Preliminary Efficacy/Feasibility Study of a Breast Cancer-Related Lymphedema Prospective Screening and Early Intervention Program at the Dana-Farber Brigham Cancer Center
Abstract
1. Introduction
2. Methods and Approach to Developing the PSM
2.1. PSM Personnel and Patient Selection
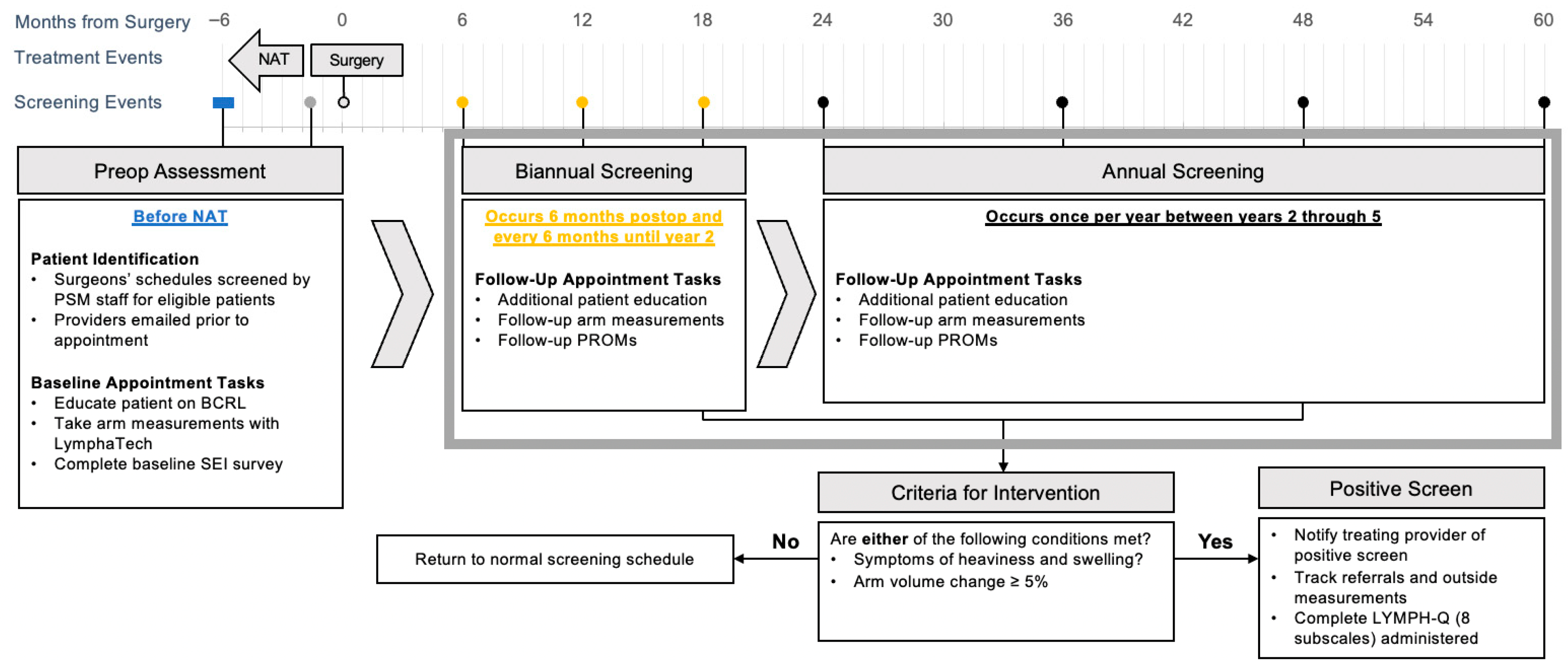
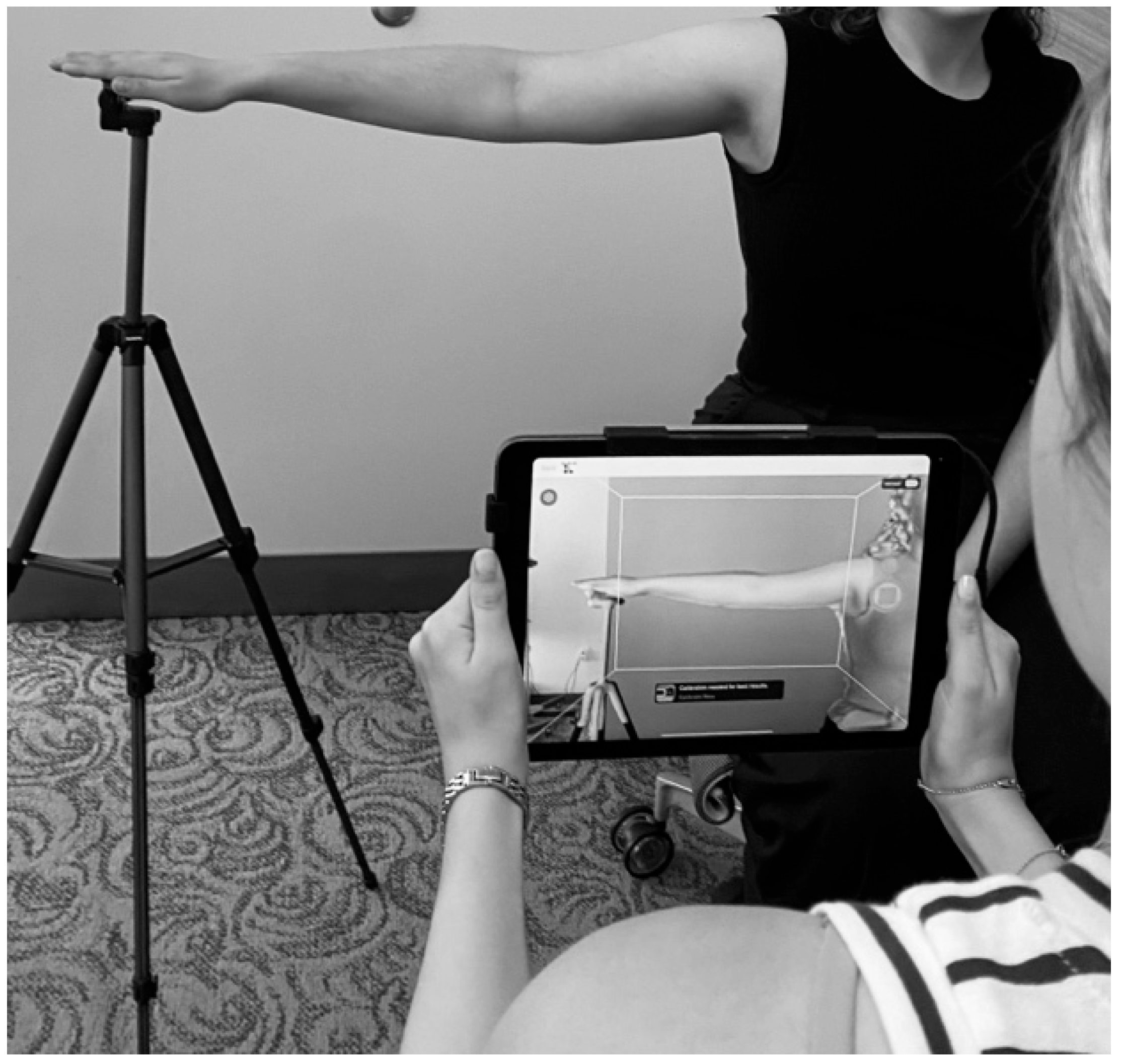
2.2. Initial Intake (Baseline) Appointment
2.3. Bi-Annual Screening: 6–24 Months Postoperatively
2.4. Annual Screening After 24 Months Postoperatively
2.5. Metrics Used in Screening
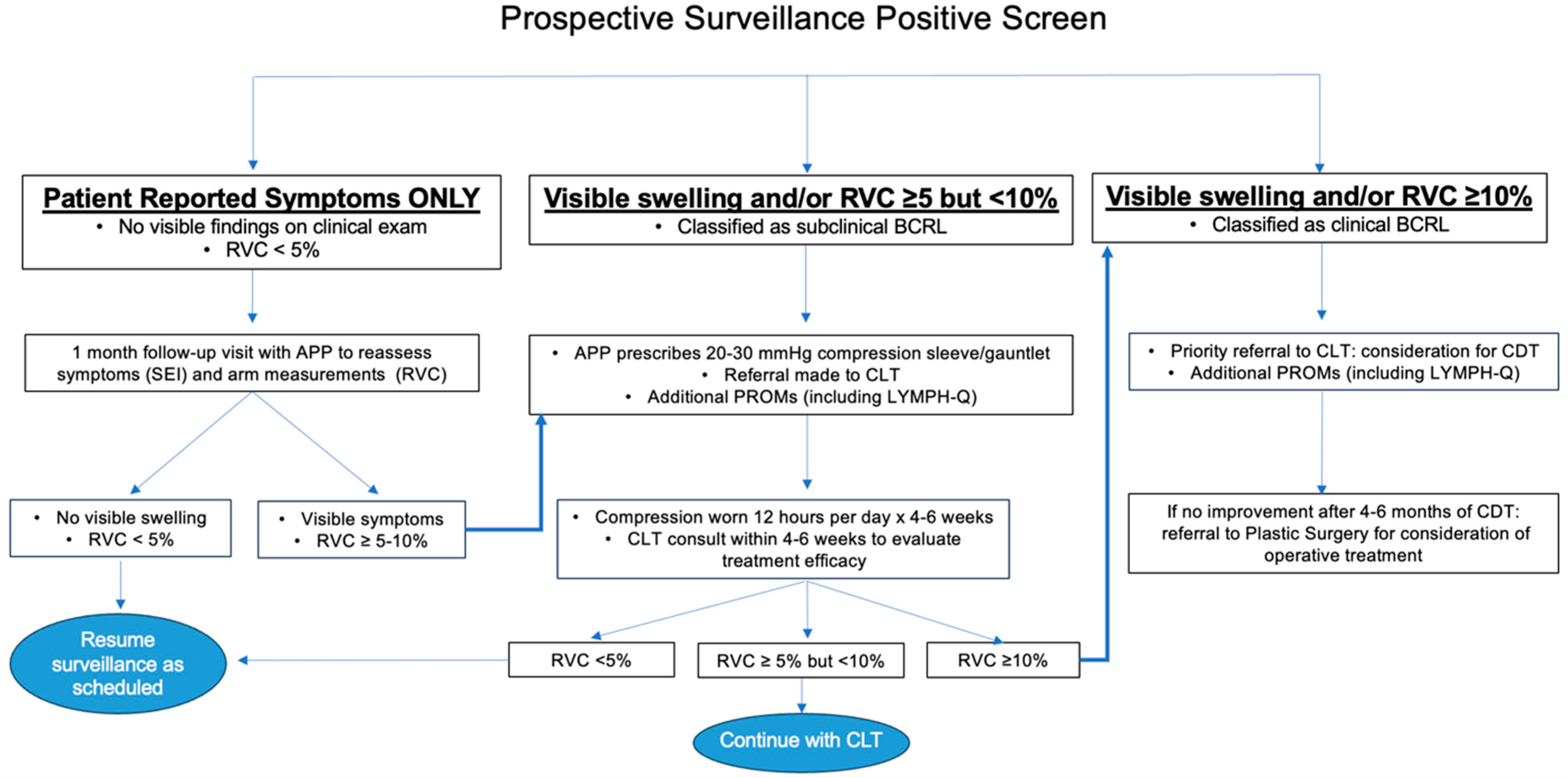
2.6. Early Intervention
3. Analysis
3.1. Assessing Feasibility, Organizational Readiness and Implementation
3.2. Evaluating Program Efficacy
3.3. Validating Relevant PROMs
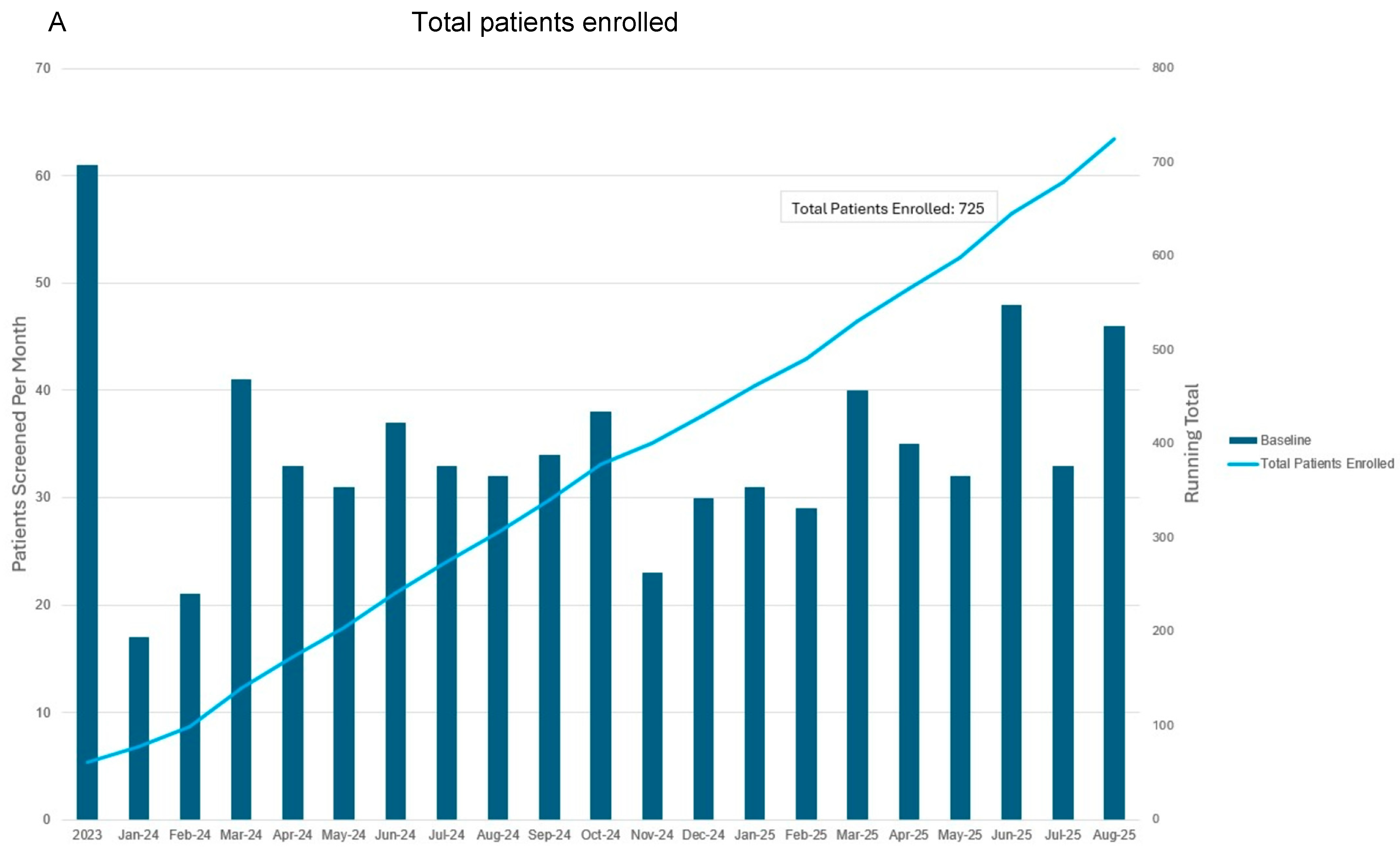
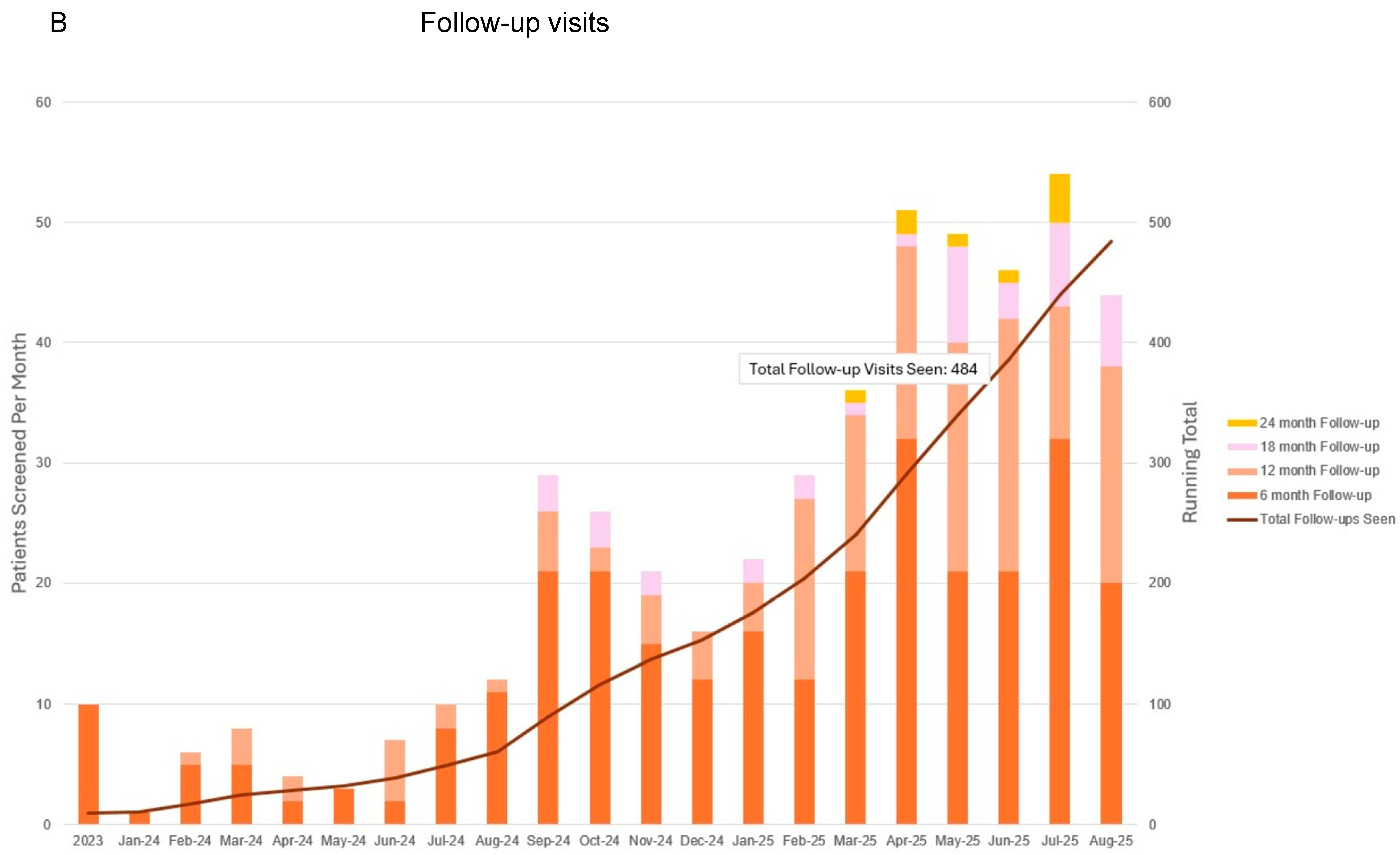
3.4. Progress to Date
4. Concluding Remarks: Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillai, U.S.; Kayal, S.; Cyriac, S.; Nisha, Y.; Dharanipragada, K.; Kamalanathan, S.K.; Halanaik, D.; Kumar, N.; Madasamy, P.; Muniswamy, D.K.; et al. Late effects of breast cancer treatment and outcome after corrective intervention. Asian Pac. J. Cancer Prev. 2019, 20, 2673–2679. [Google Scholar] [CrossRef]
- Tandra, P.; Kallam, A.; Krishnamurthy, J. Identification and management of lymphedema in patients with breast cancer. J. Oncol. Pract. 2019, 15, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, C.S.; Sanft, T.; Moslehi, J.J.; Overholser, L.; Armenian, S.; Baker, K.S.; Broderick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; et al. NCCN Guidelines Insights: Survivorship, Version 2.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 1016–2023. [Google Scholar] [CrossRef]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Danielle, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef]
