Effects of Oral Nutritional Supplementation on Body Composition and Bone Health in Undernourished Children: A Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Intervention
2.3. Study Procedure
2.4. Dual Energy X-Ray Absorptiometry
2.5. Sample Size and Statistical Analysis
3. Results
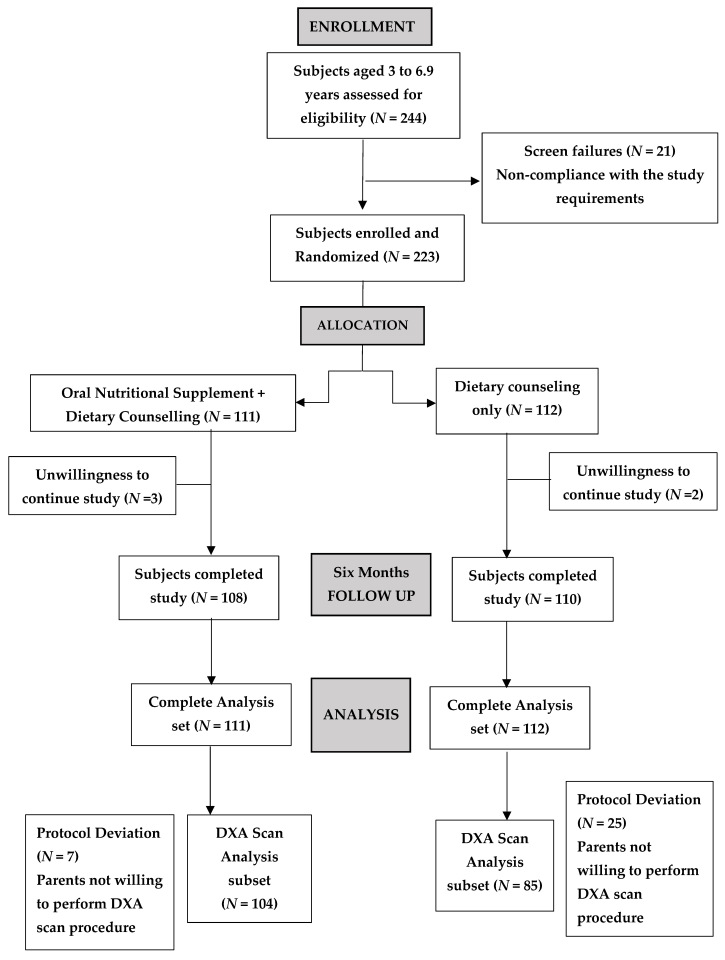
3.1. Subject Disposition
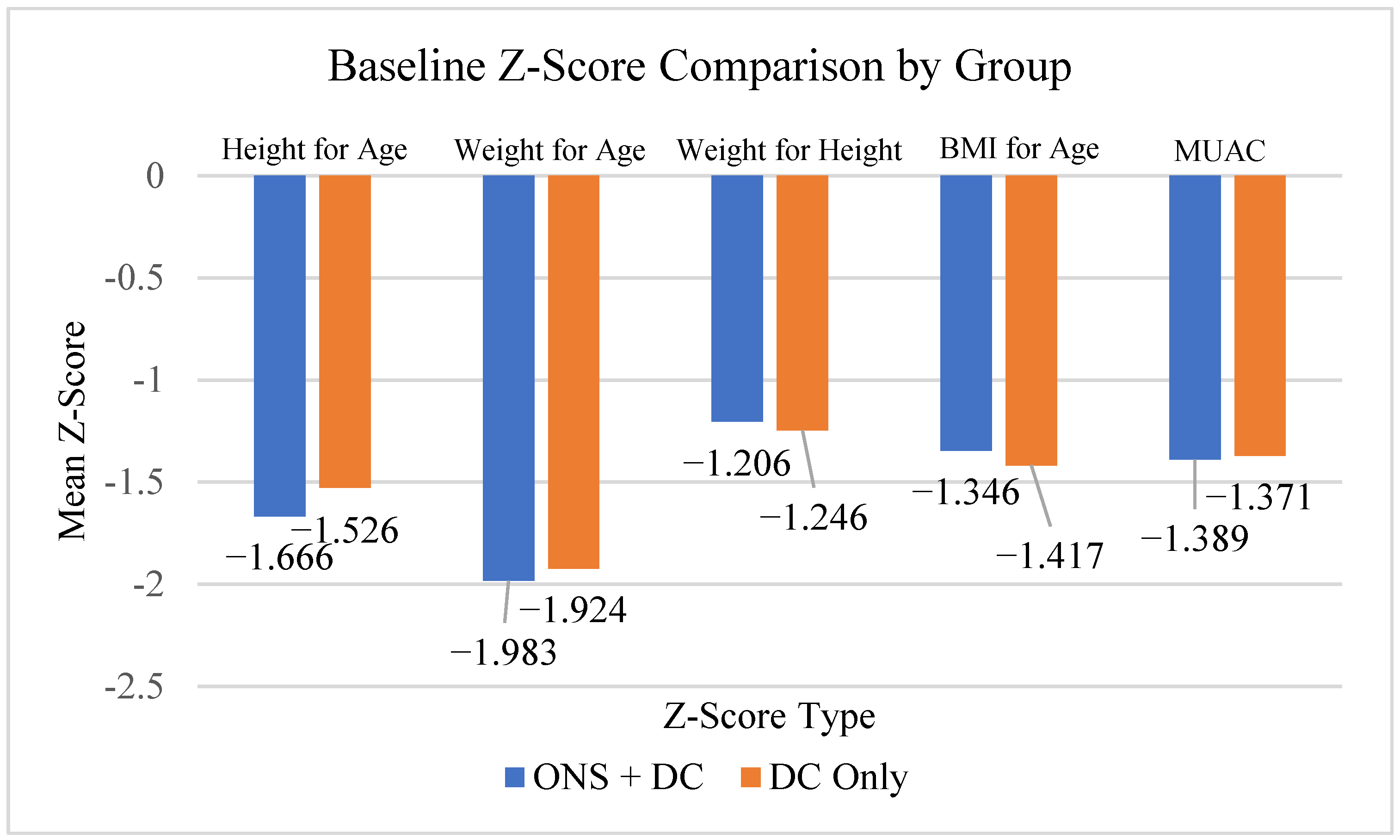
3.2. Baseline Demography
3.3. Bone Health and Body Composition
4. Discussion
4.1. Key Findings
4.2. Strengths and Limitations
4.3. Future Prospectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCOVA | Analyzed using analysis of covariance |
| BMC | Bone mineral content |
| BMD | Bone mineral density |
| BMI | Body Mass Index |
| CFB | Changes from baseline |
| CTRI | Clinical Trials Registry of India |
| DC | Dietary counselling |
| DXA | Dual-energy X-ray absorptiometry |
| FMI | Fat Mass Index |
| ICMR | Indian Council of Medical Research |
| LAR | Legal Authorized Representative |
| LMI | Lean Mass Index |
| ONS | Oral Nutritional Supplements |
| SPROUT | Supporting Pediatric GRowth and Health OUTcomes |
| WHO | World Health Organization |
References
- Saavedra, J.M.; Prentice, A.M. Nutrition in school-age children: A rationale for revisiting priorities. Nutr. Rev. 2023, 81, 823–843. [Google Scholar] [CrossRef]
- Prentice, A.; Schoenmakers, I.; Laskey, M.A.; de Bono, S.; Ginty, F.; Goldberg, G.R. Nutrition and bone growth and development. Proc. Nutr. Soc. 2006, 65, 348–360. [Google Scholar] [CrossRef]
- Joint Child Malnutrition Estimates. Available online: https://www.who.int/data/gho/data/themes/topics/joint-child-malnutrition-estimates-unicef-who-wb (accessed on 7 July 2025).
- National Family Health Survey (NFHS-5), 2019–21: India. Mumbai: International Institute for Population Sciences (IIPS). 2021. Available online: https://dhsprogram.com/pubs/pdf/FR375/FR375.pdf (accessed on 7 July 2025).
