Glenoid Radiolucent Lines and Subsidence Show Limited Impact on Clinical and Functional Long-Term Outcomes After Anatomic Total Shoulder Arthroplasty: A Retrospective Analysis of Cemented Polyethylene Glenoid Components
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Radiographic and Clinical Evauation
2.3. Surgical Technique and Postoperative Care Protocol
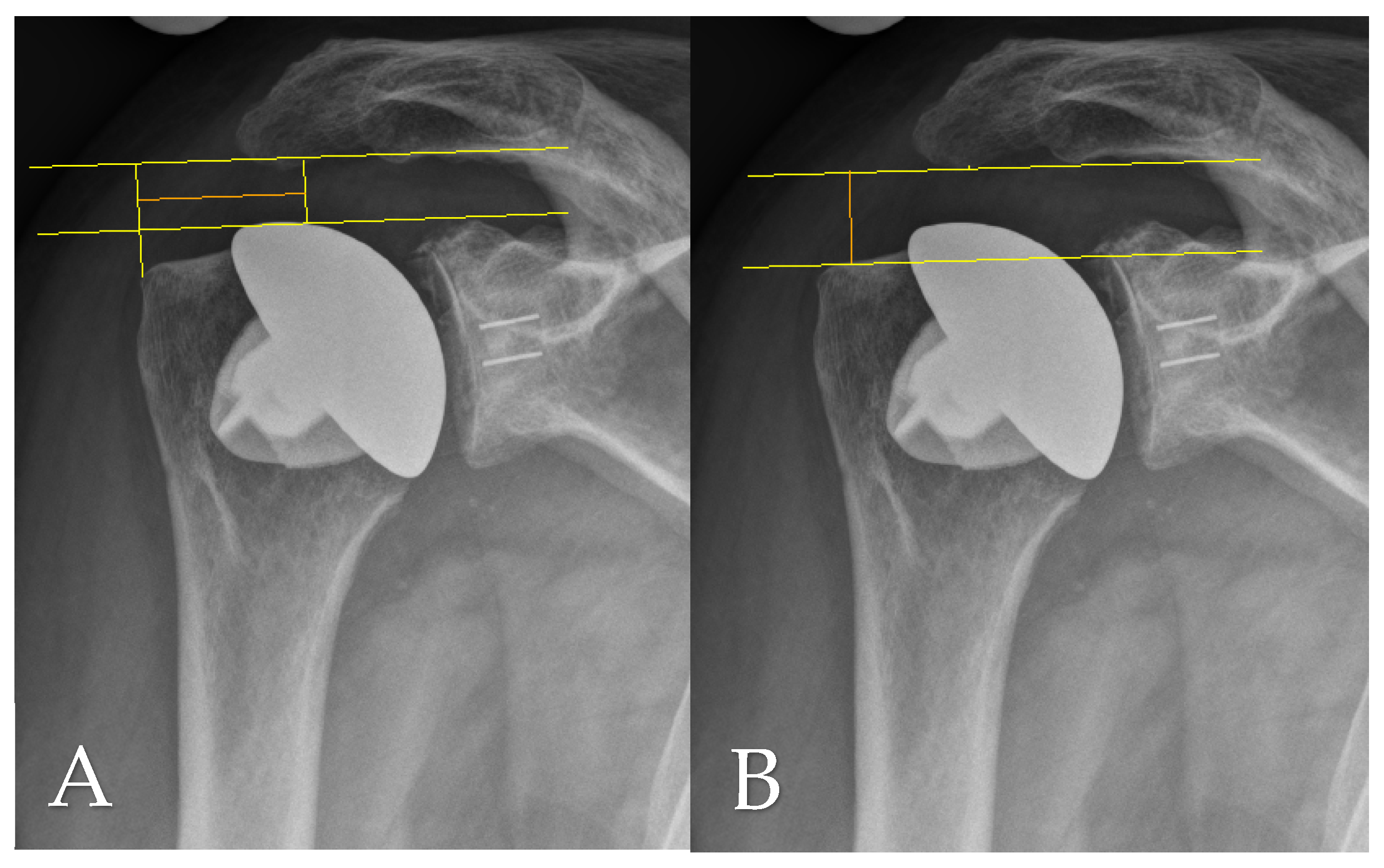
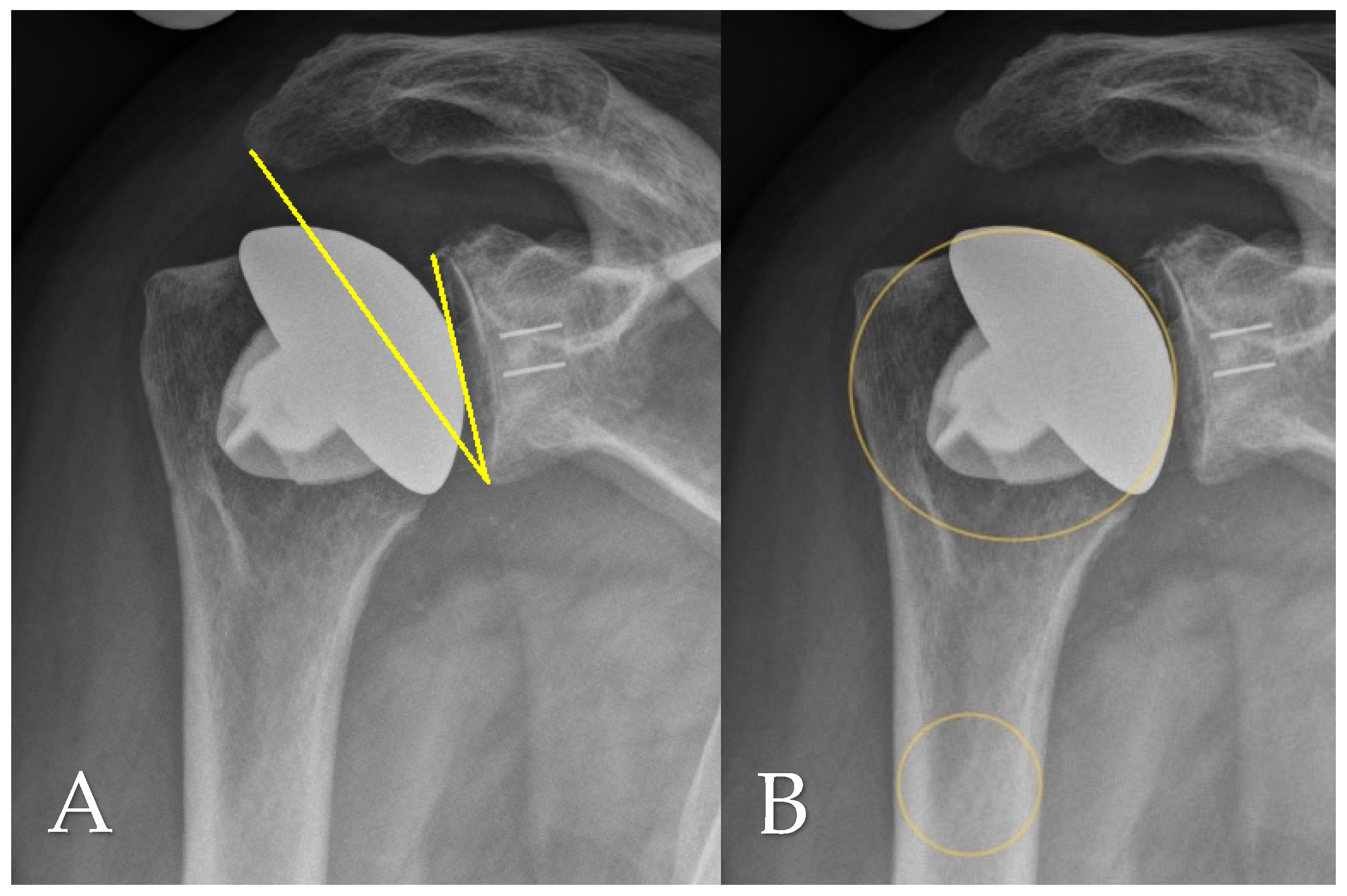
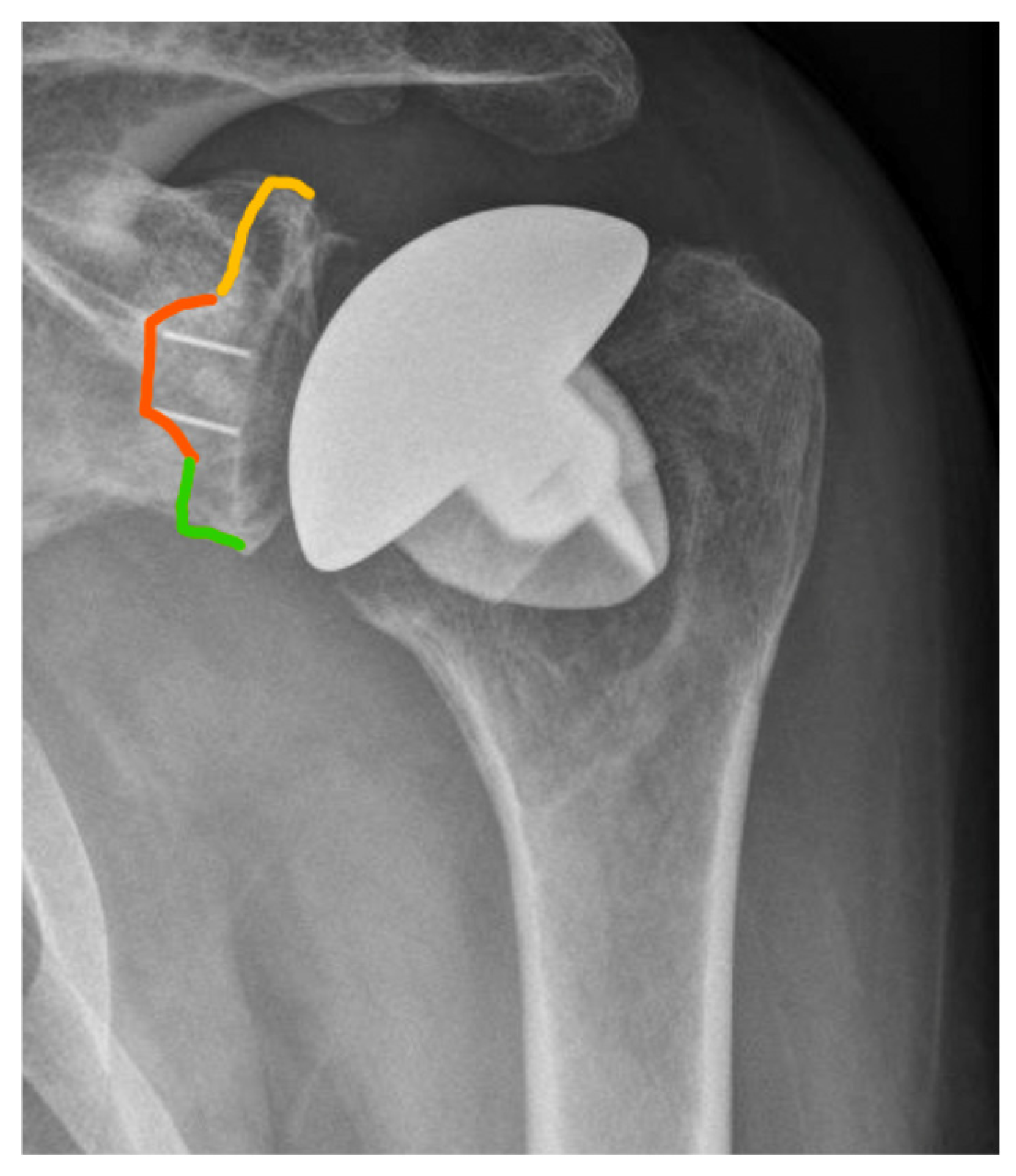
2.4. Radiographic Assessment
2.5. Data Analysis and Statistical Methods
3. Results
3.1. Patient Demographics
3.2. Evaluation of Clinical and Functional Results
3.3. Radiographic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunze, K.N.; Krivicich, L.M.; Brusalis, C.; Taylor, S.A.; Gulotta, L.V.; Dines, J.S.; Fu, M.C. Pathogenesis, Evaluation, and Management of Osteolysis After Total Shoulder Arthroplasty. Clin. Shoulder Elb. 2022, 25, 244–254. [Google Scholar] [CrossRef]
- Raiss, P.; Edwards, T.B.; Deutsch, A.; Shah, A.; Bruckner, T.; Loew, M.; Boileau, P.; Walch, G. Radiographic changes around humeral components in shoulder arthroplasty. J. Bone Jt. Surg. Am. 2014, 96, e54. [Google Scholar] [CrossRef]
- Hornung, A.L.; Cohn, M.R.; Mehta, N.; McCormick, J.R.; Menendez, M.E.; Pourzal, R.; Nicholson, G.P.; Garrigues, G.E. The Definition of Periprosthetic Osteolysis in Shoulder Arthroplasty: A Systematic Review of Grading Schemes and Criteria. JBJS Rev. 2022, 10, e22.00002. [Google Scholar] [CrossRef]
- Kepler, C.K.; Nho, S.J.; Bansal, M.; Ala, O.L.; Craig, E.V.; Wright, T.M.; Warren, R.F. Radiographic and histopathologic analysis of osteolysis after total shoulder arthroplasty. J. Shoulder Elb. Surg. 2010, 19, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Mazaleyrat, M.; Favard, L.; Boileau, P.; Berhouet, J. Humeral