Prolonging the Warm-Up Effect by Using Additional Respiratory Dead Space Volume After the Cessation of Warm-Up Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.2.1. Progressive Test with Verification Phase
2.2.2. The 3 Min Non-ARDSv Test
2.2.3. The 3 Min ARDSv Test
2.3. Statistical Analysis
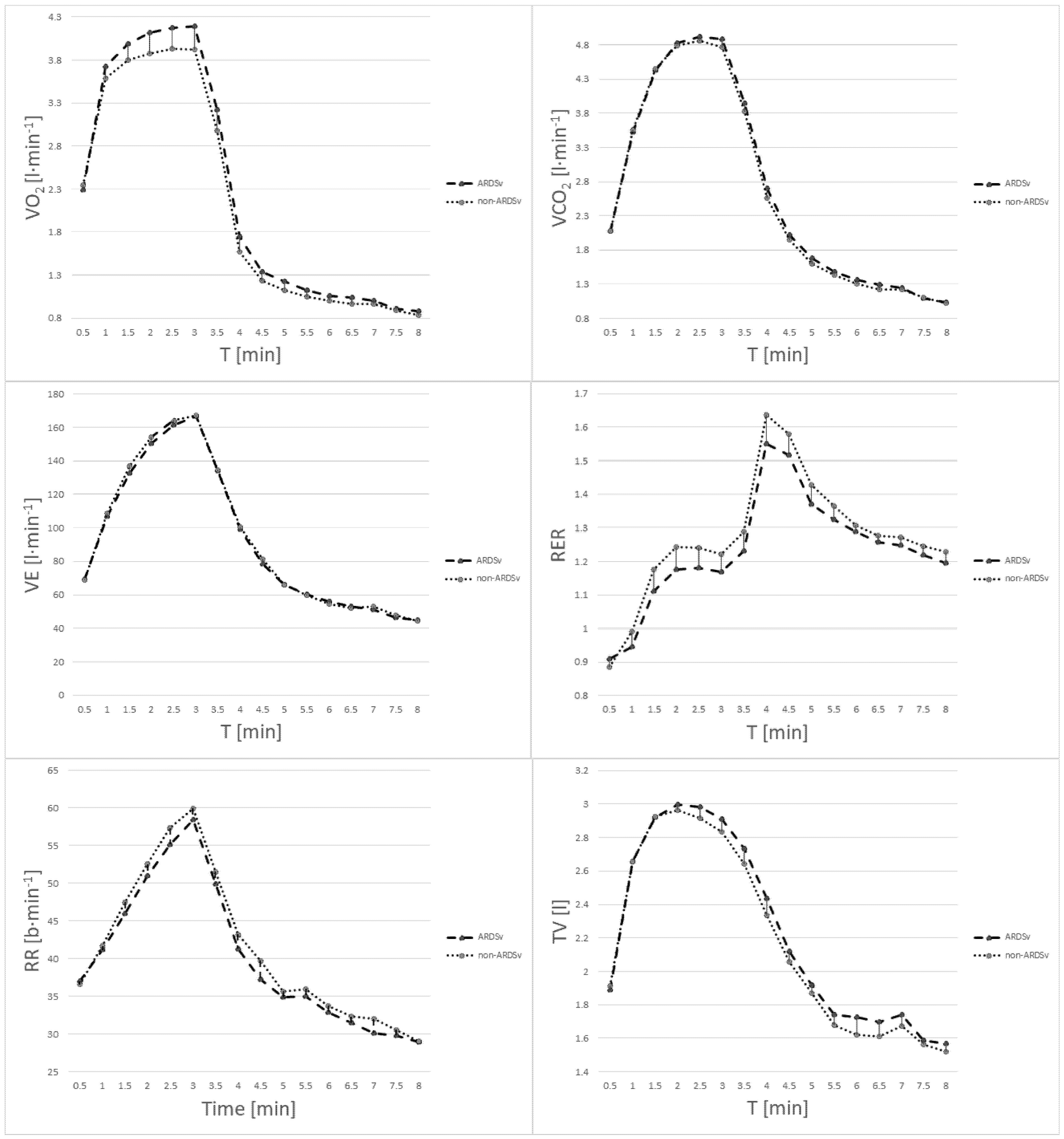
3. Results
4. Discussion
Practical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Body mass |
| BH | Body height |
| VO2max | Maximal oxygen uptake |
| Pmax | Maximal power output in the progressive test |
| VE | Minute ventilation |
| VCO2 | Carbon dioxide output |
| RER | Respiratory exchange ratio |
| RR | Respiratory rate |
| TV | Tidal volume |
| PETO2 | End-tidal partial pressure of oxygen |
| PETCO2 | End-tidal partial pressure of carbon dioxide |
| VT1 | First ventilatory threshold |
| VT2 | Second ventilatory threshold |
| VO2rec-1min | Oxygen uptake during the 1st minutes of recovery |
| VO2rec-2min | Oxygen uptake during the 2nd minute of recovery |
| VO2rec-3min | Oxygen uptake during the 3rd minute of recovery |
| VO2rec-4min | Oxygen uptake during the 4th minute of recovery |
| VO2rec-5min | Oxygen uptake during the 5th minute of recovery |
| RERrec-av | Mean RER during the 5 min recovery period |
| RERrec-tot | Peak RER value for recovery |
| SV | Stroke volume |
| HR | Heart rate |
| CO | Cardiac output |
| SBP | Systolic blood pressure measured immediately post-exercise |
| DBP | Diastolic blood pressure measured immediately post-exercise |
| SVBP | Stroke volume estimated based on post-exercise blood pressure |
| RPE | Rate of perceived exertion |
References
- Næss, S.; Sollie, O.; Gløersen, Ø.N.; Losnegard, T. Exercise Intensity and Pacing Pattern During a Cross-Country Olympic Mountain Bike Race. Front. Physiol. 2021, 12, 702415. [Google Scholar] [CrossRef] [PubMed]
- Stöggl, T.L.; Hertlein, M.; Brunauer, R.; Welde, B.; Andersson, E.P.; Swarén, M. Pacing, Exercise Intensity, and Technique by Performance Level in Long-Distance Cross-Country Skiing. Front. Physiol. 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; Abbiss, C.R.; Aubry, A.; Vauchez, Y.; Dorel, S.; Hausswirth, C.; Le Meur, Y. Power Output and Pacing During International Cross-Country Mountain Bike Cycling. Int. J. Sports Physiol. Perform. 2018, 13, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, Ø.; Gilgien, M.; Gløersen, Ø.N.; Rud, B.; Losnegard, T. Exercise Intensity During Cross-Country Skiing Described by Oxygen Demands in Flat and Uphill Terrain. Front. Physiol. 2018, 9, 846. [Google Scholar] [CrossRef]