- Wells, J.C.K. Body composition of children with moderate and severe undernutrition and after treatment: A narrative review. BMC Med. 2019, 17, 215. [Google Scholar] [CrossRef]
- van Beijsterveldt, I.A.; van der Steen, M.; de Fluiter, K.S.; Spaans, S.A.; Hokken-Koelega, A.C. Body composition and bone mineral density by Dual Energy X-ray Absorptiometry: Reference values for young children. Clin. Nutr. 2022, 41, 71–79. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.H. Proposed recommended nutrient densities for moderately malnourished children. Food Nutr. Bull. 2009, 30 (Suppl. 3), S267–S342. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T. The role of ascorbic acid in mesenchymal differentiation. Nutr. Rev. 1992, 50, 65–70. [Google Scholar] [CrossRef]
- Booth, S.L. Vitamin K: Food composition and dietary intakes. Food Nutr. Res. 2012, 56, 5505. [Google Scholar] [CrossRef]
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F.; ASPEN Board of Directors. Defining pediatric malnutrition: Aparadigm shift toward etiology-related definitions. J. Parenter. Enteral Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Berkley, J.A.; Bandsma, R.H.J.; Kerac, M.; Trehan, I.; Briend, A. Severe childhood malnutrition. Nat. Rev. Dis. Primers. 2017, 3, 17067. [Google Scholar] [CrossRef]
- Fabiansen, C.; Yaméogo, C.W.; Iuel-Brockdorf, A.S.; Cichon, B.; Rytter, M.J.; Kurpad, A.; Wells, J.C.; Ritz, C.; Ashorn, P.; Filteau, S.; et al. Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2×2×3 factorial trial in Burkina Faso. PLoS Med. 2017, 14, e1002387. [Google Scholar] [CrossRef]
- Ow, M.Y.L.; Tran, N.T.; Berde, Y.; Nguyen, T.S.; Tran, V.K.; Jablonka, M.J.; Baggs, G.E.; Huynh, D.T.T. Efficacy of long-term oral nutritional supplementation with dietary counseling on growth, body composition and bone mineralization in children with or at risk for undernutrition: A randomized controlled trial. Nutr. J. 2025, 24, 110. [Google Scholar] [CrossRef] [PubMed]
- National Osteoporosis Society. A Practical Guide to Bone Densitometry in Children; National Osteoporosis Society: London, UK, 2004. [Google Scholar]
- Khadilkar, A.; Ranade, A.; Bhosale, N.; Motekar, S.; Mehta, N. Impact of oral nutritional supplementation and dietary counseling on outcomes of linear catch-up growth in Indian children aged 3–6.9 years: Findings from a 6-month randomized controlled trial. Children 2025, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height, and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, L.K.; Gordon, C.M.; Section on Endocrinology. Bone densitometry in children and adolescents. Pediatrics 2016, 138, e20162398. [Google Scholar] [CrossRef]
- Dongare-Bhor, S.; Lohiya, N.; Maheshwari, A.; Ekbote, V.; Chiplonkar, S.; Padidela, R.; Mughal, Z.; Khadilkar, V.; Khadilkar, A. Muscle and bone parameters in underprivileged Indian children and adolescents with T1DM. Bone 2020, 130, 115074. [Google Scholar] [CrossRef]
- Lopriore, C.; Guidoum, Y.; Briend, A.; Branca, F. Spread fortified with vitamins and minerals induces catch-up growth and eradicates severe anemia in stunted refugee children aged 3–6 y. Am. J. Clin. Nutr. 2004, 80, 973–981. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Hoppe, C.; Roos, N.; Kaestel, P.; Stougaard, M.; Lauritzen, L.; Mølgaard, C.; Girma, T.; Friis, H. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr. Bull. 2009, 30 (Suppl. 3), S343–S404. [Google Scholar] [CrossRef]