- Shaitelman, S.F.; Cromwell, K.D.; Rasmussen, J.C.; Stout, N.L.; Armer, J.M.; Lasinski, B.B.; Cormier, J.N. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J. Clin. 2015, 65, 55–81. [Google Scholar] [CrossRef]
- Ridner, S.H.; Dietrich, M.S.; Boyages, J.; Koelmeyer, L.; Elder, E.; Hughes, T.M.; French, J.; Ngui, N.; Hsu, J.; Abramson, V.G.; et al. A comparison of bioimpedance spectroscopu or tape measure triggered compression intervention in chronic breast cancer lymphedema prevention. Lymphat. Res. Biol. 2022, 20, 618–628. [Google Scholar] [CrossRef]
- Gebruers, N.; Verbelen, H.; De Vrieze, T.; Vos, L.; Devoogdt, N.; Fias, L.; Tjalma, W. Current and future perspectives on the evaluation, prevention, and conservatice management of breast cancer related lymphoedema: A best practice guideline. Eur. J. Obs. Gynceol. Reprod. Biol. 2017, 216, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Armer, J.M.; Hulett, J.M.; Bernas, M.; Ostby, P.; Stewart, B.R.; Cormier, J.N. Best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Curr. Breast Cancer Rep. 2013, 5, 134–144. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Brunelle, C.L.; Taghian, A. Breast cancer-related lymphedema: Risk factors, screening, management, and the impact of locoregional treatment. J. Clin. Oncol. 2020, 38, 2341–2350. [Google Scholar] [CrossRef]
- Koelmeyer, L.; Gaitatzis, K.; Ridner, S.H.; Boyages, J.; Nelms, J.; Hughes, T.M.; Elder, E.; French, J.; Ngui, N.; Hsu, J.; et al. Implementing a prospective surveillance and early intervention model of care for breast cancer related lymphedema into clinical practice: Application of the RE-AIM framework. Support. Care Cancer 2021, 29, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.M.; Nelson, J.; Sowles, L.; Stephenson, R.G.; Robinson, K.; Cheville, A.; Sander, A.P.; Blot, W.J. Lymphedema signs, symptoms, and diagnosis in women who are in minority and low-income groups and have survived breast cancer. Phys. Ther. 2020, 100, 487–499. [Google Scholar] [CrossRef]
