Non-Invasive Hemodynamic Monitoring in Critically Ill Patients: A Guide for Emergency Physicians
Abstract
1. Introduction
2. Physiological Concepts in Hemodynamic Monitoring
- End-Diastolic Volume (EDV): the maximum ventricular volume at end of diastole, before ejection;
- End-Systolic Volume (ESV): the residual volume remaining after systole;
- Stroke Volume: the amount of blood ejected per heartbeat, calculated as SV = EDV − ESV [7].
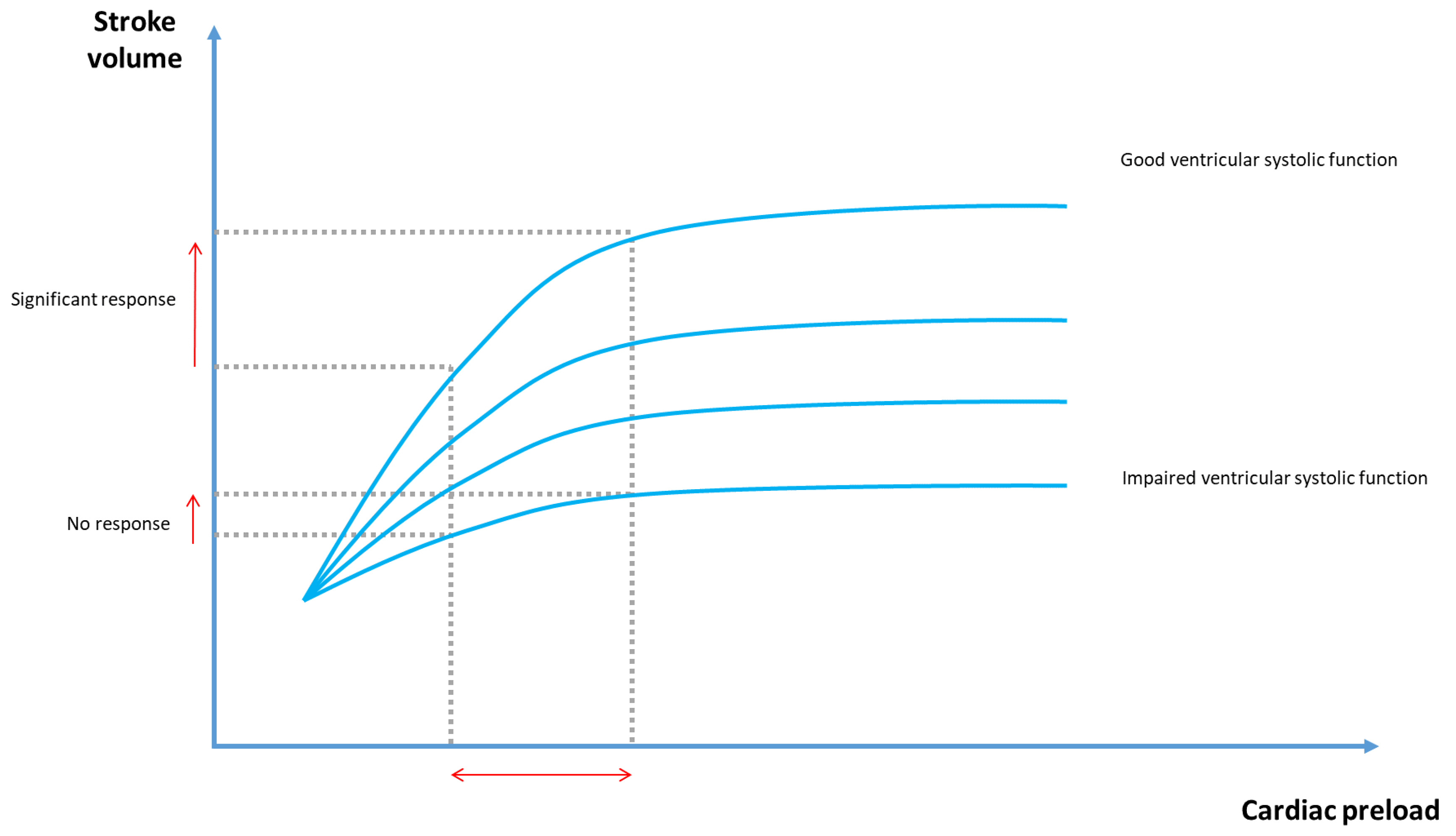
3. Evaluating Preload Responsiveness
- Fluid challenge involves the rapid infusion of 500 mL of crystalloids over 10–15 min, to assess the resulting changes in CO. An increase in CO of ≥15% is generally considered indicative of fluid responsiveness. However, if the patient is not fluid-responsive, the administered volume cannot be withdrawn and may contribute to fluid overload [17].