- Laaksonen, M.S.; Finkenzeller, T.; Holmberg, H.C.; Sattlecker, G. The Influence of Physiobiomechanical Parameters, Technical Aspects of Shooting, and Psychophysiological Factors on Biathlon Performance: A Review. J. Sport Health Sci. 2018, 7, 394–404. [Google Scholar] [CrossRef]
- Formenti, D.; Rossi, A.; Calogiuri, G.; Thomassen, T.O.; Scurati, R.; Weydahl, A. Exercise Intensity and Pacing Strategy of Cross-Country Skiers during a 10 km Skating Simulated Race. Res. Sports Med. 2015, 23, 126–139. [Google Scholar] [CrossRef]
- Creagh, U.; Reilly, T.; Nevill, A.M. Heart Rate Response to “Off-Road” Running Events in Female Athletes. Br. J. Sports Med. 1998, 32, 34–38. [Google Scholar] [CrossRef]
- Losnegard, T. Energy System Contribution During Competitive Cross-Country Skiing. Eur. J. Appl. Physiol. 2019, 119, 1675–1690. [Google Scholar] [CrossRef]
- Noordhof, D.A.; de Koning, J.J.; Foster, C. The Maximal Accumulated Oxygen Deficit Method: A Valid and Reliable Measure of Anaerobic Capacity? Sports Med. 2010, 40, 285–302. [Google Scholar] [CrossRef]
- Sahlin, K. Muscle Energetics During Explosive Activities and Potential Effects of Nutrition and Training. Sports Med. 2014, 44, S167–S173. [Google Scholar] [CrossRef]
- Woodward, M.; Debold, E.P. Acidosis and Phosphate Directly Reduce Myosin’s Force-Generating Capacity Through Distinct Molecular Mechanisms. Front. Physiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Smekal, G.; von Duvillard, S.P.; Hörmandinger, M.; Moll, R.; Heller, M.; Pokan, R.; Bacharach, D.W.; LeMura, L.M.; Arciero, P. Physiological Demands of Simulated Off-Road Cycling Competition. J. Sports Sci. Med. 2015, 14, 799–810. [Google Scholar]
- Yanaoka, T.; Iwata, R.; Yoshimura, A.; Hirose, N. A 1-Minute Re-Warm Up at High Intensity Improves Sprint Performance During the Loughborough Intermittent Shuttle Test. Front. Physiol. 2021, 11, 616158. [Google Scholar] [CrossRef]
- Racinais, S.; Cocking, S.; Périard, J.D. Sports and Environmental Temperature: From Warming-Up to Heating-Up. Temperature 2017, 4, 227–257. [Google Scholar] [CrossRef]
- McGowan, C.J.; Pyne, D.B.; Thompson, K.G.; Rattray, B. Warm-Up Strategies for Sport and Exercise: Mechanisms and Applications. Sports Med. 2015, 45, 1523–1546. [Google Scholar] [CrossRef]
- Vangsoe, M.T.; Nielsen, J.K.; Paton, C.D. A Comparison of Different Prerace Warm-Up Strategies on 1-km Cycling Time-Trial Performance. Int. J. Sports Physiol. Perform. 2020, 15, 1109–1116. [Google Scholar] [CrossRef]