- Lentz, R.; Shin, C.; Bloom, Z.; Yamada, K.; Hong, Y.; Wong, A.K.; Patel, K. From bench to bedside: The role of a multidisciplinary approach to treating patients with lymphedema. Lymphat. Res. Biol. 2021, 19, 11–16. [Google Scholar] [CrossRef]
- Shah, C.; Arthur, D.W.; Wazer, D.; Khan, A.; Ridner, S.; Vicini, F. The impact of early detection and intervention of breast cancer-related lymphedema: A systematic review. Cancer Med. 2016, 5, 1154–1162. [Google Scholar] [CrossRef]
- Cheville, A.L.; Nyman, K.A.; Pruthi, S.; Basford, J.R. Cost considerations regarding the prospective surveillance model for breast cancer survivors. Cancer 2012, 118, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Vignes, S.; Arrault, M.; Dupuy, A. Factors associated with increased breast cancer-related lymphedema and volume. Acta Oncol. 2007, 46, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kebede, M.A.; Ogunleye, A.A.; Emerson, M.A.; Evenson, K.R.; Carey, L.A.; Hayes, S.C.; Troester, M.A. Burden of lymphedema in long-term breast cancer survivors by race and age. Cancer 2022, 128, 4119–4128. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, J.L.B.; Kattan, M.W.; Changhong, Y.; Koifman, S.; Mattos, I.E.; Koifman, R.J.; Bergmann, A. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann. Surg. Oncol. 2012, 19, 2580–2589. [Google Scholar] [CrossRef]
- Sagen, A.; Kaaresen, R.; Sandvik, L.; Thune, I.; Risberg, M.A. Upper limb physical function and adverse effects after breast cancer surgery: A prospective 2.5-year follow up study and preoperative measures. Arch. Phys. Med. Rehabil. 2014, 95, 875–881. [Google Scholar] [CrossRef]