- Passive leg raising (PLR) transiently transfers venous blood from the lower limbs to the central circulation, mimicking a reversible fluid challenge. A significant increase in SV and CO following PLR (≥10%) suggests fluid responsiveness [18].
- Pulse pressure variation (PPV) and stroke volume variation (SVV) rely on heart–lung interactions during positive pressure ventilation. They evaluate the cyclic changes in arterial pressure or stroke volume induced by mechanical breaths, serving as indicators of preload responsiveness. PPV and SVV values ≥ 12% indicate preload responsiveness [19].
- The PEEP test consists in transiently decreasing the positive end-expiratory pressure (PEEP) to 5 cmH2O in mechanically ventilated patients with a PEEP ≥ 10 cmH2O; an increase in CO ≥ 8.6% reflects preload responsiveness [22].
4. Monitoring Methods Without Specialized Equipment
5. Invasive Monitoring Techniques
6. Non-Invasive Monitoring Technologies
6.1. Bioreactance
6.2. Point-of-Care Ultrasound (POCUS) for Hemodynamic Monitoring
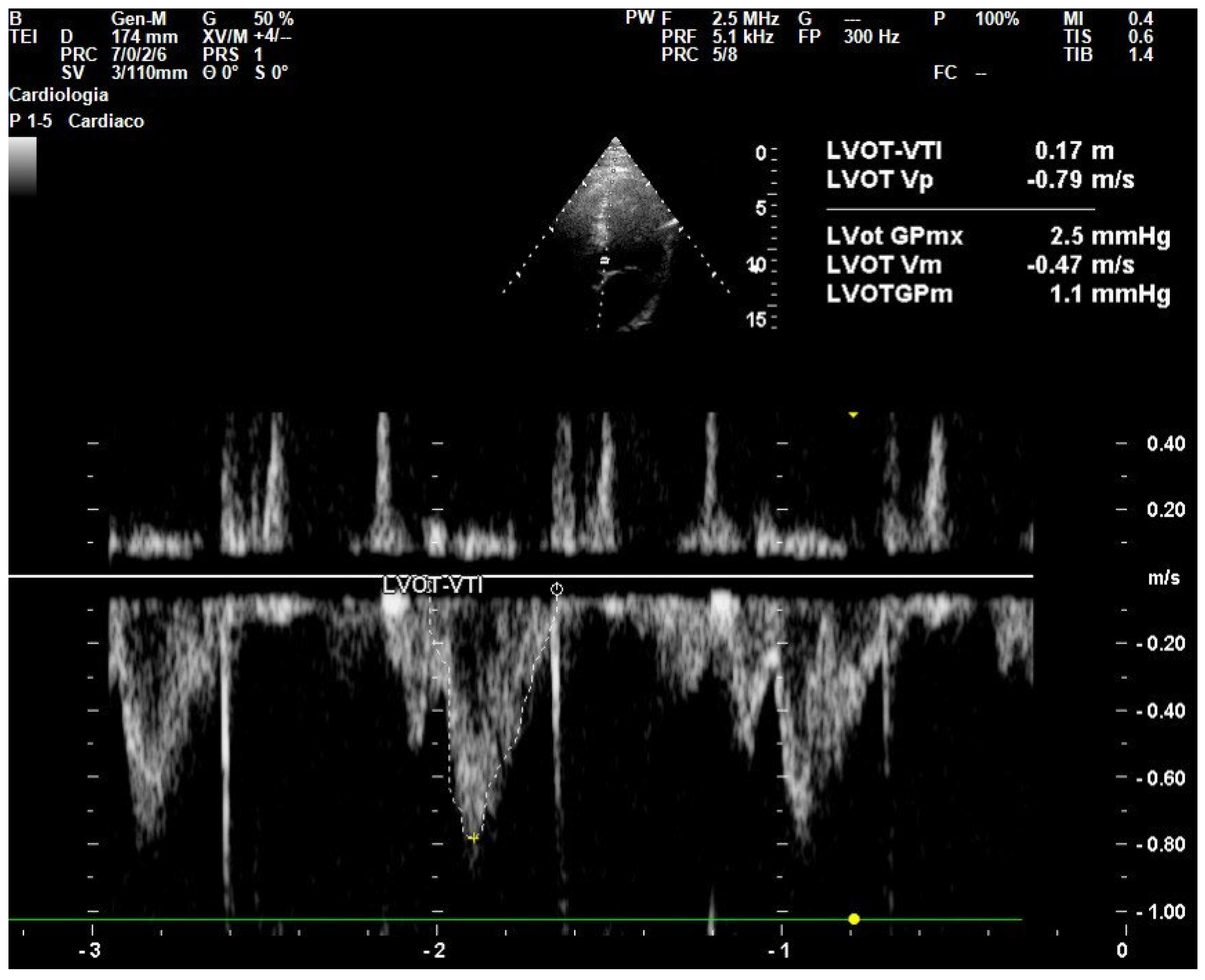
6.3. Transthoracic Echocardiography
6.4. Pulse Contour Analysis and Photoplethysmography
7. Emerging Technologies
8. Advantages and Limits in the ED Setting
9. Future Perspectives and Technological Innovation in Non-Invasive Monitoring
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Alternating Current |
| AI | Artificial Intelligence |
| CI | Cardiac Index |
| CO | Cardiac Output |
| CVP | Central Venous Pressure |
| DBP | Diastolic Blood Pressure |
| DVT | Deep Vein Thrombosis |
| EEXPO | End-Expiratory Occlusion |
| ED | Emergency Department |
| EDV | End-Diastolic Volume |
| ESV | End-Systolic Volume |
| HR | Heart Rate |
| ICU | Intensive Care Unit |
| LVOT | Left Ventricular Outflow Tract |
| MAP | Mean Arterial Pressure |
| NACA | National Advisory Committee for Aeronautics |
| NICOM | Non-Invasive Cardiac Output Monitoring |
| PAC | Pulmonary Artery Catheter |
| PATD | Pulmonary Artery Thermodilution |
| PCWP | Pulmonary Capillary Wedge Pressure |
| PEEP | Positive End-Expiratory Pressure |
| PI | Perfusion Index |
| PLR | Passive Leg Raising |
| POCUS | Point-of-Care Ultrasound |
| PP | Pulse Pressure |
| PPG | Photoplethysmography |
| PPV | Pulse Pressure Variation |
| Pmsf | Mean Systemic Filling Pressure |
| SBP | Systolic Blood Pressure |
| SV | Stroke Volume |
| SVV | Stroke Volume Variation |
| TPTD | Transpulmonary Thermodilution |
| TTE | Transthoracic Echocardiography |
| VET | Ventricular Ejection Time |
| VTI | Velocity Time Integral |
References
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M. Early Goal-Directed Therapy Collaborative Group Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Teboul, J.-L. My Patient Has Received Fluid. How to Assess Its Efficacy and Side Effects? Ann. Intensive Care 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Delicce, A.V.; Makaryus, A.N. Physiology, Frank Starling Law. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Monnet, X.; Marik, P.E.; Teboul, J.-L. Prediction of Fluid Responsiveness: An Update. Ann. Intensive Care 2016, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.-J.; Joannes-Boyau, O.; Teboul, J.-L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of Fluid Management and Stewardship in Septic Shock: It Is Time to Consider the Four D’s and the Four Phases of Fluid Therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef]