- Chorley, A.; Lamb, K.L. The Effects of a Cycling Warm-Up Including High-Intensity Heavy-Resistance Conditioning Contractions on Subsequent 4-km Time Trial Performance. J. Strength Cond. Res. 2019, 33, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.H.; Ferguson, R.A.; Hodder, S.G.; Havenith, G. External Muscle Heating During Warm-Up Does Not Provide Added Performance Benefit above External Heating in the Recovery Period Alone. Eur. J. Appl. Physiol. 2013, 113, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Sperlich, B.; Zinner, C.; Pfister, R.; Holmberg, H.C.; Michels, G. Repeated Apnea-Induced Contraction of the Spleen in Cyclists Does Not Enhance Performance in a Subsequent Time-Trial. Eur. J. Appl. Physiol. 2015, 115, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Casey, D.P. Regulation of Increased Blood Flow (Hyperemia) to Muscles During Exercise: A Hierarchy of Competing Physiological Needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Ramanlal, R.; Gupta, V. Physiology, Vasodilation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hein, T.W.; Razavi, H.M.; Xu, X.; Somvanshi, S.; Muthuchamy, M.; Kuo, L. Activation of Smooth Muscle Kir2.1 Channels and Na+/K+-ATPase Mediates Dilation of Porcine Coronary Arterioles at Physiological Levels of Potassium. Int. J. Mol. Sci. 2025, 26, 2654. [Google Scholar] [CrossRef]
- Oikawa, S.; Hirakawa, H.; Kusakabe, T.; Nakashima, Y.; Hayashida, Y. Autonomic Cardiovascular Responses to Hypercapnia in Conscious Rats: The Roles of the Chemo- and Baroreceptors. Auton. Neurosci. 2005, 117, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, G.M.; Paulson, O.B.; Hertz, M.M. Kinetic Analysis of the Human Blood-Brain Barrier Transport of Lactate and Its Influence by Hypercapnia. J. Cereb. Blood Flow Metab. 1991, 11, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; Wilson, B.A.; Sample, M.; Van Dijk, J.; Goslin, B. The Effects of Hypercapnia on the Metabolic Response to Steady-State Exercise. Med. Sci. Sports Exerc. 1982, 14, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Smolka, L.; Borkowski, J.; Zatoń, M. The Effect of Additional Dead Space on Respiratory Exchange Ratio and Carbon Dioxide Production Due to Training. J. Sports Sci. Med. 2014, 13, 36–43. [Google Scholar]
- Danek, N.; Michalik, K.; Zatoń, M. Warm-Up with Added Respiratory Dead Space Volume Mask Improves the Performance of the Cycling Sprint Interval Exercise: Crossover Study. Front. Physiol. 2022, 13, 812221. [Google Scholar] [CrossRef]
- Zatoń, M.W.; Hebisz, R.G.; Hebisz, P. The Effect of Training with Additional Respiratory Dead Space on Haematological Elements of Blood. Isokinet. Exerc. Sci. 2010, 18, 137–143. [Google Scholar] [CrossRef]
- Meihua, S.; Jiahui, J.; Yujia, L.; Shuang, Z.; Jingjing, Z. Research on Sweat Metabolomics of Athlete’s Fatigue Induced by High-Intensity Interval Training. Front. Physiol. 2023, 14, 1269885. [Google Scholar] [CrossRef]
