteristic intracranial calcification. J. Clin. Pathol. 1988, 41, 220–222. [Google Scholar]
- Comi, A.M. Pathophysiology of Sturge-Weber syndrome. J. Child Neurol. 2004, 19, 906–914. [Google Scholar]
- Grossman, R.; Buchbinder, B. A prospective study of the role of cutaneous trigeminal innervation in corneal sensation and ocular surface homeostasis. Am. J. Ophthalmol. 2014, 157, 816–823. [Google Scholar]
- Waelchli, R.; Aylett, S.; Robinson, K.; Chong, W.; Martinez, A.; Kinsler, V. New vascular classification of port-wine stains: Improving prediction of Sturge-Weber risk. Br. J. Dermatol. 2014, 171, 861–867. [Google Scholar] [CrossRef]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.-C.; et al. Vascular anomalies classification: Recommendations from the international society for the study of vascular anomalies. Pediatrics 2015, 136, e203–e214. [Google Scholar] [CrossRef]
- Yeom, S.; Comi, A.M. Updates on Sturge-Weber. Stroke 2022, 53, 3769–3779. [Google Scholar] [CrossRef]
- Su, W.W. Acute primary angle-closure in Sturge-Weber syndrome. Am. J. Ophthalmol. Case Rep. 2018, 10, 101–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piram, M.; Lorette, G.; Sirinelli, D.; Herbreteau, D.; Giraudeau, B.; Maruani, A. Sturge-Weber Syndrome in patients with facial port-wine stain. Pediatr. Dermatol. 2012, 29, 32–37. [Google Scholar] [CrossRef]
- Uram, M.; Zubillaga, C. The cutaneous manifestations of Sturge-Weber syndrome. J. Clin. Neurooftalmol. 1982, 2, 245–248. [Google Scholar]
- Sabeti, S.; Ball, K.L.; Bhattacharya, S.K.; Bitrian, E.; Blieden, L.S.; Brandt, J.D.; Burkhart, C.; Chugani, H.T.; Falchek, S.J.; Jain, B.G.; et al. Consensus statement for the management and treatment of Sturge-Weber syndrome: Neurology, neuroimaging, and ophthalmology recommendations. Pediatr. Neurol. 2021, 121, 59–66. [Google Scholar] [CrossRef]
- Cibis, G.W.; Tripathi, R.C.; Tripathi, B.J. Glaucoma in Sturge-Weber syndrome. Ophthalmology 1977, 84, 205–209. [Google Scholar] [CrossRef]
- Ossoinig, K.C. Anomalies of the angle of the anterior chamber in Sturge-Weber syndrome. Trans. Am. Ophthalmol. Soc. 1978, 76, 108–131. [Google Scholar]
- Madge, S.N.; Porges, Y. Glaucoma in Sturge-Weber syndrome. Clin. Exp. Ophthalmol. 1998, 26, 181–185. [Google Scholar]
- Phelps, C.D. The pathogenesis of glaucoma in Sturge-Weber syndrome. Ophthalmology 1978, 85, 276–286. [Google Scholar] [CrossRef]
- Weiss, D.I. Glaucoma in the Sturge-Weber syndrome. Trans. Am. Ophthalmol. Soc. 1978, 76, 101–107. [Google Scholar]
- Muci-Mendoza, R.; Ramella, M. Anterior chamber angle in Sturge-Weber syndrome. Ophthalmology 1994, 101, 446–452. [Google Scholar]
- Shields, C.L.; Atalay, H.T.; Wuthisiri, W.; Levin, A.V.; Lally, S.E.; Shields, J.A. Sector iris hemangioma in association with diffuse choroidal hemangioma. J. AAPOS 2015, 19, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Sharan, S.; Swamy, B.; Taranath, D.A.; Jamieson, R.; Yu, T.; Wargon, O.; Grigg, J.R. Port-wine vascular malformations and glaucoma risk in Sturge-Weber syndrome. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2009, 13, 374–378. [Google Scholar] [CrossRef]
- Hennedige, A.A.; Quaba, A.A.; Al-Nakib, K. Sturge-Weber syndrome and dermatomal facial port-wine stains: Incidence, association with glaucoma, and pulsed tunable dye laser treatment efectiveness. Plast. Reconstr. Surg. 2008, 121, 1173–1180. [Google Scholar] [CrossRef]
- Kabra, N.; Pfaffenbach, D.D. Sturge-Weber Syndrome. In StatPearls [Internet]; StatPearls Publishing: Petersburg, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459265/ (accessed on 23 March 2025).