- Chheda, E.; Gurumaani, S.; Gangwar, V.; Saxena, A.; Lomore, K.; Marathe, M.; Rahul, P.; Kareenhalli, V. Effect of oral nutrition supplement on growth in preschool children–a systems physiology-based in silico analysis. Indian J. Child Health 2022, 9, 204–213. [Google Scholar]
- Bonjour, J.P. Protein intake and bone health. Int. J. Vitam. Nutr. Res. 2011, 81, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Zemel, B.S.; Wren, T.A.; Leonard, M.B.; Bachrach, L.K.; Rauch, F.; Gilsanz, V.; Rosen, C.J.; Winer, K.K. The determinants of peak bone mass. J. Pediatr. 2017, 180, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Abrams, S.A.; Daniels, S.R.; Corkins, M.R.; de Ferranti, S.D.; Magge, S.N.; Schwarzenberg, S.J.; Committee on Nutrition. Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [PubMed]
- Kozioł-Kozakowska, A.; Maresz, K. The impact of vitamin K2 (menaquinones) in children’s health and diseases: A review of the literature. Children 2022, 9, 78. [Google Scholar] [CrossRef]
- Tenenbaum, M.; Deracinois, B.; Dugardin, C.; Matéos, A.; Romelard, A.; Auger, J.; Boulier, A.; Ravallec, R.; Flahaut, C.; Cudennec, B. Identification, production and bioactivity of casein phosphopeptides—A review. Food Res. Int. 2022, 157, 111360. [Google Scholar] [CrossRef]
- Khadilkar, A.V.; Sanwalka, N.J.; Chiplonkar, S.A.; Khadilkar, V.V.; Mughal, M.Z. Normative data and percentile curves for dual energy X-ray absorptiometry in healthy Indian girls and boys aged 5–17 years. Bone 2011, 48, 810–819. [Google Scholar] [CrossRef]
- Lakshmi, S.; Metcalf, B.; Joglekar, C.; Yajnik, C.S.; Fall, C.H.; Wilkin, T.J. Differences in body composition and metabolic status between white U.K. and Asian Indian children (EarlyBird 24 and the Pune Maternal Nutrition Study). Pediatr. Obes. 2012, 7, 347–354. [Google Scholar] [CrossRef]
- Nakavachara, P.; Pooliam, J.; Weerakulwattana, L.; Kiattisakthavee, P.; Chaichanwattanakul, K.; Manorompatarasarn, R.; Chokephaibulkit, K.; Viprakasit, V. A normal reference of bone mineral density measured by dual energy X-ray absorptiometry in healthy Thai children and adolescents aged 5–18 years: A new reference for Southeast Asian populations. PLoS ONE 2014, 9, e97218. [Google Scholar] [CrossRef]
- Shatrugna, V.; Balakrishna, N.; Krishnaswamy, K. Effect of micronutrient supplement on health and nutritional status of schoolchildren: Bone health and body composition. Nutrition 2006, 22 (Suppl. 1), S33–S39. [Google Scholar] [CrossRef]
- Levine, M.A. Assessing bone health in children and adolescents. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. 2), S205–S212. [Google Scholar] [CrossRef]
- Greer, F.R.; Krebs, N.F.; Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics 2006, 117, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Kontulainen, S.A.; Khan, K.M.; McKay, H.A. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J. Bone Miner. Res. 2007, 22, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.J.; Vatanparast, H.; Baxter-Jones, A.; Faulkner, R.A.; Mirwald, R.; Bailey, D.A. Factors that affect bone mineral accrual in the adolescent growth spurt. J. Nutr. 2004, 134, 696S–700S. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Fuller, N.J.; Dewit, O.; Fewtrell, M.S.; Elia, M.; Cole, T.J. Four-component model of body composition in children: Density and hydration of fat-free mass and comparison with simpler models. Am. J. Clin. Nutr. 1999, 69, 904–912. [Google Scholar] [CrossRef]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef]