- Opialla, T.; Gollasch, B.; Kuich, P.H.J.L.; Klug, L.; Rahn, G.; Busjahn, A.; Spuler, S.; Boschmann, M.; Kirwan, J.A.; Luft, F.C.; et al. Exercise Blood-Drop Metabolic Profiling Links Metabolism with Perceived Exertion. Front. Mol. Biosci. 2022, 9, 1042231. [Google Scholar] [CrossRef]
- De Pauw, K.; Roelands, B.; Cheung, S.S.; de Geus, B.; Rietjens, G.; Meeusen, R. Guidelines to Classify Subject Groups in Sport-Science Research. Int. J. Sports Physiol. Perform. 2013, 8, 111–122. [Google Scholar] [CrossRef]
- Hebisz, R.; Hebisz, P. Greater Improvement in Aerobic Capacity after a Polarised Training Program Including Cycling Interval Training at Low Cadence (50–70 RPM) than Freely Chosen Cadence (above 80 RPM). PLoS ONE 2024, 19, e0311833. [Google Scholar] [CrossRef]
- Hebisz, P.; Jastrzębska, A.D.; Hebisz, R. Real Assessment of Maximum Oxygen Uptake as a Verification after an Incremental Test versus Without a Test. Front. Physiol. 2021, 12, 739745. [Google Scholar] [CrossRef]
- Pallarés, J.G.; Morán-Navarro, R.; Ortega, J.F.; Fernández-Elías, V.E.; Mora-Rodriguez, R. Validity and Reliability of Ventilatory and Blood Lactate Thresholds in Well-Trained Cyclists. PLoS ONE 2016, 11, e0163389. [Google Scholar] [CrossRef]
- Hebisz, P.; Hebisz, R.; Zatoń, M.; Ochmann, B.; Mielnik, N. Concomitant Application of Sprint and High-Intensity Interval Training on Maximal Oxygen Uptake and Work Output in Well-Trained Cyclists. Eur. J. Appl. Physiol. 2016, 116, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Danek, N.; Michalik, K.; Smolarek, M.; Zatoń, M. Acute Effects of Using Added Respiratory Dead Space Volume in a Cycling Sprint Interval Exercise Protocol: A Crossover Study. Int. J. Environ. Res. Public Health 2020, 17, 9485. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Smith, J.A.B.; Murach, K.A.; Dyar, K.A.; Zierath, J.R. Exercise Metabolism and Adaptation in Skeletal Muscle. Nat. Rev. Mol. Cell Biol. 2023, 24, 607–632. [Google Scholar] [CrossRef]
- Kinnear, W.J.M.; Hull, J.H. Respiratory Exchange Ratio. In A Practical Guide to the Interpretation of Cardiopulmonary Exercise Tests, 2nd ed.; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Álvarez-Herms, J. Summatory Effects of Anaerobic Exercise and a Westernised Athletic Diet on Gut Dysbiosis and Chronic Low-Grade Metabolic Acidosis. Microorganisms 2024, 12, 1138. [Google Scholar] [CrossRef]
- Scott, C.B. Contribution of Anaerobic Energy Expenditure to Whole Body Thermogenesis. Nutr. Metab. 2005, 2, 14. [Google Scholar] [CrossRef]