osteolysis after reverse shoulder arthroplasty using cemented or cementless stems comparative retrospective study with a mean follow-up of 9 years. Orthop. Traumatol. Surg. Res. 2021, 107, 102916. [Google Scholar] [CrossRef] [PubMed]
- Streck, L.E.; Gaal, C.; Gohlke, F.; Rudert, M.; List, K. Does radiolucency really predict loose components in revision shoulder arthroplasty? Skelet. Radiol. 2023, 52, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, D.; Birch, A.; Haines, J.F.; Watts, A.C.; Trail, I.A. Early migration of a partially cemented fluted glenoid component inserted using a cannulated preparation system. Bone Joint J. 2017, 99-B, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, D.; Haines, J.F.; Trail, I.A. The early migration of a partially cemented fluted pegged glenoid component using radiostereometric analysis. J. Shoulder Elbow. Surg. 2012, 21, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Razfar, N.; Reeves, J.M.; Langohr, D.G.; Willing, R.; Athwal, G.S.; Johnson, J.A. Comparison of proximal humeral bone stresses between stemless, short stem, and standard stem length: A finite element analysis. J. Shoulder Elb. Surg. 2016, 25, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Nuthalapati, P.; Feeley, B.T.; Lansdown, D.A. Outcomes of anatomic and reverse total shoulder arthroplasty in patients over the age of 70: A systematic review. JSES Rev. Rep. Tech. 2023, 3, 181–188. [Google Scholar] [CrossRef]
- Moor, B.K.; Bouaicha, S.; Rothenfluh, D.A.; Sukthankar, A.; Gerber, C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint?: A radiological study of the critical shoulder angle. Bone Joint J. 2013, 95-B, 935–941. [Google Scholar] [CrossRef]