- Soran, A.E.; Menekse, E.; Girgis, M.; DeGore, L.; Johnson, R. Brease cancer-related lymphedema after axllary lymph node dissection: Does early postoperative prediction model work. Support. Care Cancer 2014, 24, 1413–1419. [Google Scholar] [CrossRef]
- Warren, L.E.; Miller, C.L.; Horick, N.; Skolny, M.N.; Jammallo, L.S.; Sadek, B.T.; Shenouda, M.N.; O’TOole, J.A.; MacDonald, S.M.; Specht, M.C.; et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: A prospective cohort study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 565–571. [Google Scholar] [CrossRef]
- Walsh, V.L.; Fox, L.M.; Brady, M.; King, J.; Worrell, C.M.; Hübner, M.P. A Delphi consultation to assess indicators of readiness to provide quality health facility-based lymphoedema management services. PLoS Negl. Trop. Dis. 2018, 12, e0006699. [Google Scholar] [CrossRef]
- Given, C.W.; Sikorskii, A.; Tamkus, D.; Given, B.; You, M.; McCorksle, R.; Champion, V.; Decker, D. Managing symptoms among patients with breast cancer during chemotherapy:results of a two-arm behavioral trial. J. Clin. Oncol. 2008, 26, 5855–5862. [Google Scholar] [CrossRef]
- Swaroop, M.N.; Ferguson, C.M.; Horick, N.K.; Skolny, M.N.; Miller, C.L.; Jammallo, L.S.; Brunelle, C.L.; O’Toole, B.A.; Isakoff, S.J.; Specht, M.C.; et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: Results from a large prospective cohort. Breast Cancer Res. Treat. 2015, 151, 393–403. [Google Scholar] [CrossRef] [PubMed]
- McDuff, S.G.R.; Mina, A.I.; Brunelle, C.L.; Salama, L.; Warren, L.E.G.; Abouegylah, M.; Swaroop, M.; Skolny, M.N.; Asdourian, M.; Gillespie, T.; et al. Timing of lymphedema following treatment for breast cancer: When are patients most at-risk? Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 62–70. [Google Scholar] [CrossRef]
