A 20-Year Real-World Study of Small Bowel Cancers: Histologic Subtypes, Clinical Features, and Survival Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Selection
2.3. Data Collection
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
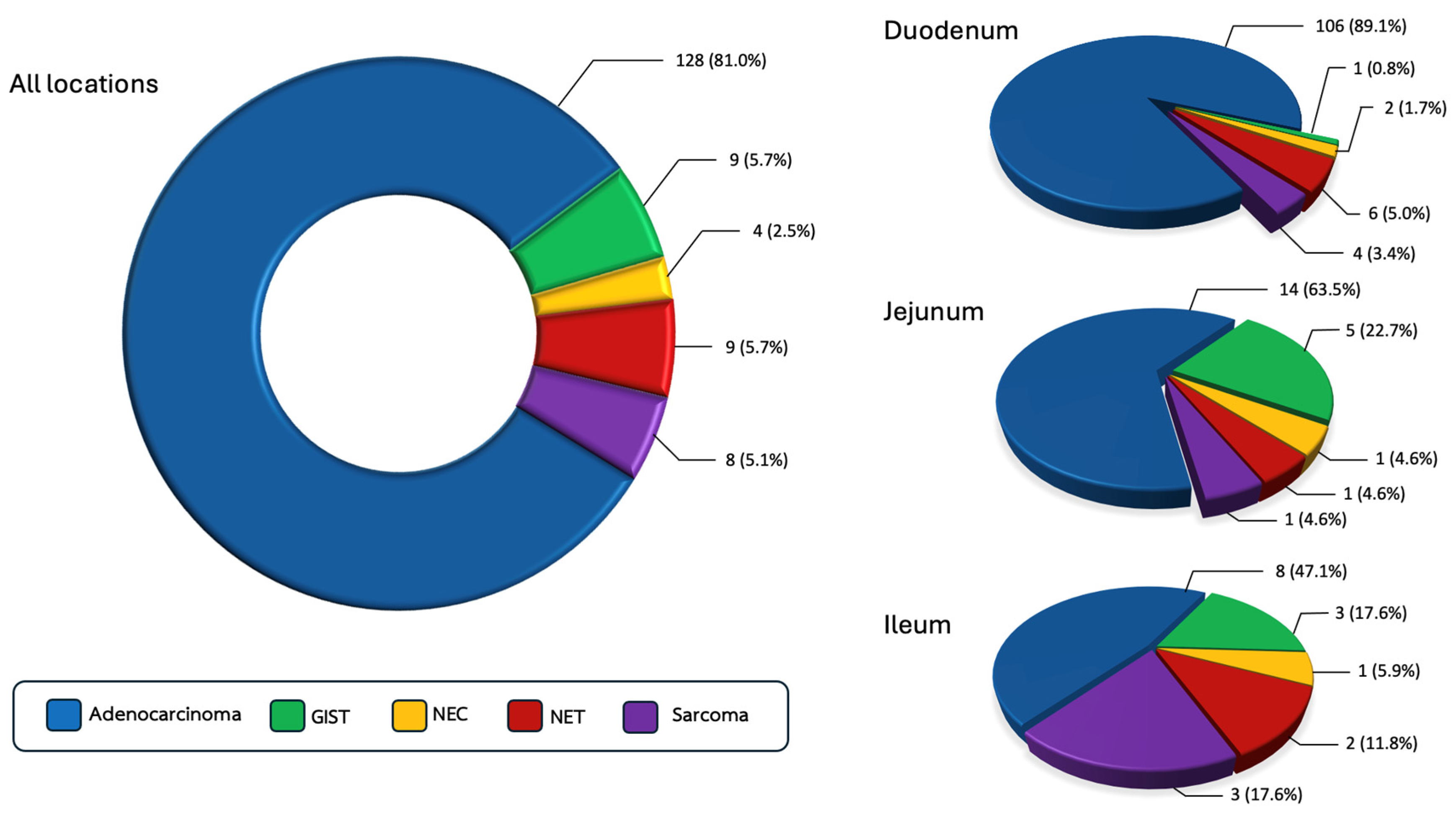
3.1. Prevalence of Small Bowel Cancer Across Histologic Subtypes