- Sirveaux, F.; Favard, L.; Oudet, D.; Huquet, D.; Walch, G.; Mole, D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J. Bone Jt. Surg. Br. 2004, 86, 388–395. [Google Scholar] [CrossRef]
- Lévigne, C.F.J. Rheumatoid arthritis of the shoulder: Radiological presentation and results of arthroplasty. In Shoulder Arthroplasty; Walch, G., Boileau, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 221–230. [Google Scholar]
- Berthold, D.P.; Morikawa, D.; Muench, L.N.; Baldino, J.B.; Cote, M.P.; Creighton, R.A.; Denard, P.J.; Gobezie, R.; Lederman, E.; Romeo, A.A.; et al. Negligible Correlation between Radiographic Measurements and Clinical Outcomes in Patients Following Primary Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Hochberger, F.; Weth, B.; Heinz, T.; Boehm, D.; Rudert, M.; List, K. Outcomes of anatomic total shoulder arthroplasty: Evaluation of implant-related, radiographic, and demographic factors influencing durability and revision rates. Int. Orthop. 2025, 49, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Simovitch, R.W.; Friedman, R.J.; Cheung, E.V.; Flurin, P.H.; Wright, T.; Zuckerman, J.D.; Roche, C. Rate of Improvement in Clinical Outcomes with Anatomic and Reverse Total Shoulder Arthroplasty. J. Bone Jt. Surg. Am. 2017, 99, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Sarah, J.; Sanjay, G.; Sanjay, S.; Carolyn, A.; Emery, R.; Andrew, A.; Ulrich, H. Failure mechanism of the all-polyethylene glenoid implant. J. Biomech. 2010, 43, 714–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gohlke, F.; Werner, B.; Wiese, I. Glenoidrekonstruktion bei Wechseloperationen an der Schulter [Glenoid reconstruction in revision shoulder arthroplasty]. Oper. Orthop. Traumatol. 2019, 31, 98–114. (In German) [Google Scholar] [CrossRef] [PubMed]