- Hassanpour, K.; Nourinia, R.; Gerami, E.; Mahmoudi, G.; Esfandiari, H. Ocular manifestations of the Sturge-Weber syndrome. J. Ophthalmic Vis. Res. 2021, 16, 415–431. [Google Scholar] [CrossRef]
- Silverstein, M.; Salvin, J. Ocular manifestations of Sturge-Weber syndrome. Curr. Opin. Ophthalmol. 2019, 30, 301–305. [Google Scholar] [CrossRef]
- Formisano, M.; di Pippo, M.C.; Scuderi, L.; Abdolrahimzadeh, S. Current concepts on diffuse choroidal hemangioma in Sturge Weber syndrome. Ophthalmic Genet. 2021, 42, 375–382. [Google Scholar] [CrossRef]
- Scott, I.U.; Alexandrakis, G.; Cordahi, G.J.; Murray, T.G. Difuse and circumscribed choroidal hemangiomas in a patient with SturgeWeber syndrome. Arch. Ophthalmol. 1999, 117, 406–407. [Google Scholar]
- Ch’ng, S.; Tan, S.T. Facial port-wine stains: Clinical stratifications and risks of neuro-ocular involvement. Plast. Reconstr. Aesthetic Surg. 2008, 61, 889–893. [Google Scholar] [CrossRef]
- Pascual-Castroviejo, I.; Pascual-Pascual, S.-I.; Velazquez-Fragua, R.; Viaño, J. Sturge-Weber syndrome. Study of 55 patients. Can. J. Neurol. Sci. 2008, 35, 301–307. [Google Scholar] [CrossRef]
- Jagtap, S.; Srinivas, G.; Harsha, K.J.; Radhakrishnan, N.; Radhakrishnan, A. Sturge-Weber syndrome: Clinical spectrum, disease course, and outcome of 30 patients. J. Child Neurol. 2013, 28, 725–731. [Google Scholar] [CrossRef]
- Dave, T.; Shah, G.; Pappuru, R.R. Diffuse choroidal hemangioma masquerading as central serous chorioretinopathy treated with oral propranolol. Retin. Cases Brief Rep. 2016, 10, 11–14. [Google Scholar] [CrossRef]
- Singh, N.; Fonkeu, Y.; Lorek, B.H.; Singh, A.D. Diagnostic A-Scan of Choroidal Tumors: Comparison of Quantified Parameters. Ocul. Oncol. Pathol. 2019, 5, 358–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amirikia, A.; Scott, I.U.; Capo, H.; Murray, T.G. Increasing hyperopia and esotropia as the presenting signs of bilateral diffuse choroidal hemangiomas in a patient with Sturge-Weber syndrome. J. Pediatr. Ophthalmol. Strabismus. 2013, 38, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Horgan, N.; O’KEefe, M.; McLoone, E.; Lanigan, B. Fundus fluorescein angiographic characterization of diffuse choroidal hemangiomas. J. Pediatr. Ophthalmol. Strabismus 2008, 45, 26–30. [Google Scholar] [CrossRef]