- Teboul, J.-L.; Saugel, B.; Cecconi, M.; De Backer, D.; Hofer, C.K.; Monnet, X.; Perel, A.; Pinsky, M.R.; Reuter, D.A.; Rhodes, A.; et al. Less Invasive Hemodynamic Monitoring in Critically Ill Patients. Intensive Care Med. 2016, 42, 1350–1359. [Google Scholar] [CrossRef]
- Bruss, Z.S.; Raja, A. Volume. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Varpula, M.; Tallgren, M.; Saukkonen, K.; Voipio-Pulkki, L.-M.; Pettilä, V. Hemodynamic Variables Related to Outcome in Septic Shock. Intensive Care Med. 2005, 31, 1066–1071. [Google Scholar] [CrossRef]
- LeDoux, D.; Astiz, M.E.; Carpati, C.M.; Rackow, E.C. Effects of Perfusion Pressure on Tissue Perfusion in Septic Shock. Crit. Care Med. 2000, 28, 2729–2732. [Google Scholar] [CrossRef]
- Asfar, P.; Meziani, F.; Hamel, J.-F.; Grelon, F.; Megarbane, B.; Anguel, N.; Mira, J.-P.; Dequin, P.-F.; Gergaud, S.; Weiss, N.; et al. High versus Low Blood-Pressure Target in Patients with Septic Shock. N. Engl. J. Med. 2014, 370, 1583–1593. [Google Scholar] [CrossRef]
- Russell, J.A.; Walley, K.R.; Singer, J.; Gordon, A.C.; Hébert, P.C.; Cooper, D.J.; Holmes, C.L.; Mehta, S.; Granton, J.T.; Storms, M.M.; et al. Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock. N. Engl. J. Med. 2008, 358, 877–887. [Google Scholar] [CrossRef]
- Chemla, D.; Hébert, J.L.; Coirault, C.; Zamani, K.; Suard, I.; Colin, P.; Lecarpentier, Y. Total Arterial Compliance Estimated by Stroke Volume-to-Aortic Pulse Pressure Ratio in Humans. Am. J. Physiol. 1998, 274, H500–H505. [Google Scholar] [CrossRef]
- Persichini, R.; Lai, C.; Teboul, J.-L.; Adda, I.; Guérin, L.; Monnet, X. Venous Return and Mean Systemic Filling Pressure: Physiology and Clinical Applications. Crit. Care 2022, 26, 150. [Google Scholar] [CrossRef]
- Marik, P.E.; Monnet, X.; Teboul, J.-L. Hemodynamic Parameters to Guide Fluid Therapy. Ann. Intensive Care 2011, 1, 1. [Google Scholar] [CrossRef]
- Gavelli, F.; Patrucco, F.; DE Vita, N.; Solidoro, P.; Avanzi, G.C. Central Venous Pressure in Critically Ill Patients: Do We Still Need It? Panminerva Med. 2024, 66, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on Circulatory Shock and Hemodynamic Monitoring. Task Force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Calabrò, L.; Pugliese, L.; Lulja, A.; Sopuch, A.; Rosalba, D.; Morenghi, E.; Hernandez, G.; Monnet, X.; Cecconi, M. Fluid Challenge in Critically Ill Patients Receiving Haemodynamic Monitoring: A Systematic Review and Comparison of Two Decades. Crit. Care 2022, 26, 186. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Shi, R.; Teboul, J.-L. Prediction of Fluid Responsiveness. What’s New? Ann. Intensive Care 2022, 12, 46. [Google Scholar] [CrossRef]
- Grassi, P.; Lo Nigro, L.; Battaglia, K.; Barone, M.; Testa, F.; Berlot, G. Pulse Pressure Variation as a Predictor of Fluid Responsiveness in Mechanically Ventilated Patients with Spontaneous Breathing Activity: A Pragmatic Observational Study. HSR Proc. Intensive Care Cardiovasc. Anesth. 2013, 5, 98–109. [Google Scholar]