- Flatow, E.L.; Soslowsky, L.J.; Ticker, J.B.; Pawluk, R.J.; Hepler, M.; Ark, J.; Mow, V.C.; Bigliani, L.U. Excursion of the rotator cuff under the acromion. Patterns of subacromial contact. Am. J. Sports Med. 1994, 22, 779–788. [Google Scholar] [CrossRef]
- Razmjou, H.; Palinkas, V.; Christakis, M.; Kennedy, D.; Robarts, S. Diagnostic Value of Acromiohumeral Distance in Rotator Cuff Pathology: Implications for Advanced-Practice Physiotherapists. Physiother. Can. 2020, 72, 52–62. [Google Scholar] [CrossRef]
- Duey, A.H.; Dieterich, J.D.; Patel, A.V.; White, C.A.; Cirino, C.M.; Li, T.; Galatz, L.M.; Parsons, B.O.; Flatow, E.L.; Cagle, P.J. Superior migration of the humeral head does not significantly affect outcomes at an average of 11 years after total shoulder arthroplasty. J. Shoulder Elb. Surg. 2023, 32, 2493–2500. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Chen, J.; Hua, Y.; Chen, S. Large Critical Shoulder Angle Has Higher Risk of Tendon Retear After Arthroscopic Rotator Cuff Repair. Am. J. Sports Med. 2018, 46, 1892–1900. [Google Scholar] [CrossRef]
- Monteiro, H.L.; Antunes, M.; Sarmento, M.; Quental, C.; Folgado, J. Influence of age-related bone density changes on primary stability in stemless shoulder arthroplasty: A multi-implant finite element study. J. Shoulder Elb. Surg. 2025, 34, 557–566. [Google Scholar] [CrossRef]
- Crowley, M.; Ghattas, Y.; Collins, A.P.; Levin, S.; Service, B.C. Systematic Review and Meta-Analysis of Locked Posterior Dislocation of the Shoulder Treated with Shoulder Arthroplasty: Improved Outcomes for Total Shoulder Arthroplasty are Associated with Increased Age. Orthop. Surg. 2023, 15, 1730–1741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiater, J.M.; Levy, J.C.; Wright, S.A.; Brockmeier, S.F.; Duquin, T.R.; Wright, J.O.; Codd, T.P. Prospective, Blinded, Randomized