- Kubicka-Trząska, A.; Karska-Basta, I.; Oleksy, P.; Romanowska-Dixon, B. Management of diffuse choroidal hemangioma in Sturge-Weber syndrome with Ruthenium-106 plaque radiotherapy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, A.; Shields, C.L.; Harmon, S.A.; Shields, J.A. Autofluorescence of choroidal hemangioma in 34 consecutive eyes. Retina 2010, 30, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Callaway, N.F.; Mruthyunjaya, P. Widefield imaging of retinal and choroidal tumors. Int. J. Retin. Vitr. 2019, 5, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, F.; Wu, D. Indocyanine green angiographic findings in diffuse choroidal hemangioma associated with Sturge-Weber syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 625–627. [Google Scholar] [CrossRef]
- Schalenbourg, A.; Piguet, B.; Zografos, L. Indocyanine green angiographic findings in choroidal hemangiomas: A study of 75 cases. Ophthalmologica 2000, 214, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Gold, A.S.; Villegas, V.M.; Wildner, A.C.; Ehlies, F.J.; Murray, T.G. Spontaneous exudative retinal detachment in a patient with sturge-weber syndrome after taking arginine, a supplement for erectile dysfunction. Eye Vis. 2014, 1, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amirikia, A.; Scott, I.U.; Murray, T.G. Bilateral diffuse choroidal hemangiomas with unilateral facial nevus flammeus in Sturge-Weber syndrome. Am. J. Ophthalmol. 2000, 130, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Konana, V.K.; Shanmugam, P.M.; Ramanjulu, R.; Mishra, K.C.D.; Sagar, P. Optical coherence tomography angiography features of choroidal hemangioma. Indian J. Ophthalmol. 2018, 66, 581–583. [Google Scholar] [CrossRef]