- Gavelli, F.; Teboul, J.-L.; Monnet, X. The End-Expiratory Occlusion Test: Please, Let Me Hold Your Breath! Crit. Care 2019, 23, 274. [Google Scholar] [CrossRef]
- Gavelli, F.; Shi, R.; Teboul, J.-L.; Azzolina, D.; Monnet, X. The End-Expiratory Occlusion Test for Detecting Preload Responsiveness: A Systematic Review and Meta-Analysis. Ann. Intensive Care 2020, 10, 65. [Google Scholar] [CrossRef]
- Lai, C.; Shi, R.; Beurton, A.; Moretto, F.; Ayed, S.; Fage, N.; Gavelli, F.; Pavot, A.; Dres, M.; Teboul, J.-L.; et al. The Increase in Cardiac Output Induced by a Decrease in Positive End-Expiratory Pressure Reliably Detects Volume Responsiveness: The PEEP-Test Study. Crit. Care 2023, 27, 136. [Google Scholar] [CrossRef]
- Leszczyński, P.; Mioduski, M.; Gałązkowski, R. The NACA Score as a Predictor of Ventricular Cardiac Arrhythmias—A Retrospective Six-Year Study. Am. J. Emerg. Med. 2020, 38, 2249–2253. [Google Scholar] [CrossRef]
- Sevransky, J. Clinical Assessment of Hemodynamically Unstable Patients. Curr. Opin. Crit. Care 2009, 15, 234–238. [Google Scholar] [CrossRef]
- Sheridan, D.C.; Cloutier, R.; Kibler, A.; Hansen, M.L. Cutting-Edge Technology for Rapid Bedside Assessment of Capillary Refill Time for Early Diagnosis and Resuscitation of Sepsis. Front. Med. 2020, 7, 612303. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Goodall, M.; Dumville, J.; Hill, J.; Norman, G.; Hamer, O.; Clegg, A.; Watkins, C.L.; Georgiou, G.; Hodkinson, A.; et al. The Accuracy of Pulse Oximetry in Measuring Oxygen Saturation by Levels of Skin Pigmentation: A Systematic Review and Meta-Analysis. BMC Med. 2022, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- McGregor, D.; Sharma, S.; Gupta, S.; Ahmad, S.; Godec, T.; Harris, T. Emergency Department Non-Invasive Cardiac Output Study (EDNICO): A Feasibility and Repeatability Study. Scand. J. Trauma. Resusc. Emerg. Med. 2019, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- Fagnoul, D.; Vincent, J.-L.; Backer, D.D. Cardiac Output Measurements Using the Bioreactance Technique in Critically Ill Patients. Crit. Care 2012, 16, 460. [Google Scholar] [CrossRef]
- Poelaert, J.; Roosens, C. Echocardiography and Assessing Fluid Responsiveness: Acoustic Quantification Again into the Picture? Crit. Care 2007, 11, 105. [Google Scholar] [CrossRef]
- Critchley, L.A. Pulse Contour Analysis: Is It Able to Reliably Detect Changes in Cardiac Output in the Haemodynamically Unstable Patient? Crit. Care 2011, 15, 106. [Google Scholar] [CrossRef][Green Version]
- Keren, H.; Burkhoff, D.; Squara, P. Evaluation of a Noninvasive Continuous Cardiac Output Monitoring System Based on Thoracic Bioreactance. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H583–H589. [Google Scholar] [CrossRef]
- Gavelli, F.; Beurton, A.; Teboul, J.-L.; De Vita, N.; Azzolina, D.; Shi, R.; Pavot, A.; Monnet, X. Bioreactance Reliably Detects Preload Responsiveness by the End-Expiratory Occlusion Test When Averaging and Refresh Times Are Shortened. Ann. Intensive Care 2021, 11, 133. [Google Scholar] [CrossRef]