Controlled Trial of Stemless Versus Stemmed Humeral Components in Anatomic Total Shoulder Arthroplasty: Results at Short-Term Follow-up. J. Bone Joint Surg. 2020, 102, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Looney, A.M.; Day, J.; Johnson, J.L.; Johnston, P.S. Outcomes Between Stemmed and Stemless Total Shoulder Arthroplasty: A Systematic Review and Meta-Analysis. JAAOS Glob. Res. Rev. 2022, 6, e22.00077. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, Y.S.; Lee, S.H.; Jeong, H.J.; Kim, Y.K.; Oh, J.H. Can convertible metal-backed glenoid components replace cemented polyethylene glenoid components in anatomical total shoulder arthroplasty? BMC Surg. 2023, 23, 193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


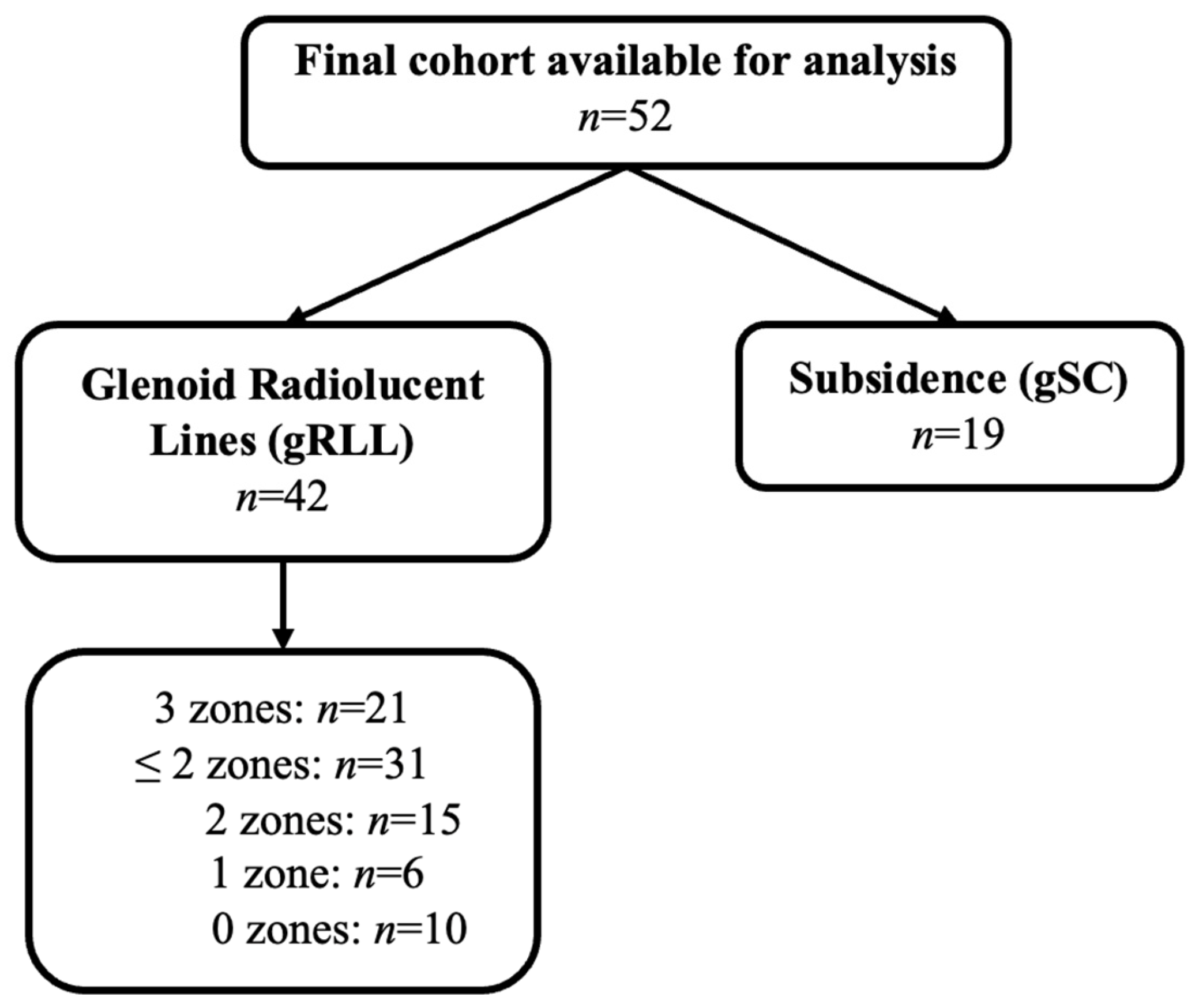
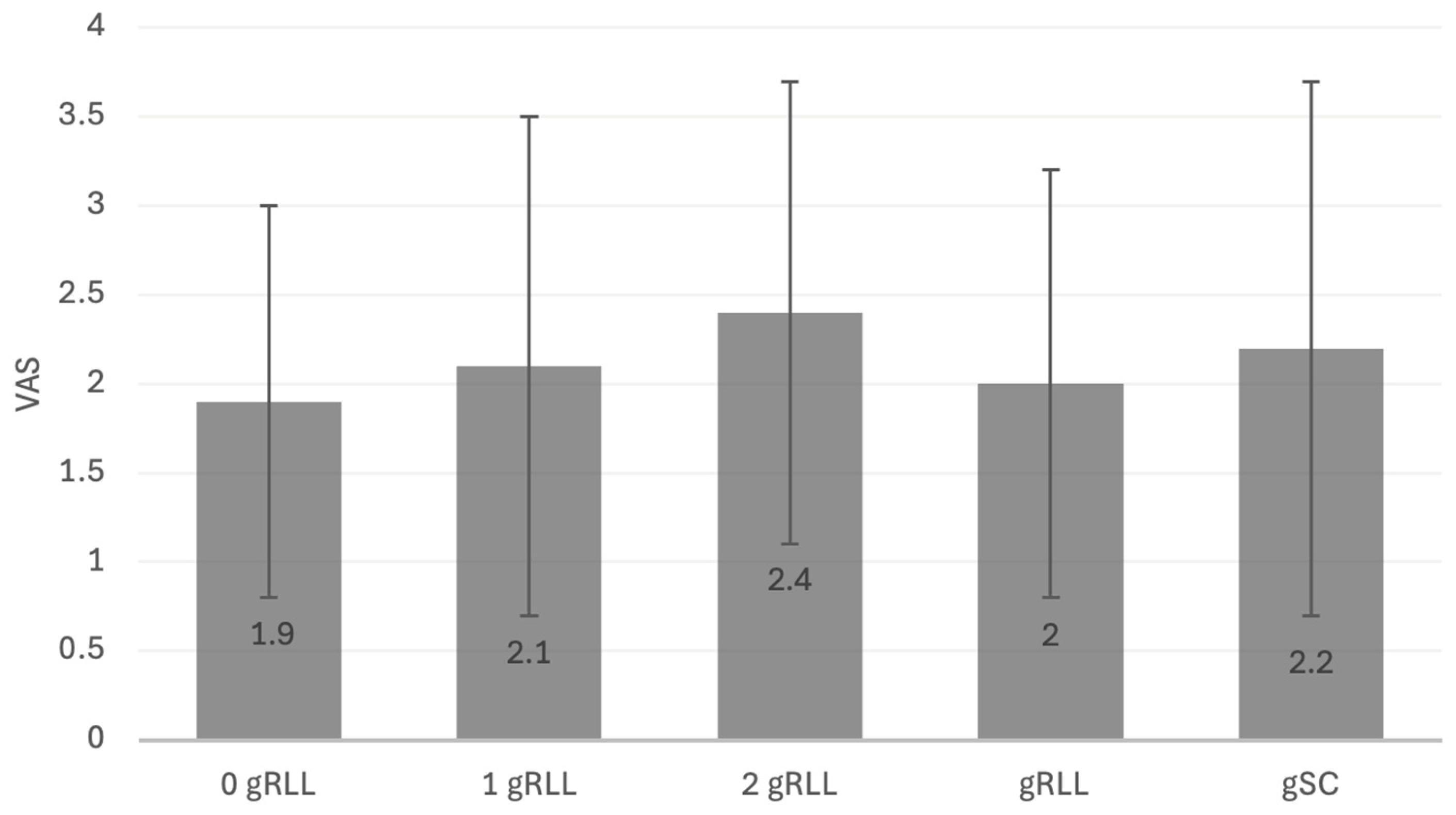
| Glenoid Type | 0 gRLL | 1 gRLL | 2 gRLL | 3 gRLL | gSC |
|---|---|---|---|---|---|
| Perform™ Glenoid, Stryker/Tornier (n = 12) | 4 (33%) | 1 (8%) | 4 (33%) | 3 (25%) | 3 (25%) |
| Arthrex Keel Glenoid® (n = 4) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (100%) | 3 (75%) |
| Aequalis™ Glenoid, Tornier (n = 36) | 6 (16%) | 5 (14%) | 11 (31%) | 14 (39%) | 13 (36%) |
| Walch Group | ≤2 Zones | 3 Zones | Total | Mean Follow-Up (m) | p-Value |
|---|---|---|---|---|---|
| A1/A2 | 28 | 15 | 43 | 125.5 ± 35.1 | 0.09 |
| B1/B2 | 2 | 7 | 9 | 105.3 ± 34.3 | 0.14 |
| Parameter | ≤2 Zones (n = 31) (Mean ± SD, 95% CI) | 3 Zones (n = 21) (Mean ± SD, 95% CI) | p-Value |
|---|---|---|---|
| Age at surgery (years) | 62.2 ± 8.0 (59.2–65.1) | 62.4 ± 6.9 (59.3–65.6) | 0.76 |
| Sex (male/female) | 13 (M)/18 (F) | 10 (M)/11 (F) | 0.69 |
| Side (right/left) | 12 (R)/19 (L) | 12 (R)/9 (L) | 0.19 |
| Follow-up (months) | 122.1 ± 32.8 (110.0–134.1) | 121.9 ± 40.0 (103.6–140.0) | 0.77 |