- Cacciamani, A.; Scarinci, F.; Parravano, M.; Giorno, P.; Varano, M. Choroidal thickness changes with photodynamic therapy for a diffuse choroidal hemangioma in Sturge–Weber syndrome. Int. Ophthalmol. 2014, 34, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Koizumi, H.; Pozzoni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.; Spaide, R.F. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.S.; Quigley, H.A.; Comi, A.M.; Miller, R.B.; Jampel, H.D. Increased choroidal thickness in patients with Sturge-Weber syndrome. JAMA Ophthalmol. 2013, 131, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Surve, A.; Azad, S.; Venkatesh, P.; Kumar, V.; Chawla, R.; Gupta, V.; Vohra, R. Choroidal Vascular Pattern in Cases of Sturge-Weber Syndrome. Ophthalmol Retin. 2019, 3, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D. Sturge-Weber syndrome revisited: The role of neuroradiology. Neuropediatrics 1996, 27, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Courtie, E.; Veenith, T.; Logan, A.; Denniston, A.K.; Blanch, R.J. Retinal blood flow in critical illness and systemic disease: A review. Ann. Intensiv Care 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hohberger, B.; Mardin, C.Y. OCT Angiography as an Interdisciplinary Diagnostic Tool for Systemic Diseases. Klin. Monatsblatter fur Augenheilkd. 2021, 238, 1294–1298, (In English and German). [Google Scholar] [CrossRef] [PubMed]
- Kellner, R.L.; Harris, A.; Ciulla, L.; Guidoboni, G.; Verticchio Vercellin, A.; Oddone, F.; Carnevale, C.; Zaid, M.; Antman, G.; Kuvin, J.T.; et al. The Eye as the Window to the Heart: Optical Coherence Tomography Angiography Biomarkers as Indicators of Cardiovascular Disease. J. Clin. Med. 2024, 13, 829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, T.E.; Jampol, L.M.; Ferris, F.L.; Tadayoni, R.; Sadda, S.R.; Chong, V.; Domalpally, A.; Blodi, B.L.; Duh, E.J.; Curcio, C.A.; et al. Imaging Modalities for Assessing the Vascular Component of Diabetic Retinal Disease: Review and Consensus for an Updated Staging System. Ophthalmol Sci. 2023, 4, 100449. [Google Scholar] [CrossRef]