- Ancukiewicz, M.; Russell, T.A.; Otoole, J.; Specht, M.; Singer, M.; Kelada, A.; Murphy, C.D.; Pogachar, J.; Gioioso, V.; Patel, M.; et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Binkley, J.M.; Weiler, M.J.; Frank, N.; Bober, L.; Dixon, J.B.; Stratford, P.W. Assessing arm volume in people during and after treatment for breast cancer: Reliability and convergent validity of the LymphaTech system. Phys. Ther. 2020, 100, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Houwen, F.; Stemkens, J.; de Schipper, P.J.; van der Wouw, P.; Heitink, M.; van Langen, H. Estimate for assessment of lymphedema: Reliability and validity of extremity measurements. Lymphat. Res. Biol. 2022, 20, 48–52. [Google Scholar] [CrossRef]
- Miller, C.L.; Specht, M.C.; Horick, N.; Skolny, M.N.; Jammallo, L.S.; O’Toole, J.; Taghian, A.G. A novel, validated method to quantify breast cancer-related lymphedema (BCRL) following bilateral breast surgery. Lymphology 2013, 46, 65–74. [Google Scholar] [PubMed]
- Sun, F.; Skolny, M.N.; Swaroop, M.N.; Rawal, B.; Catalano, P.J.; Brunelle, C.L.; Miller, C.L.; Taghian, A.G. The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema. Breast Cancer Res. Treat. 2016, 157, 229–240. [Google Scholar] [CrossRef]
- DiSipio, T.; Rue, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Brunelle, C.L.; Roberts, S.A.; Horick, N.K.; Gillespie, T.C.; Jacobs, J.M.; Daniell, K.M.; Naoum, G.E.; Taghian, A.G. Integrating symptoms into the diagnostic criteria for breast cancer-related lymphedema: Applying results from a prospective surveillance program. Phys. Ther. 2020, 100, 2186–2197. [Google Scholar] [CrossRef]
- Bian, J.; Shen, A.; Yang, W.; Zhang, L.; Qiang, W. Financial toxicity experienced by patients with breast cancer-related lymphedema: A systematic review. Support. Care Cancer 2023, 31, 354. [Google Scholar] [CrossRef]