| ASA Classification | 2.1 ± 0.5 (1.9–2.3) | 2.4 ± 0.7 (2.0–2.7) | 0.14 |
| BMI | 29.4 ± 5.4 (27.5–31.4) | 31.2 ± 6.5 (28.3–34.2) | 0.31 |
| Osteoporosis (yes/no) | 2/29 | 3/18 | 0.35 |
| Rheumatoid arthritis (yes/no) | 1/30 | 2/19 | 0.34 |
| Diabetes mellitus (yes/no) | 2/29 | 3/18 | 0.35 |
| Smoker (yes/no) | 4/27 | 5/16 | 0.30 |
| Fixation (cemented/uncemented) | 30/1 | 21/0 | 0.40 |
| Indication (OA/HN) Implant type (stemless/short-stem) | 31/0 14/17 | 19/2 8/13 | 0.06 0.61 |
| Parameter | ≤2 Zones (n = 31) (Mean ± SD, 95% CI) | 3 Zones (n = 21) (Mean ± SD, 95% CI) | p-Value |
|---|---|---|---|
| FE (°) | 121 ± 9 (118–124) | 118 ± 9 (114–122) | 0.27 |
| ABD (°) | 114 ± 10 (110–118) | 111 ± 7 (108–114) | 0.26 |
| ER (°) | 47 ± 4 (45–48) | 44 ± 4 (42–46) | <0.05 |
| IR (°) | 34 ± 5 (32–36) | 35 ± 6 (32–38) | 0.60 |
| VAS (0–10) | 2.4 ± 1.3 (1.7–1.5) | 2.1 ± 0.9 (1.9–1.4) | 0.09 |
| Constant | 66 ± 21 (58–74) | 64 ± 12 (59–70) | 0.78 |
| DASH | 34 ± 24 (25–43) | 25 ± 20 (15–34) | 0.14 |
| Parameter | 0 Zones (n = 10) (Mean ± SD, 95% CI) | 1 Zone (n = 6) (Mean ± SD, 95% CI) | p-Value |
|---|---|---|---|
| FE (°) | 121 ± 12 (113–130) | 122 ± 11 (111–134) | 0.75 |
| ABD (°) | 120 ± 7 (115–125) | 112.8 ± 12.3 (100–126) | 0.23 |
| ER (°) | 49 ± 2 (47–50) | 46 ± 4 (42–51) | 0.33 |
| IR (°) | 36 ± 5 (33–39) | 33 ± 5 (27–38) | 0.19 |
| VAS (0–10) | 1.9 ± 1.1 (1.5–1.7) | 2.1 ± 1.4 (1.7–1.3) | 0.07 |
| Constant | 55.4 ± 25.6 (37.0–73.7) | 69.7 ± 15.1 (53.8–85.6) | 0.33 |
| DASH | 45.4 ± 20.9 (30.5–60.4) | 24.4 ± 22.5 (0.8–48.0) | 0.05 |
| Parameter | 0 Zones (n = 10) (Mean ± SD, 95% CI) | 3 Zones (n = 21) (Mean ± SD, 95% CI) | p-Value |
|---|---|---|---|
| FE (°) | 121 ± 11 (113–124) | 118 ± 9 (114–122) | 0.44 |
| ABD (°) | 114 ± 10 (110–118) | 120 ± 7 (115–125) | <0.05 |