- Yi-Dan, H.; Ying-Xin, Z.; Shi-Wei, Y.; Yu-Jie, Z. High-Energy Phosphates and Ischemic Heart Disease: From Bench to Bedside. Front. Cardiovasc. Med. 2021, 8, 675608. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Maeda, T.; Takahashi, T.; Ashikaga, K.; Tanaka, S.; Sumi, Y.; Itoh, H. Changes in Oxygen Uptake Kinetics after Exercise Caused by Differences in Loading Pattern and Exercise Intensity. ESC Heart Fail. 2020, 7, 1109–1117. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Brooks, G.A. Metabolic Bases of Excess Post-Exercise Oxygen Consumption: A Review. Med. Sci. Sports Exerc. 1984, 16, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Korzeniewski, B.; Kulinowski, P.; Zapart-Bukowska, J.; Majerczak, J.; Jasiński, A. Phosphocreatine Recovery Overshoot after High-Intensity Exercise in Human Skeletal Muscle Is Associated with Extensive Muscle Acidification and a Significant Decrease in Phosphorylation Potential. J. Physiol. Sci. 2010, 60, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, D.L.; Wenger, H.A. The Relationship between Aerobic Fitness and Recovery from High-Intensity Intermittent Exercise. Sports Med. 2001, 31, 1–11. [Google Scholar] [CrossRef]
- Børsheim, E.; Bahr, R. Effect of Exercise Intensity, Duration and Mode on Post-Exercise Oxygen Consumption. Sports Med. 2003, 33, 1037–1060. [Google Scholar] [CrossRef]
- Jung, W.S.; Park, H.Y.; Kim, S.W.; Kim, J.; Hwang, H.; Lim, K. Estimating Excess Post-Exercise Oxygen Consumption Using Multiple Linear Regression in Healthy Korean Adults: A Pilot Study. Phys. Act. Nutr. 2021, 25, 35–41. [Google Scholar] [CrossRef]
- Gifford, J.R.; Blackmon, C.; Hales, K.; Hinkle, L.J.; Richards, S. Overdot and Overline Annotation Must Be Understood to Accurately Interpret VO2max Physiology with the Fick Formula. Front. Physiol. 2024, 15, 1359119. [Google Scholar] [CrossRef]
- Brinkman, J.E.; Toro, F.; Sharma, S. Physiology, Respiratory Drive. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Migliaccio, G.M.; Russo, L.; Maric, M.; Padulo, J. Sports Performance and Breathing Rate: What Is the Connection? A Narrative Review on Breathing Strategies. Sports 2023, 11, 103. [Google Scholar] [CrossRef]
- Danek, N.; Szczepan, S.; Wróblewska, Z.; Michalik, K.; Zatoń, M. Hypercapnic Warm-Up and Re-Warm-Up—A Novel Experimental Approach in Swimming Sprint. PLoS ONE 2025, 20, e0314089. [Google Scholar] [CrossRef]
- Woorons, X.; Bourdillon, N.; Vandewalle, H.; Lamberto, C.; Mollard, P.; Richalet, J.P.; Pichon, A. Exercise with Hypoventilation Induces Lower Muscle Oxygenation and Higher Blood Lactate Concentration: Role of Hypoxia and Hypercapnia. Eur. J. Appl. Physiol. 2010, 110, 367–377. [Google Scholar] [CrossRef]