- Fu, M.R.; Rosedale, M. Breast cancer survivors’ experiences of lymphedema-related symptoms. J. Pain Symptom Manag. 2009, 38, 849–859. [Google Scholar] [CrossRef]
- Armer, J.M.; Radina, M.E.; Porock, D.; Culbertson, S.D. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs. Res. 2003, 52, 370–379. [Google Scholar] [CrossRef]
- Klassen, A.F.; Tsangaris, E.; Kaur, M.N.; Poulsen, L.; Beelen, L.M.; Jacobsen, A.L.; Jørgensen, M.G.; Sørensen, J.A.; Vasilic, D.; Dayan, J.; et al. Development and Psychometric Validation of a Patient-Reported Outcome Measure for Arm Lymphedema: The LYMPH-Q Upper Extremity Module. Ann. Surg. Oncol. 2021, 28, 5166–5182. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.P.; Laws, A.; Dominici, L.S.; Lagendijk, M.; Grossmith, S.; Mittendorf, E.A.; King, T.A. Arm morbidity and financial difficulty in breast cancer survivors. J. Cancer Surviv. 2024, 1–8, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.P.; Zheng, Y.; Dibble, K.; Mittendorf, E.A.; King, T.A.; Ruddy, K.J.; Peppercorn, J.M.; Schapira, L.; Borges, V.F.; Come, S.E.; et al. Financial difficulty over time in a multisite prospective cohort of young adults with breast cancer: A secondary analysis of data from the Young Women’s Breast Cancer Study. JAMA Network Open. 2024, 7, e2446091. [Google Scholar] [CrossRef]
- de Souza, J.A.; Yap, B.J.; Wroblewski, K.; Blinder, V.; Araújo, F.S.; Hlubocky, F.J.; Nicholas, L.H.; O’Connor, J.M.; Brockstein, B.; Ratain, M.J.; et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer 2017, 123, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Successful Qualitative Research: A Practice Guide for Beginners; SAGE publications, Ltd.: London, UK, 2013. [Google Scholar]
- Barrett, G.V.; Phillips, J.S.; Alexander, R.A. Concurrent and predictive validity designs: A critical reanalysis. J. Appl. Psychol. 1981, 66, 1–6. [Google Scholar] [CrossRef]
| Criteria for Enrollment | Details |