| ER (°) | 48 ± 2 (47–50) | 44 ± 4 (42–46) | <0.05 |
| IR (°) | 35.9 ± 4.5 (33–39) | 35 ± 6 (32–38) | 0.62 |
| VAS (0–10) | 1.9 ± 1.1 (1.5–1.7) | 2.0 ± 1.2 (1.7–1.8) | 0.07 |
| Constant | 55.4 ± 25.6 (37.0–73.7) | 64.3 ± 12.4 (58.7–69.9) | 0.32 |
| DASH | 45.4 ± 20.9 (30.5–60.4) | 24.6 ± 20.2 (15.3–33.8) | <0.05 |
| Parameter | gSC (n = 19) (Mean ± SD, 95% CI) | No gSC (n = 33) (Mean ± SD, 95% CI) | p-Value |
|---|---|---|---|
| FE (°) | 116 ± 10 (112–121) | 122 ± 8 (119–125) | <0.05 |
| ABD (°) | 111 ± 9 (107–115) | 114 ± 10 (110–117) | 0.40 |
| ER (°) | 44 ± 5 (42–46) | 46 ± 4 (45–48) | <0.05 |
| IR (°) | 33 ± 6 (31–36) | 35 ± 6 (33–37) | 0.27 |
| VAS (0–10) | 2.2 ± 1.5 (1.7–2.7) | 2.0 ± 0.4 (1.9–2.1) | 0.07 |
| Constant | 66.0 ± 13.3 (59.6–72.5) | 64.7 ± 20.3 (57.5–71.9) | 0.80 |
| DASH | 22.7 ± 18.6 (13.7–31.6) | 34.6 ± 24.3 (25.9–43.2) | 0.07 |
| Parameter | Decentration (n = 19) (Mean ± SD, 95% CI) | No decentration (n = 33) (Mean ± SD, 95% CI) | p-Value |
|---|---|---|---|
| FE (°) | 120 ± 9 (116–124) | 120 ± 10 (116–123) | 0.96 |
| ABD (°) | 112 ± 9 (108–116) | 113 ± 9 (110–116) | 0.78 |
| ER (°) | 44 ± 4 (42–46) | 46 ± 4 (45–48) | 0.06 |
| IR (°) | 34 ± 5 (32–36) | 35 ± 6 (33–37) | 0.63 |
| VAS (0–10) | 1.8 ± 0.8 (1.3–1.4) | 2.1 ± 0.7 (1.4–1.1) | 0.07 |
| Constant | 64.9 ± 18.1 (56.7–73.1) | 65.4 ± 18.2 (58.8–72.0) | 0.92 |
| DASH | 31.0 ± 25.0 (19.6–42.4) | 29.7 ± 21.8 (21.7–37.7) | 0.85 |
| Parameter | ≤2 Zones (n = 31) (Mean ± SD, 95% CI) | 3 Zones (n = 21) (Mean ± SD, 95% CI) | p-Value |
|---|---|---|---|
| CSA° | 33 ± 7 (30–36) | 31 ± 6 (29–33) | 0.19 |
| LO | 9 ± 8 (6–12) | 12 ± 8 (9–15) | 0.10 |
| AHD | 8.5 ± 4.6 (6.5–10.5) | 10.5 ± 4.4 (8.8–12.2) | 0.13 |
| HSI | 0.4 ± 0.0 (0.4–0.5) | 0.4 ± 0.0 (0.4–0.5) | 0.95 |