- Bailey, D.A.; Martin, A.D. Physical activity and skeletal health in adolescents. Pediatr. Exerc. Sci. 1994, 6, 330–347. [Google Scholar] [CrossRef]
- Córdoba-Rodríguez, D.P.; Iglesia, I.; Gomez-Bruton, A.; Rodríguez, G.; Casajús, J.A.; Morales-Devia, H.; Moreno, L.A. Fat-free/lean body mass in children with insulin resistance or metabolic syndrome: A systematic review and meta-analysis. BMC Pediatr. 2022, 22, 58. [Google Scholar] [CrossRef]
- Deng, K.L.; Yang, W.Y.; Hou, J.L.; Li, H.; Feng, H.; Xiao, S.M. Association between body composition and bone mineral density in children and adolescents: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 12126. [Google Scholar] [CrossRef]
- Nguyen, H.G.; Pham, M.T.; Ho-Pham, L.T.; Nguyen, T.V. Lean mass and peak bone mineral density. Osteoporos. Sarcopenia 2020, 6, 212–216. [Google Scholar] [CrossRef]
- Binkovitz, L.A.; Henwood, M.J. Pediatric DXA: Technique and interpretation. Pediatr. Radiol. 2007, 37, 21–31. [Google Scholar] [CrossRef]
- Zemel, B.S.; Leonard, M.B.; Kelly, A.; Lappe, J.M.; Gilsanz, V.; Oberfield, S.; Mahboubi, S.; Shepherd, J.A.; Hangartner, T.N.; Frederick, M.M.; et al. Height adjustment in assessing dual energy X-ray absorptiometry measurements of bone mass and density in children. J. Clin. Endocrinol. Metab. 2010, 95, 1265–1273. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; British Paediatric and Adolescent Bone Group. Bone densitometry in children assessed by dual X-ray absorptiometry: Uses and pitfalls. Arch. Dis. Child. 2003, 88, 795–798. [Google Scholar] [CrossRef]
- Fan, B.; Shepherd, J.A.; Levine, M.A.; Steinberg, D.; Wacker, W.; Barden, H.S.; Ergun, D.; Wu, X.P. National Health and Nutrition Examination Survey whole-body dual-energy X-ray absorptiometry reference data for GE Lunar systems. J. Clin. Densitom. 2014, 17, 344–377. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about/index.html (accessed on 2 January 2025).
| Parameters | Statistics | ONS + Dietary Counselling (N = 111) | Dietary Counselling (N = 112) | Total (N = 223) | |
|---|---|---|---|---|---|
| Gender | Male | n (%) | 59 (53.2) | 53 (47.3) | 112 (50.2) |
| Female | n (%) | 52 (46.8) | 59 (52.7) | 111 (49.8) | |
| Age (Years) | Mean (SD) | 4.8 (0.9) | 4.8 (0.9) | 4.86 (0.9) | |
| Average Height (cm) | Mean (SD) | 101.0 (5.8) | 101.7 (5.6) | 101.3 (5.7) | |
| Average Body Weight (kg) | Mean (SD) | 13.9 (1.3) | 14.0 (1.3) | 13.9(1.3) | |
| BMI (kg/m2) | Mean (SD) | 13.6 (0.5) | 13.5 (0.6) | 13.5 (0.5) | |
| Parameters Visits | ONS + Dietary Counselling (N = 111) | LS Mean Difference [95% CI] | p-Value | Dietary Counselling (N = 112) | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| BMD (g/cm2) | ||||||
| Baseline | 111 | 0.424 (0.0417) | 112 | 0.424 (0.0436) | ||
| Visit 4 (6 Months) | 104 | 0.445 (0.0422) | 85 | 0.434 (0.0437) | ||
| CFB of BMD (g/cm2) Visit 4 (6 Months) | 104 | 0.023 (0.0168) | 0.007 [0.002, 0.011] | 0.0040 * | 85 | 0.017 (0.0138) |
| BMC (g) | ||||||
| Baseline | 111 | 294.051 (62.4857) | 112 | 299.647 (61.5589) | ||
| Visit 4 (6 Months) | 104 | 328.338 (65.1734) | 85 | 321.561 (64.7891) | ||