|---|---|
| Tumor size > 5 cm | Any receptor status |
| Lymph node involvement | Any receptor status |
| HER2 + disease | Tumor size > 2 cm |
| ER/PR-HER2- | Tumor size > 1.5–2 cm |
| Characteristic | n/N (%) n = 348 |
|---|---|
| Age at diagnosis (years) | |
| ≤40 | 93 (26.7%) |
| 40–65 | 186 (53.4%) |
| ≥65 | 69 (19.8%) |
| BMI | |
| <30 | 244 (70.1%) |
| ≥30 | 104 (29.9%) |
| Race | |
| White | 266 (76.4%) |
| Black | 37 (10.6%) |
| Asian/Pacific Islander | 25 (7.2%) |
| American Indian/Aleutian | 1 (3%) |
| Other | 2 (0.6%) |
| Unknown | 17 (4.9%) |
| Ethnicity | |
| Not Hispanic/Latino | 318 (91.4%) |
| Hispanic/Latino | 22 (6.3%) |
| Unknown | 8 (2.3%) |
| Sex at birth | |
| Female | 341 (98.0%) |
| Male | 7 (2.0%) |
| Handedness | |
| Left | 21 (6.0%) |
| Right | 268 (77.0%) |
| Unknown | 59 (17.0%) |
| Laterality of breast cancer | |
| Right | 171 (49.1%) |
| Left | 165 (47.4%) |
| Bilateral | 12 (3.4%) |
| Clinical T stage | |
| T0 | 3 (0.9%) |
| T1 | 77 (22.1%) |
| T2 | 189 (54.3%) |
| T3 | 50 (14.4%) |
| T4 | 26 (7.5%) |
| Clinical N stage | |
| N0 | 126 (36.2%) |
| N1 | 187 (53.7%) |
| N2/3 | 33 (9.5%) |
| Nx | 2 (0.6%) |
| Treatment | |
| Neoadjuvant chemotherapy | 324 (93.1%) |
| Neoadjuvant endocrine therapy | 7 (2.0%) |
| Upfront surgery | 15 (4.3%) |
| Other | 2 (0.6%) |
| Type of surgery | |
| Partial mastectomy | 110 (31.6%) |
| Total mastectomy with reconstruction | 76 (21.8%) |
| Total mastectomy without reconstruction | 65 (18.7%) |
| Other | 5 (1.4%) |
| Surgery not yet completed | 92 (26.4%) |
| Axillary staging | |
| Sentinel lymph node biopsy | 119 (34.2%) |
| Axillary lymph node dissection | 134 (38.5%) |
| None | 3 (0.9%) |
| Surgery not yet completed | 92 (26.4%) |
| Prophylactic lymphovenous bypass | 4 (1.2%) |
| Radiation regimen | |
| None | 28 (8.0%) |
| WBRT with RNI | 44 (12.6%) |