| Levigne | 1.6 ± 0.6 (1.3–1.8) | 1.5 ± 0.6 (1.2–1.7) | 0.53 |
| Sirveaux | 0.9 ± 0.9 (0.6–1.3) | 0.7 ± 0.6 (0.5–1.0) | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochberger, F.; Limmer, J.; Muhmann, J.; Gohlke, F.; Streck, L.E.; Rudert, M.; List, K. Glenoid Radiolucent Lines and Subsidence Show Limited Impact on Clinical and Functional Long-Term Outcomes After Anatomic Total Shoulder Arthroplasty: A Retrospective Analysis of Cemented Polyethylene Glenoid Components. J. Clin. Med. 2025, 14, 7058. https://doi.org/10.3390/jcm14197058
Hochberger F, Limmer J, Muhmann J, Gohlke F, Streck LE, Rudert M, List K. Glenoid Radiolucent Lines and Subsidence Show Limited Impact on Clinical and Functional Long-Term Outcomes After Anatomic Total Shoulder Arthroplasty: A Retrospective Analysis of Cemented Polyethylene Glenoid Components. Journal of Clinical Medicine. 2025; 14(19):7058. https://doi.org/10.3390/jcm14197058
Chicago/Turabian StyleHochberger, Felix, Jonas Limmer, Justus Muhmann, Frank Gohlke, Laura Elisa Streck, Maximilian Rudert, and Kilian List. 2025. "Glenoid Radiolucent Lines and Subsidence Show Limited Impact on Clinical and Functional Long-Term Outcomes After Anatomic Total Shoulder Arthroplasty: A Retrospective Analysis of Cemented Polyethylene Glenoid Components" Journal of Clinical Medicine 14, no. 19: 7058. https://doi.org/10.3390/jcm14197058
APA StyleHochberger, F., Limmer, J., Muhmann, J., Gohlke, F., Streck, L. E., Rudert, M., & List, K. (2025). Glenoid Radiolucent Lines and Subsidence Show Limited Impact on Clinical and Functional Long-Term Outcomes After Anatomic Total Shoulder Arthroplasty: A Retrospective Analysis of Cemented Polyethylene Glenoid Components. Journal of Clinical Medicine, 14(19), 7058. https://doi.org/10.3390/jcm14197058