- Tan, P.E.Z.; Yu, P.K.; Cringle, S.J.; Yu, D.-Y. Quantitative assessment of the human retinal microvasculature with or without vascular comorbidity. Investig. Opthalmology. Vis. Sci. 2014, 55, 8439–8452. [Google Scholar] [CrossRef]
- Henkind, P. Radial peripapillary capillaries of the retina. I. Anatomy: Human and comparative. Br. J. Ophthalmol. 1967, 51, 115–123. [Google Scholar] [CrossRef]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 42201. [Google Scholar] [CrossRef]
- Hirano, T.; Chanwimol, K.; Weichsel, J.; Tepelus, T.; Sadda, S. Distinct Retinal Capillary Plexuses in Normal Eyes as Observed in Optical Coherence Tomography Angiography Axial Profile Analysis. Sci. Rep. 2018, 8, 9380. [Google Scholar] [CrossRef]
- Thanos, A.; Young, J.; Fortune, B.; Tang, S.J. The retinal deep capillary plexus as a venous outflow system; insights from Sturge Weber Syndrome. Retin. Cases Brief Reports. 2023, 18, 660–664. [Google Scholar] [CrossRef]
- Ciancimino, C.; Di Pippo, M.; Rullo, D.; Ruggeri, F.; Grassi, F.; Scuderi, G.; Abdolrahimzadeh, S. An Update on Multimodal Ophthalmological Imaging of Diffuse Choroidal Hemangioma in Sturge-Weber Syndrome. Vision 2023, 7, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, B.; Li, Y.; Xie, L.; Chiu, K.; Hao, X.; Xu, J.; Luo, J.; Sham, P.C. Computational Retinal Microvascular Biomarkers from an OCTA Image in Clinical Investigation. Biomedicines 2024, 12, 868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Z.; Xu, L.; Ding, X.; Wu, Y.; Zhu, X.; Fu, Y.; Guo, W. Optical Coherence Tomography Angiography of Perilimbal Vasculature in Port-Wine Stain and Sturge-Weber Syndrome Patients. Investig. Ophthalmol. Vis. Sci. 2020, 61, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takkar, B.; Azad, S.; Shakrawal, J.; Gaur, N.; Venkatesh, P. Blood flow pattern in a choroidal hemangioma imaged on swept-source-optical coherence tomography angiography. Indian J. Ophthalmol. 