- Galarza, L.; Mercado, P.; Teboul, J.-L.; Girotto, V.; Beurton, A.; Richard, C.; Monnet, X. Estimating the Rapid Haemodynamic Effects of Passive Leg Raising in Critically Ill Patients Using Bioreactance. Br. J. Anaesth. 2018, 121, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Beurton, A.; Gavelli, F.; Teboul, J.-L.; De Vita, N.; Monnet, X. Changes in the Plethysmographic Perfusion Index During an End-Expiratory Occlusion Detect a Positive Passive Leg Raising Test. Crit. Care Med. 2021, 49, e151–e160. [Google Scholar] [CrossRef] [PubMed]
- Beurton, A.; Teboul, J.-L.; Gavelli, F.; Gonzalez, F.A.; Girotto, V.; Galarza, L.; Anguel, N.; Richard, C.; Monnet, X. The Effects of Passive Leg Raising May Be Detected by the Plethysmographic Oxygen Saturation Signal in Critically Ill Patients. Crit. Care 2019, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Duus, N.; Shogilev, D.J.; Skibsted, S.; Zijlstra, H.W.; Fish, E.; Oren-Grinberg, A.; Lior, Y.; Novack, V.; Talmor, D.; Kirkegaard, H.; et al. The Reliability and Validity of Passive Leg Raise and Fluid Bolus to Assess Fluid Responsiveness in Spontaneously Breathing Emergency Department Patients. J. Crit. Care 2015, 30, 217.e1–217.e5. [Google Scholar] [CrossRef]
- Squara, P.; Denjean, D.; Estagnasie, P.; Brusset, A.; Dib, J.C.; Dubois, C. Noninvasive Cardiac Output Monitoring (NICOM): A Clinical Validation. Intensive Care Med. 2007, 33, 1191–1194. [Google Scholar] [CrossRef]
- Osterwalder, J.; Polyzogopoulou, E.; Hoffmann, B. Point-of-Care Ultrasound-History, Current and Evolving Clinical Concepts in Emergency Medicine. Medicina 2023, 59, 2179. [Google Scholar] [CrossRef]
- Acharya, Y.; Witt, E. An Atypical Type-A Dissection of Aorta: Case Report. Crit. Care Innov. 2022, 5, 33–41. [Google Scholar] [CrossRef]
- Popat, A.; Harikrishnan, S.; Seby, N.; Sen, U.; Patel, S.K.; Mittal, L.; Patel, M.; Vundi, C.; Patel, Y.; Kumar, A.; et al. Utilization of Point-of-Care Ultrasound as an Imaging Modality in the Emergency Department: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e52371. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, D.; Gao, Y.; Wu, Z.; Wang, X.; Lv, C. Effect of VTILVOT Variation Rate on the Assessment of Fluid Responsiveness in Septic Shock Patients. Medicine 2020, 99, e22702. [Google Scholar] [CrossRef]
- Dinh, V.A.; Ko, H.S.; Rao, R.; Bansal, R.C.; Smith, D.D.; Kim, T.E.; Nguyen, H.B. Measuring Cardiac Index with a Focused Cardiac Ultrasound Examination in the ED. Am. J. Emerg. Med. 2012, 30, 1845–1851. [Google Scholar] [CrossRef]
- Chanthawatthanarak, S.; Boonasa, K.; Apiratwarakul, K.; Cheung, L.W.; Tiamkao, S.; Ienghong, K. Agreement between Carotid and LVOT Non-Invasive Cardiac Output Measurements in ED Septic Shock Patients: A Prospective Observational Study. Sci. Rep. 2025, 15, 19911. [Google Scholar] [CrossRef]
- Parker, C.W.; Kolimas, A.M.; Kotini-Shah, P. Velocity-Time Integral: A Bedside Echocardiography Technique Finding a Place in the Emergency Department. J. Emerg. Med. 2022, 63, 382–388. [Google Scholar] [CrossRef]
- Douglas, I.S.; Elwan, M.H.; Najarro, M.; Romagnoli, S. Dynamic Monitoring Tools for Patients Admitted to the Emergency Department with Circulatory Failure: Narrative Review with Panel-Based Recommendations. Eur. J. Emerg. Med. 2024, 31, 98–107. [Google Scholar] [CrossRef]