3.2. Demographics
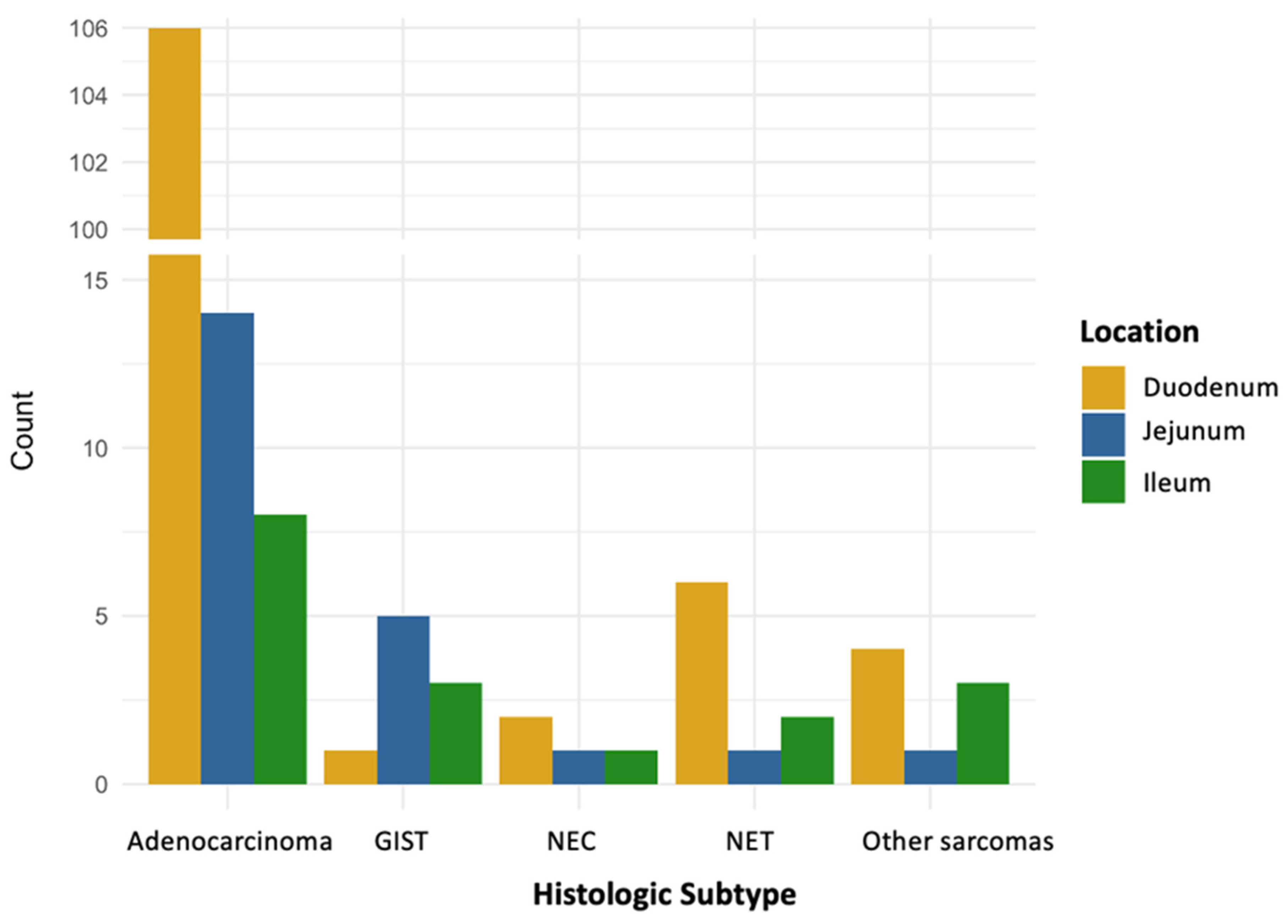
3.3. Tumor Location
3.4. Clinical Presentation
3.5. Radiologic Characteristics