- Esposito, F.; Cè, E.; Limonta, E. Cycling Efficiency and Time to Exhaustion Are Reduced after Acute Passive Stretching Administration. Scand. J. Med. Sci. Sports 2012, 22, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Doust, J.H.; Burnley, M. Determination of Critical Power Using a 3-min All-Out Cycling Test. Med. Sci. Sports Exerc. 2007, 39, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F.; Stickford, J.L.; Levine, B.D. Altitude Training Considerations for the Winter Sport Athlete. Exp. Physiol. 2010, 95, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Wilson, M.G.; Tebeck, S.T.; Stanley, J.; Girard, O. Health Status and Heat Preparation at a UCI World Tour Multistage Cycling Race. J. Sci. Med. Sport 2025, 28, 77–83. [Google Scholar] [CrossRef]


| Variables | ± SD |
|---|---|
| Age [years] | 20.3 ± 1.5 |
| BM [kg] | 70.1 ± 8.5 |
| BH [m] | 1.79 ± 0.07 |
| VO2max [mL·kg−1·min−1] | 57.7 ± 6.7 |
| Pmax [W] | 357.8 ± 43 |
| Pmax [W·kg−1] | 5.13 ± 0.55 |
| Variables | Non-ARDSv | ARDSv | p Value q Value | Cohen’s d (r) | ||
|---|---|---|---|---|---|---|
| ± SD | Lower CI Upper CI | ± SD | Lower CI Upper CI | |||
| RRpeak [b·min−1] | 60.5 ± 11.1 | 55.3 | 59.5 ± 11.2 | 53.2 | 0.067 | (0.409) |
| 65.7 | 63.7 | 0.025 | ||||
| RRav [b·min−1] | 49.7 ± 9.1 | 45.4 | 48.0 ± 9.4 | 43.6 | 0.028 | 0.184 |
| 54.0 | 52.4 | 0.021 | ||||
| TVpeak [L] | 3.01 ± 0.39 | 2.83 | 3.04 ± 0.38 | 2.86 | 0.266 | 0.078 |
| 3.20 | 3.22 | 0.037 | ||||
| TVav [L] | 2.73 ± 0.36 | 2.56 | 2.73 ± 0.34 | 2.57 | 0.996 | 0.000 |
| 2.89 | 2.88 | 0.050 | ||||
| VEpeak [L·min−1] | 170.3 ± 24.6 | 158.7 | 168.2 ± 21.7 | 158.1 | 0.341 | 0.091 |
| 181.8 | 178.4 | 0.042 | ||||
| VEav [L·min−1] | 135.4 ± 20.0 | 126.0 | 130.9 ± 17.5 | 122.7 | 0.086 | 0.239 |
| 144.8 | 139.1 | 0.029 | ||||
| VO2peak [L·min−1] | 3.98 ± 0.42 | 3.79 | 4.22 ± 0.40 | 4.03 | 0.002 * | 0.585 |
| 4.18 | 4.41 | 0.004 | ||||
| VO2av [L·min−1] | 3.60 ± 0.38 | 3.42 | 3.75 ± 0.35 | 3.58 | 0.023 | 0.411 |
| 3.78 | 3.91 | 0.017 | ||||
| VCO2peak [L·min−1] | 4.91 ± 0.43 | 4.71 | 4.97 ± 0.44 | 4.76 | 0.168 | 0.138 |
| 5.11 | 5.18 | 0.033 | ||||
| VCO2av [L·min−1] | 4.13 ± 0.42 | 3.94 | 4.11 ± 0.37 | 3.93 | 0.654 | 0.051 |
| 4.33 | 4.28 | 0.046 | ||||
| RERpeak | 1.26 ± 0.07 | 1.23 | 1.19 ± 0.07 | 1.16 | 0.016 | (0.539) |
| 1.30 | 1.22 | 0.012 | ||||
| RERav | 1.13 ± 0.06 | 1.11 | 1.08 ± 0.06 | 1.06 | 0.0081 * | 0.833 |
| 1.16 | 1.11 | 0.0083 | ||||
| Variables | Non-ARDSv | ARDSv | p Value q Value | Cohen’s d | ||
|---|---|---|---|---|---|---|
| ± SD | Lower CI Upper CI | ± SD | Lower CI Upper CI | |||
| VO2rec-1min [L] | 2.13 ± 0.45 | 1.92 | 2.47 ± 0.28 | 2.34 | 0.001 * | 0.907 |
| 2.34 | 2.60 | 0.012 | ||||
| VO2rec-2min [L] | 1.17 ± 0.18 | 1.09 | 1.28 ± 0.19 | 1.20 | 0.000 * | 0.594 |
| 1.26 | 1.37 | 0.006 | ||||
| VO2rec-3min [L] | 1.01 ± 0.18 | 0.93 | 1.09 ± 0.18 | 1.01 | 0.002 * | 0.444 |
| 1.10 | 1.18 | 0.019 | ||||