- Pour-Ghaz, I.; Manolukas, T.; Foray, N.; Raja, J.; Rawal, A.; Ibebuogu, U.N.; Khouzam, R.N. Accuracy of Non-Invasive and Minimally Invasive Hemodynamic Monitoring: Where Do We Stand? Ann. Transl. Med. 2019, 7, 421. [Google Scholar] [CrossRef]
- Gellert, G.; Bramlage, P. Use of the ClearSight® System for Continuous Noninvasive Hemodynamic Monitoring during Heart Valve Interventions: Review of the Literature and Single-Site Experience. Heart Surg. Forum 2018, 21, E476–E483. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.E.; Meram, S.; Li, X.; White, M.B.; Henry, S.; Gupta, S.; Zhu, D.; Pang, P.; Levy, P. Hemodynamic Profiles by Non-Invasive Monitoring of Cardiac Index and Vascular Tone in Acute Heart Failure Patients in the Emergency Department: External Validation and Clinical Outcomes. PLoS ONE 2022, 17, e0265895. [Google Scholar] [CrossRef] [PubMed]
- Musu, M.; Guddelmoni, L.; Murgia, F.; Mura, S.; Bonu, F.; Mura, P.; Finco, G. Prediction of Fluid Responsiveness in Ventilated Critically Ill Patients. J. Emerg. Crit. Care Med. 2020, 4, 26. [Google Scholar] [CrossRef]
- Pirneskoski, J.; Harjola, V.-P.; Jeskanen, P.; Linnamurto, L.; Saikko, S.; Nurmi, J. Critically Ill Patients in Emergency Department May Be Characterized by Low Amplitude and High Variability of Amplitude of Pulse Photoplethysmography. Scand. J. Trauma. Resusc. Emerg. Med. 2013, 21, 48. [Google Scholar] [CrossRef]
- Mager, C.M.; Jwayyed, S.; Wilber, S.T.; Stiffler, K. Digital Photoplethysmography to Detect Deep Venous Thrombosis in the Emergency Department. Ann. Emerg. Med. 2004, 44, S17–S18. [Google Scholar] [CrossRef]
- Angelucci, A.; Greco, M.; Cecconi, M.; Aliverti, A. Wearable Devices for Patient Monitoring in the Intensive Care Unit. Intensive Care Med. Exp. 2025, 13, 26. [Google Scholar] [CrossRef]
- Weller, G.B.; Mault, J.; Ventura, M.E.; Adams, J.; Campbell, F.J.; Tremper, K.K. A Retrospective Observational Study of Continuous Wireless Vital Sign Monitoring via a Medical Grade Wearable Device on Hospitalized Floor Patients. J. Clin. Med. 2024, 13, 4747. [Google Scholar] [CrossRef]
- Ahmad, R.U.S.; Khan, W.U.; Khan, M.S.; Cheung, P. Emerging Rapid Detection Methods for the Monitoring of Cardiovascular Diseases: Current Trends and Future Perspectives. Mater. Today Bio 2025, 32, 101663. [Google Scholar] [CrossRef]
- Lee, G.H.; Kang, H.; Chung, J.W.; Lee, Y.; Yoo, H.; Jeong, S.; Cho, H.; Kim, J.-Y.; Kang, S.-G.; Jung, J.Y.; et al. Stretchable PPG Sensor with Light Polarization for Physical Activity-Permissible Monitoring. Sci. Adv. 2022, 8, eabm3622. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.K.; Garcia, J.O.; Saha, A.K.; Harris, L.; Baruch, M.; Martin, R.S. Agreement between Cardiac Output Estimation with a Wireless, Wearable Pulse Decomposition Analysis Device and Continuous Thermodilution in Post Cardiac Surgery Intensive Care Unit Patients. J. Clin. Monit. Comput. 2024, 38, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gratz, I.; Baruch, M.; Awad, A.; McEniry, B.; Allen, I.; Seaman, J. A New Continuous Noninvasive Finger Cuff Device (Vitalstream) for Cardiac Output That Communicates Wirelessly via Bluetooth or Wi-Fi. BMC Anesth. 2023, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.D.; Binns, S.; McFann, K.; Nudell, N.; Dunn, J.A. A Direct Assessment of Noninvasive Continuous Blood Pressure Monitoring in the Emergency Department and Intensive Care Unit. J. Emerg. Nurs. 2024, 50, 503–515. [Google Scholar] [CrossRef]