| WBRT without RNI | 36 (10.3%) |
| PMRT with RNI | 69 (19.8%) |
| PMRT without RNI | 11 (3.2%) |
| Radiation not yet completed | 160 (46.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myers, S.P.; Jasper, J.M.; Higgins, T.; Serig, A.; Faust, A.C.; Tappan, L.J.; Nakhlis, F.; Taylor, E.M.; Agarwal, S.; Mittendorf, E.A.; et al. Preliminary Efficacy/Feasibility Study of a Breast Cancer-Related Lymphedema Prospective Screening and Early Intervention Program at the Dana-Farber Brigham Cancer Center. J. Clin. Med. 2025, 14, 7051. https://doi.org/10.3390/jcm14197051
Myers SP, Jasper JM, Higgins T, Serig A, Faust AC, Tappan LJ, Nakhlis F, Taylor EM, Agarwal S, Mittendorf EA, et al. Preliminary Efficacy/Feasibility Study of a Breast Cancer-Related Lymphedema Prospective Screening and Early Intervention Program at the Dana-Farber Brigham Cancer Center. Journal of Clinical Medicine. 2025; 14(19):7051. https://doi.org/10.3390/jcm14197051
Chicago/Turabian StyleMyers, Sara P., Jacob M. Jasper, Tessa Higgins, Angela Serig, Amanda C. Faust, Lila J. Tappan, Faina Nakhlis, Erin M. Taylor, Shailesh Agarwal, Elizabeth A. Mittendorf, and et al. 2025. "Preliminary Efficacy/Feasibility Study of a Breast Cancer-Related Lymphedema Prospective Screening and Early Intervention Program at the Dana-Farber Brigham Cancer Center" Journal of Clinical Medicine 14, no. 19: 7051. https://doi.org/10.3390/jcm14197051
APA StyleMyers, S. P., Jasper, J. M., Higgins, T., Serig, A., Faust, A. C., Tappan, L. J., Nakhlis, F., Taylor, E. M., Agarwal, S., Mittendorf, E. A., & King, T. A. (2025). Preliminary Efficacy/Feasibility Study of a Breast Cancer-Related Lymphedema Prospective Screening and Early Intervention Program at the Dana-Farber Brigham Cancer Center. Journal of Clinical Medicine, 14(19), 7051. https://doi.org/10.3390/jcm14197051