| VO2rec-4min [L] | 0.96 ± 0.17 | 0.89 | 1.02 ± 0.15 | 0.95 | 0.010 * | 0.374 |
| 1.04 | 1.09 | 0.031 | ||||
| VO2rec-5min [L] | 0.86 ± 0.17 | 0.79 | 0.89 ± 0.16 | 0.82 | 0.208 | 0.182 |
| 0.94 | 0.97 | 0.050 | ||||
| RERrec-av | 1.37 ± 0.10 | 1.32 | 1.32 ± 0.10 | 1.27 | 0.014 * | 0.500 |
| 1.41 | 1.37 | 0.037 | ||||
| RERrec-peak | 1.67 ± 0.16 | 1.59 | 1.57 ± 0.12 | 1.51 | 0.015 * | 0.707 |
| 1.74 | 1.63 | 0.044 | ||||
| RPE | 18.9 ± 1.1 | 18.4 | 18.0 ± 1.7 | 17.3 | 0.009 | (0.583) |
| 19.4 | 18.8 | 0.025 | ||||
| Variables | Non-ARDSv | ARDSv | p Value q Value | Cohen’s d (r) | ||
|---|---|---|---|---|---|---|
| ± SD | Lower CI Upper CI | ± SD | Lower CI Upper CI | |||
| SVpeak [mL] | 147.1 ± 27.3 | 134.3 | 147.0 ± 22.5 | 136.5 | 0.974 | 0.004 |
| 159.9 | 157.6 | 0.044 | ||||
| SVav [mL] | 128.5 ± 24.6 | 116.9 | 130.6 ± 22.0 | 120.3 | 0.314 | 0.090 |
| 140.0 | 140.9 | 0.011 | ||||
| HRpeak [bpm] | 193.3 ± 12.9 | 187.3 | 193.9 ± 11.2 | 188.7 | 0.379 | (0.197) |
| 199.4 | 199.2 | 0.022 | ||||
| HRav [bpm] | 179 ± 12.7 | 173.1 | 180.5 ± 11.7 | 175.0 | 0.528 | (0.118) |
| 184.9 | 185.0 | 0.028 | ||||
| COpeak [L] | 27.5 ± 4.7 | 25.3 | 27.9 ± 3.8 | 26.2 | 0.545 | 0.094 |
| 29.7 | 29.7 | 0.033 | ||||
| COav [L] | 23.0 ± 3.8 | 21.2 | 23.5 ± 3.5 | 21.9 | 0.153 | 0.137 |
| 24.8 | 25.2 | 0.006 | ||||
| SBP [mmHg] | 167.5 ± 27.1 | 154.8 | 171.0 ± 22.5 | 160.5 | 0.324 | (0.221) |
| 180.2 | 181.5 | 0.017 | ||||
| DBP [mmHg] | 34.5 ± 24.4 | 23.1 | 35.0 ± 23.7 | 23.9 | 1.000 | (0.000) |
| 45.9 | 46.1 | 0.050 | ||||
| SVBP [mL] | 134.8 ± 31.8 | 119.9 | 136.0 ± 29.5 | 122.2 | 0.836 | 0.039 |
| 149.7 | 149.8 | 0.039 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hebisz, P.; Hebisz, R.; Danek, N. Prolonging the Warm-Up Effect by Using Additional Respiratory Dead Space Volume After the Cessation of Warm-Up Exercise. J. Clin. Med. 2025, 14, 7049. https://doi.org/10.3390/jcm14197049
Hebisz P, Hebisz R, Danek N. Prolonging the Warm-Up Effect by Using Additional Respiratory Dead Space Volume After the Cessation of Warm-Up Exercise. Journal of Clinical Medicine. 2025; 14(19):7049. https://doi.org/10.3390/jcm14197049
Chicago/Turabian StyleHebisz, Paulina, Rafał Hebisz, and Natalia Danek. 2025. "Prolonging the Warm-Up Effect by Using Additional Respiratory Dead Space Volume After the Cessation of Warm-Up Exercise" Journal of Clinical Medicine 14, no. 19: 7049. https://doi.org/10.3390/jcm14197049
APA StyleHebisz, P., Hebisz, R., & Danek, N. (2025). Prolonging the Warm-Up Effect by Using Additional Respiratory Dead Space Volume After the Cessation of Warm-Up Exercise. Journal of Clinical Medicine, 14(19), 7049. https://doi.org/10.3390/jcm14197049