- Hassan, M.; Frasier, K.; Li, V.; Kakarla, S.; Rezaei, M.; Vinagolu-Baur, J.; Cuevas, S.; Deehan, E. Innovative Wearable Technology for Early Detection of Cardiac Tamponade. ARC J. Cardiol. 2025, 10, 1–10. [Google Scholar] [CrossRef]
- Hegarty-Craver, M.; Davis-Wilson, H.; Gaur, P.; Walls, H.; Dausch, D.; Temple, D. Wearable Sensors for Service Members and First Responders: Considerations for Using Commercially Available Sensors in Continuous Monitoring; RTI Press Occasional Papers; RTI Press: Research Triangle Park, NC, USA, 2024.
- Tao, Q.; Liu, S.; Zhang, J.; Jiang, J.; Jin, Z.; Huang, Y.; Liu, X.; Lin, S.; Zeng, X.; Li, X.; et al. Clinical Applications of Smart Wearable Sensors. iScience 2023, 26, 107485. [Google Scholar] [CrossRef]
- Lin, W.; Demirel, B.U.; Al Faruque, M.A.; Li, G.P. Energy-Efficient Blood Pressure Monitoring Based on Single-Site Photoplethysmogram on Wearable Devices. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2021, 504–507. [Google Scholar] [CrossRef]
- Khan Mamun, M.M.R.; Sherif, A. Advancement in the Cuffless and Noninvasive Measurement of Blood Pressure: A Review of the Literature and Open Challenges. Bioengineering 2022, 10, 27. [Google Scholar] [CrossRef]
- Miller, J.C.; Shepherd, J.; Rinderknecht, D.; Cheng, A.L.; Pahlevan, N.M. Proof-of-Concept for a Non-Invasive, Portable, and Wireless Device for Cardiovascular Monitoring in Pediatric Patients. PLoS ONE 2020, 15, e0227145. [Google Scholar] [CrossRef]
- Aneja, S.; Nanda, P. Pitfalls of Noninvasive Hemodynamic Monitoring. Apollo Med. 2011, 8, 110–117. [Google Scholar] [CrossRef]
- Quan, X.; Liu, J.; Roxlo, T.; Siddharth, S.; Leong, W.; Muir, A.; Cheong, S.-M.; Rao, A. Advances in Non-Invasive Blood Pressure Monitoring. Sensors 2021, 21, 4273. [Google Scholar] [CrossRef] [PubMed]
- Saugel, B.; Thiele, R.H.; Hapfelmeier, A.; Cannesson, M. Technological Assessment and Objective Evaluation of Minimally Invasive and Noninvasive Cardiac Output Monitoring Systems. Anesthesiology 2020, 133, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Boly, C.A.; Schraverus, P.; van Raalten, F.; Coumou, J.-W.; Boer, C.; van Kralingen, S. Pulse-Contour Derived Cardiac Output Measurements in Morbid Obesity: Influence of Actual, Ideal and Adjusted Bodyweight. J. Clin. Monit. Comput. 2018, 32, 423–428. [Google Scholar] [CrossRef]
- Dheman, K.; Mayer, P.; Eggimann, M.; Schuerle, S.; Magno, M. ImpediSense:A Long Lasting Wireless Wearable Bio-Impedance Sensor Node. Sustain. Comput. Inform. Syst. 2021, 30, 100556. [Google Scholar] [CrossRef]
- Hyun, C.M.; Jang, T.J.; Nam, J.; Kwon, H.; Jeon, K.; Lee, K. Machine Learning-Based Signal Quality Assessment for Cardiac Volume Monitoring in Electrical Impedance Tomography. Mach. Learn. Sci. Technol. 2023, 4, 015034. [Google Scholar] [CrossRef]