2017, 65, 1240–1242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basiony, A.I.; Mohamed Gad Marey, H.; Ezzat Abdel Fattah, A.M.; Zaky, M.A. Predictive value of optical coherence tomography angiography in management of diabetic macular edema. BMC Ophthalmol. 2024, 24, 429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malvasi, M.; Pascale, E.; Locuratolo, N.; Fattapposta, F.; Pauletti, C.; Finoia, M.G.; Conti, M.E.; Pacella, E. Implementing potential biomarkers for early detection of retinal changes in Parkinson’s disease: Preliminary study on BDNF role. Eur. J. Ophthalmol. 2025, 35, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- Hayati, A.; Abdol Homayuni, M.R.; Sadeghi, R.; Asadigandomani, H.; Dashtkoohi, M.; Eslami, S.; Soleimani, M. Advancing Diabetic Retinopathy Screening: A Systematic Review of Artificial Intelligence and Optical Coherence Tomography Angiography Innovations. Diagnostics 2025, 15, 737. [Google Scholar] [CrossRef]
- Gao, M.; Hormel, T.T.; Wang, J.; Guo, Y.; Bailey, S.T.; Hwang, T.S.; Jia, Y. An Open-Source Deep Learning Network for Reconstruction of High-Resolution OCT Angiograms of Retinal Intermediate and Deep Capillary Plexuses. Transl. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, M.; Guo, Y.; Hormel, T.T.; Sun, J.; Hwang, T.S.; Jia, Y. Reconstruction of high-resolution 6×6-mm OCT angiograms using deep learning. Biomed. Opt. Express 2020, 11, 3585–3600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadomoto, S.; Uji, A.; Muraoka, Y.; Akagi, T.; Tsujikawa, A. Enhanced Visualization of Retinal Microvasculature in Optical Coherence Tomography Angiography Imaging via Deep Learning. J. Clin. Med. 2020, 9, 1322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
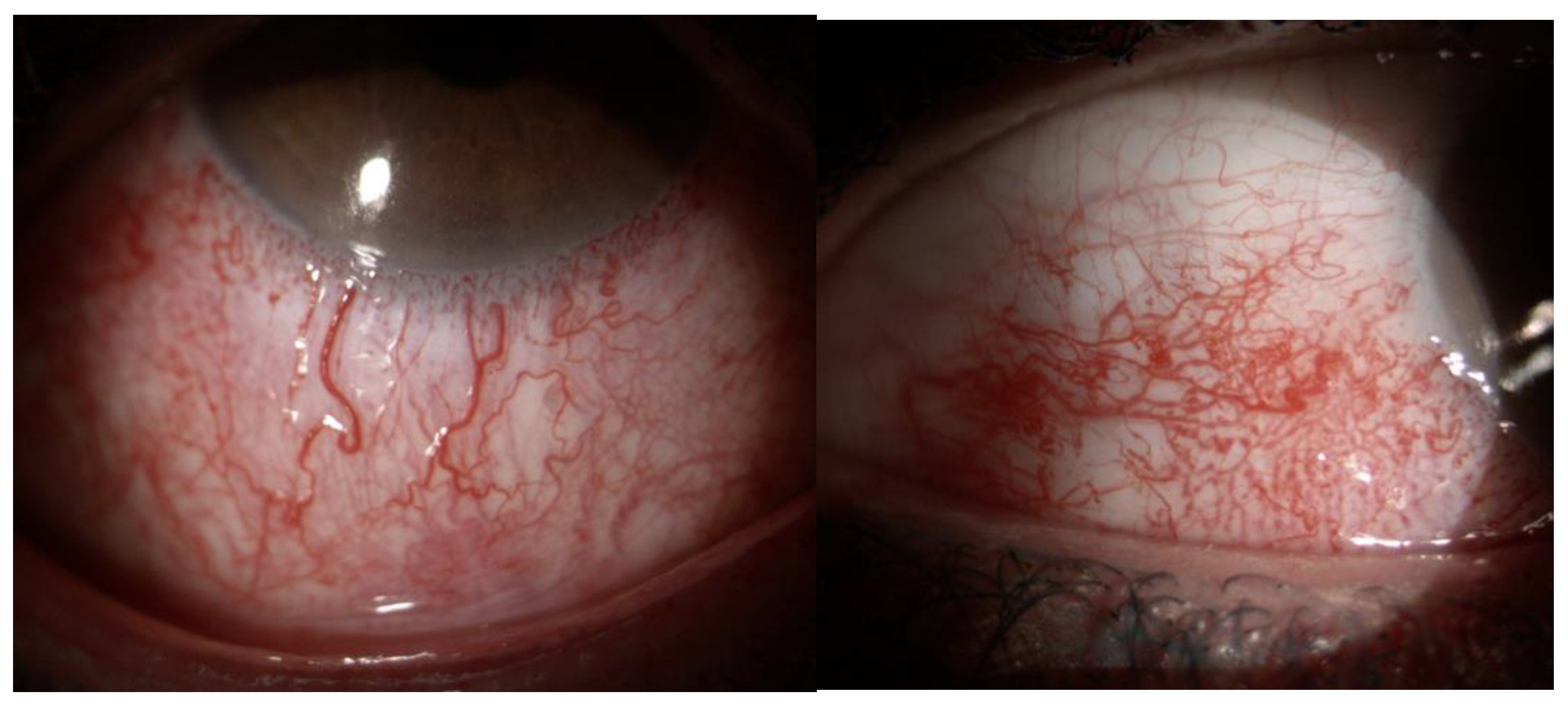
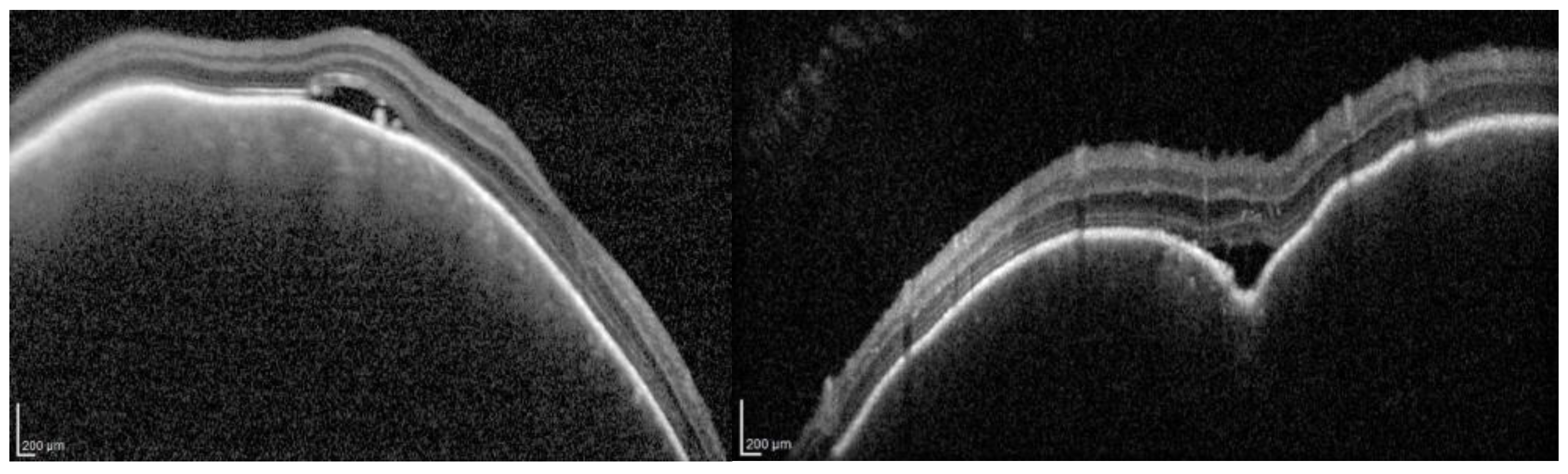

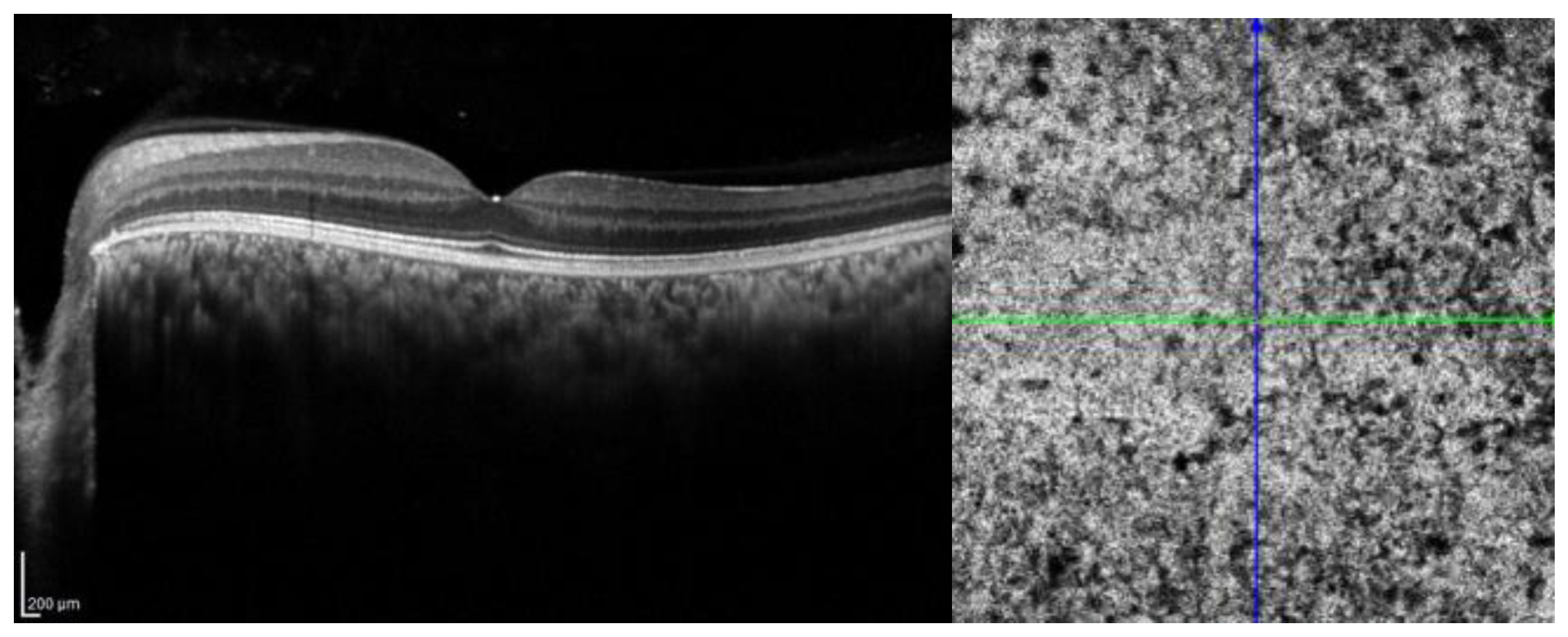

| Layer | CTL Mean ± SD | SWS Mean ± SD | p-Value |
|---|---|---|---|
| SVC | 0.2584 ± 0.0530 | 0.2385 ± 0.0611 | 0.27 |
| SVP | 0.3400 ± 0.0663 | 0.3173 ± 0.0720 | 0.29 |
| DVC | 0.2521 ± 0.0454 | 0.2644 ± 0.0608 | 0.43 |
| ICP | 0.2028 ± 0.0434 | 0.2079 ± 0.0508 | 0.72 |
| DCP | 0.2180 ± 0.0591 | 0.2591 ± 0.0612 | 0.03 |