| CFB of BMC (g)Visit 4 (6 Months) | 104 | 36.595 (18.9604) | 8.34 [3.58, 13.10] | 0.0007 * | 85 | 28.483 (12.7077) |
| Area (cm2) | ||||||
| Baseline | 111 | 680.091 (89.9563) | 112 | 693.557 (88.0579) | ||
| Visit 4 (6 Months) | 104 | 728.365 (90.0855) | 85 | 730.937 (88.7503) | ||
| CFB of Area (cm2) Visit 4 (6 Months) | 104 | 50.622 (32.3477) | 6.97 [−1.00, 14.94] | 0.0862 | 85 | 42.287 (21.5326) |
| Total Fat (g) | ||||||
| Baseline | 111 | 3852.883 (838.1879) | 112 | 3994.836 (1040.1678) | ||
| Visit 4 (6 Months) | 104 | 3683.358 (899.4342) | 85 | 3935.513 (1121.9666) | ||
| CFB of Total Fat (g) Visit 4 (6 Months) | 104 | −171.418 (454.2310) | −57.95 [−192.38, 76.48] | 0.3961 | 85 | −114.597 (471.8246) |
| Total Fat (%) | ||||||
| Baseline | 111 | 26.627 (3.8024) | 112 | 27.141 (4.9222) | ||
| Visit 4 (6 Months) | 104 | 24.183 (4.2023) | 85 | 25.442 (4.7672) | ||
| CFB of Total Fat (%) Visit 4 (6 Months) | 104 | −2.546 (2.3283) | −0.52 [−1.18, 0.14] | 0.1211 | 85 | −2.134 (2.3610) |
| Total Lean Mass (g) | ||||||
| Baseline | 111 | 10,090.978 (1453.6623) | 112 | 10,117.221 (1298.8145) | ||
| Visit 4 (6 Months) | 104 | 10,979.006 (1571.6173) | 85 | 10,861.615 (1480.8834) | ||
| CFB of Total Lean Mass (g) Visit 4 (6 Months) | 104 | 926.331 (513.2353) | 135.14 [6.18, 264.11] | 0.0401 * | 85 | 801.482 (358.7196) |
| Total Body BMD $ Z-score (+/−) | ||||||
| Baseline | 52 | −1.523 (0.7944) | 51 | −1.434 (0.7491) | ||
| Visit 4 (6 Months) | 61 | −1.479 (0.7321) | 50 | −1.463 (0.7054) | ||
| CFB of Total Body Z-score (+/−) Visit 4 (6 Months) | 47 | −0.014 (0.2081) | 0.00 [−0.10, 0.11] | 0.9715 | 34 | −0.044 (0.2995) |
| LMI (kg/m2) | ||||||
| Baseline | 111 | 9.861 (0.8788) | 112 | 9.764 (0.8256) | ||
| Visit 4 (6 Months) | 104 | 9.590 (0.7809) | 85 | 10.067 (0.8040) | ||
| CFB of Lean Mass Index (kg/m2) | 104 | −0.257 (0.6028) | −0.53 [−0.67, −0.39] | <0.0001 | 85 | 0.293 (0.4014) |
| FMI (kg/m2) | ||||||
| Baseline | 111 | 3.775 (0.7210) | 112 | 3.862 (0.9271) | ||
| Visit 4 (6 Months) | 104 | 3.231 (0.7258) | 85 | 3.650 (0.9454) | ||
| CFB of Fat Mass Index (kg/m2) | 104 | −0.557 (0.3795) | −0.28 [−0.40, −0.16] | <0.0001 | 85 | −0.289 (0.4370) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadilkar, A.; Ranade, A.; Bhosale, N.; Hiremath, S.; Mehta, N. Effects of Oral Nutritional Supplementation on Body Composition and Bone Health in Undernourished Children: A Randomized Controlled Study. J. Clin. Med. 2025, 14, 6972. https://doi.org/10.3390/jcm14196972
Khadilkar A, Ranade A, Bhosale N, Hiremath S, Mehta N. Effects of Oral Nutritional Supplementation on Body Composition and Bone Health in Undernourished Children: A Randomized Controlled Study. Journal of Clinical Medicine. 2025; 14(19):6972. https://doi.org/10.3390/jcm14196972
Chicago/Turabian StyleKhadilkar, Anuradha, Arati Ranade, Neelambari Bhosale, Swati Hiremath, and Nirali Mehta. 2025. "Effects of Oral Nutritional Supplementation on Body Composition and Bone Health in Undernourished Children: A Randomized Controlled Study" Journal of Clinical Medicine 14, no. 19: 6972. https://doi.org/10.3390/jcm14196972
APA StyleKhadilkar, A., Ranade, A., Bhosale, N., Hiremath, S., & Mehta, N. (2025). Effects of Oral Nutritional Supplementation on Body Composition and Bone Health in Undernourished Children: A Randomized Controlled Study. Journal of Clinical Medicine, 14(19), 6972. https://doi.org/10.3390/jcm14196972