- Nizami, S.; Green, J.R.; McGregor, C. Implementation of Artifact Detection in Critical Care: A Methodological Review. IEEE Rev. Biomed. Eng. 2013, 6, 127–142. [Google Scholar] [CrossRef]
- Cömert, A.; Hyttinen, J. Investigating the Possible Effect of Electrode Support Structure on Motion Artifact in Wearable Bioelectric Signal Monitoring. Biomed. Eng. Online 2015, 14, 44. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Y.; Gao, W. Skin-Interfaced Sensors in Digital Medicine: From Materials to Applications. Matter 2020, 2, 1414–1445. [Google Scholar] [CrossRef]
- Dang, T.H.; Jang, G.Y.; Lee, K.; Oh, T.I. Motion Artifacts Reduction for Noninvasive Hemodynamic Monitoring of Conscious Patients Using Electrical Impedance Tomography: A Preliminary Study. Sensors 2023, 23, 5308. [Google Scholar] [CrossRef]
- Nowak, R.M.; Nanayakkara, P.; DiSomma, S.; Levy, P.; Schrijver, E.; Huyghe, R.; Autunno, A.; Sherwin, R.L.; Divine, G.; Moyer, M. Noninvasive Hemodynamic Monitoring in Emergency Patients with Suspected Heart Failure, Sepsis and Stroke: The Premium Registry. West. J. Emerg. Med. 2014, 15, 786–794. [Google Scholar] [CrossRef]
- Couture, E.J.; Laferrière-Langlois, P.; Denault, A. New Developments in Continuous Hemodynamic Monitoring of the Critically Ill Patient. Can. J. Cardiol. 2023, 39, 432–443. [Google Scholar] [CrossRef]
- Thomas, L.B.; Mastorides, S.M.; Viswanadhan, N.A.; Jakey, C.E.; Borkowski, A.A. Artificial Intelligence: Review of Current and Future Applications in Medicine. Fed. Pr. 2021, 38, 527–538. [Google Scholar] [CrossRef]
| Technique | Advantages | Disadvantages | Available Parameters | Procedural Requirements | Cost |
|---|---|---|---|---|---|
| Bioreactance |
|
| CO, cardiac index, stroke volume (SV) | Application of thoracic electrodes | Moderate to high |
| Transthoracic Echocardiography (TTE) |
|
| VTI, SV, CO, ventricular function | Portable ultrasound device, trained operator | Moderate to high |
| Pulse Contour Analysis & Photoplethysmography (PPG) |
|
| SV, CO, dynamic indices (e.g., SVV), perfusion index (PI), dynamic changes in PI | Finger cuff device or pulse oximeter with integrated sensor | Low to moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrame, M.; Bellan, M.; Patrucco, F.; Gavelli, F. Non-Invasive Hemodynamic Monitoring in Critically Ill Patients: A Guide for Emergency Physicians. J. Clin. Med. 2025, 14, 7002. https://doi.org/10.3390/jcm14197002
Beltrame M, Bellan M, Patrucco F, Gavelli F. Non-Invasive Hemodynamic Monitoring in Critically Ill Patients: A Guide for Emergency Physicians. Journal of Clinical Medicine. 2025; 14(19):7002. https://doi.org/10.3390/jcm14197002
Chicago/Turabian StyleBeltrame, Michela, Mattia Bellan, Filippo Patrucco, and Francesco Gavelli. 2025. "Non-Invasive Hemodynamic Monitoring in Critically Ill Patients: A Guide for Emergency Physicians" Journal of Clinical Medicine 14, no. 19: 7002. https://doi.org/10.3390/jcm14197002
APA StyleBeltrame, M., Bellan, M., Patrucco, F., & Gavelli, F. (2025). Non-Invasive Hemodynamic Monitoring in Critically Ill Patients: A Guide for Emergency Physicians. Journal of Clinical Medicine, 14(19), 7002. https://doi.org/10.3390/jcm14197002