| AVASCULAR | 0.2460 ± 0.0369 | 0.2457 ± 0.0467 | 0.98 |
| CHORIOCAPILLARIS | 0.3832 ± 0.0910 | 0.3126 ± 0.1012 | 0.16 |
| CHOROID | 0.3705 ± 0.0525 | 0.3530 ± 0.1166 | 0.48 |
| RET | 0.2320 ± 0.0506 | 0.2343 ± 0.0965 | 0.96 |
| FULL | 0.3101 ± 0.0454 | 0.3114 ± 0.0935 | 0.94 |
| Layer | CTL Mean ± SD | SWS Mean ± SD | p-Value |
|---|---|---|---|
| SVC | 4.0340 ± 0.5318 | 4.1998 ± 0.5337 | 0.34 |
| SVP | 3.9783 ± 0.3928 | 4.0895 ± 0.4155 | 0.41 |
| DVC | 3.4600 ± 0.1835 | 3.6589 ± 0.3883 | 0.02 |
| ICP | 3.5068 ± 0.1654 | 3.4719 ± 0.1944 | 0.51 |
| DCP | 3.5160 ± 0.1997 | 2.7260 ± 0.0643 | 0.00000001 |
| AVASCULAR | No skeletonization | - | - |
| CHORIOCAPILLARIS | No skeletonization | - | - |
| CHOROID | No skeletonization | - | - |
| RET | 3.1523 ± 0.1878 | 3.1334 ± 0.1958 | 0.75 |
| FULL | 4.2806 ± 0.3945 | 4.5014 ± 0.5621 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, R.; Gusson, E.; Lorenzetto, E.; Polinelli, L.; Malvasi, M.; Panozzo, G.; Marchini, G. Retinal Vascular Density and Vessel Diameter in Sturge–Weber Syndrome Assessed by OCT-Angiography. J. Clin. Med. 2025, 14, 7061. https://doi.org/10.3390/jcm14197061
Longo R, Gusson E, Lorenzetto E, Polinelli L, Malvasi M, Panozzo G, Marchini G. Retinal Vascular Density and Vessel Diameter in Sturge–Weber Syndrome Assessed by OCT-Angiography. Journal of Clinical Medicine. 2025; 14(19):7061. https://doi.org/10.3390/jcm14197061
Chicago/Turabian StyleLongo, Rosa, Elena Gusson, Erika Lorenzetto, Luca Polinelli, Mariaelena Malvasi, Giacomo Panozzo, and Giorgio Marchini. 2025. "Retinal Vascular Density and Vessel Diameter in Sturge–Weber Syndrome Assessed by OCT-Angiography" Journal of Clinical Medicine 14, no. 19: 7061. https://doi.org/10.3390/jcm14197061
APA StyleLongo, R., Gusson, E., Lorenzetto, E., Polinelli, L., Malvasi, M., Panozzo, G., & Marchini, G. (2025). Retinal Vascular Density and Vessel Diameter in Sturge–Weber Syndrome Assessed by OCT-Angiography. Journal of Clinical Medicine, 14(19), 7061. https://doi.org/10.3390/jcm14197061