3.6. Staging and Metastatic Pattern
3.7. Laboratory Findings and Tumor Markers
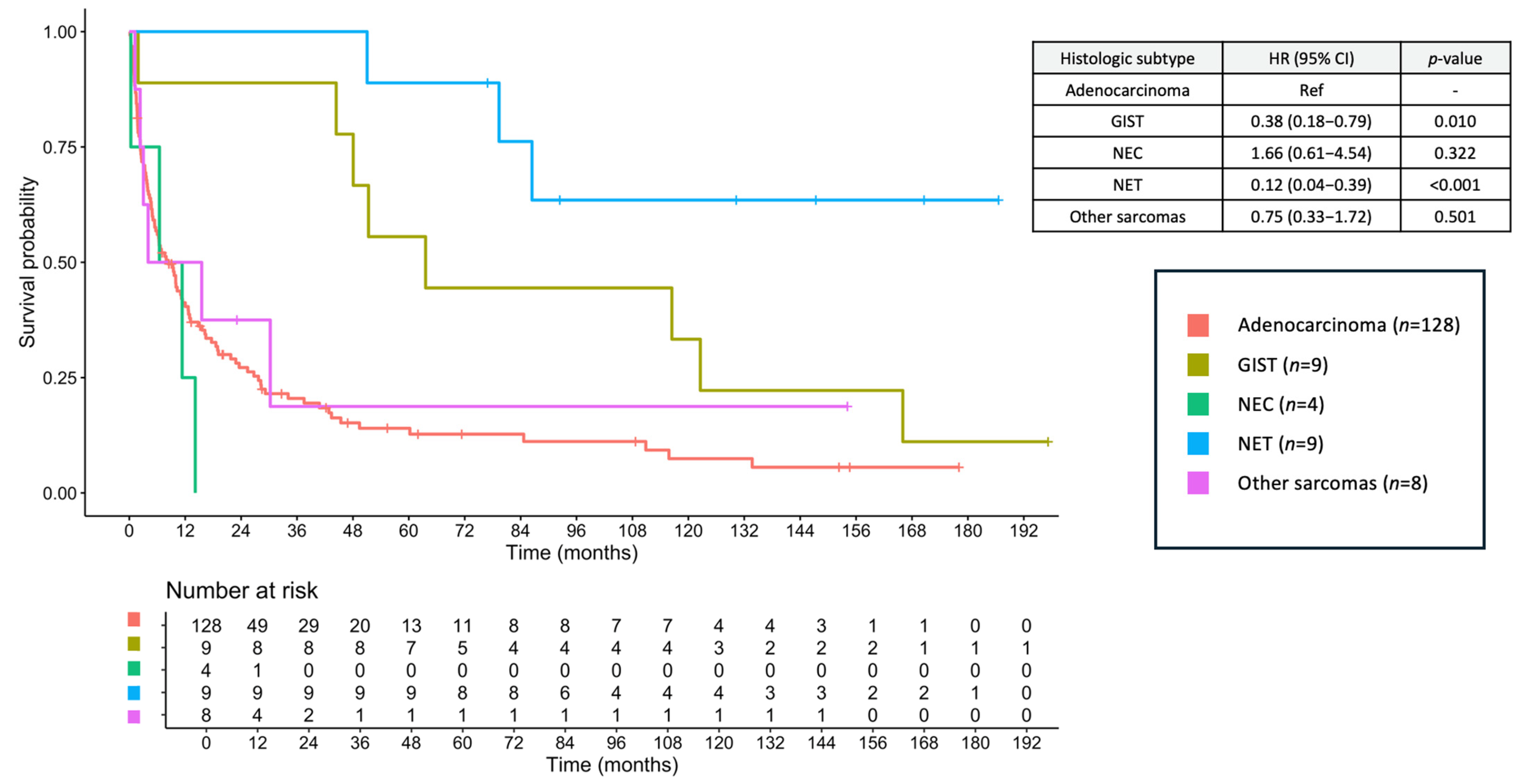
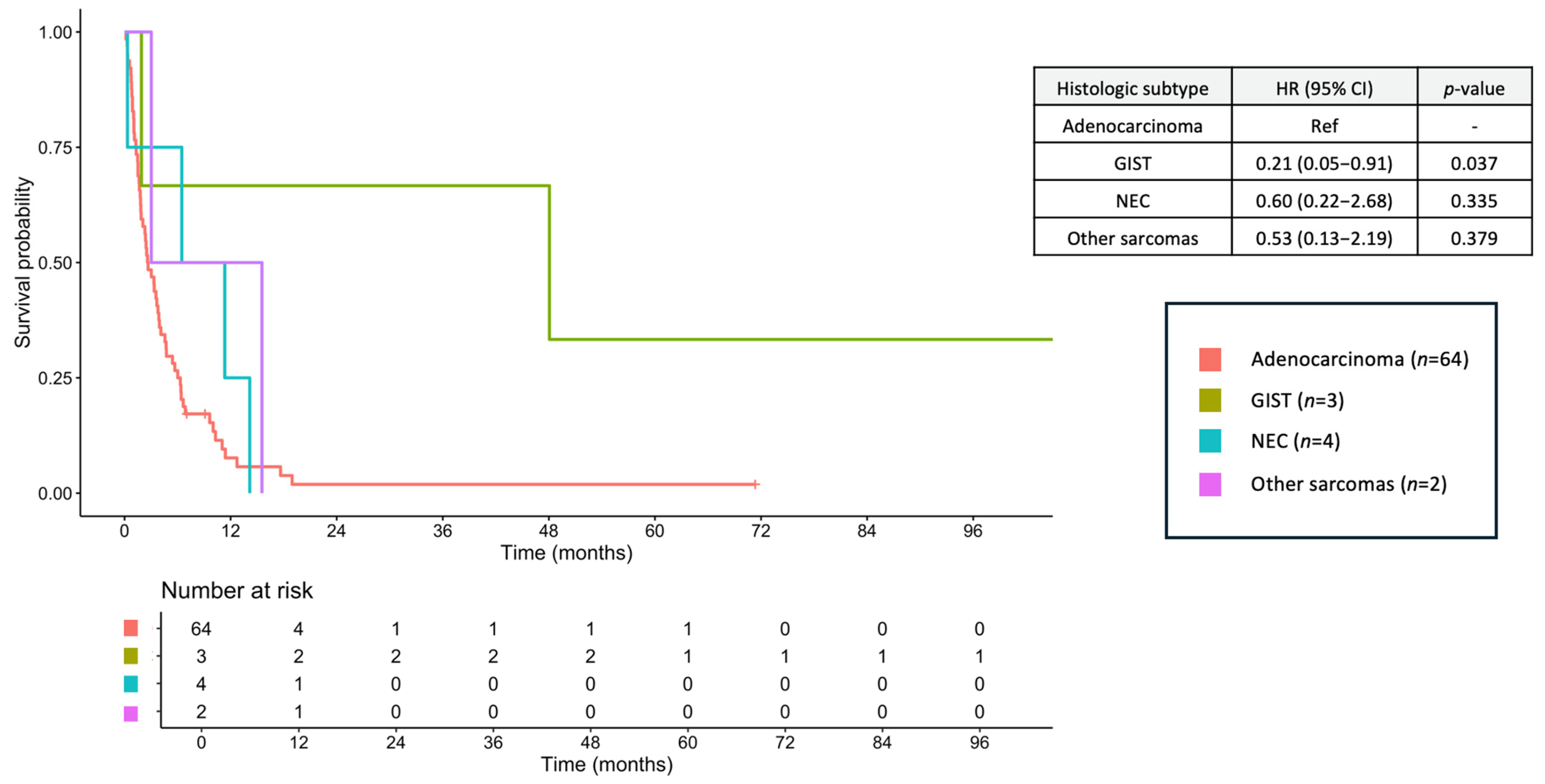
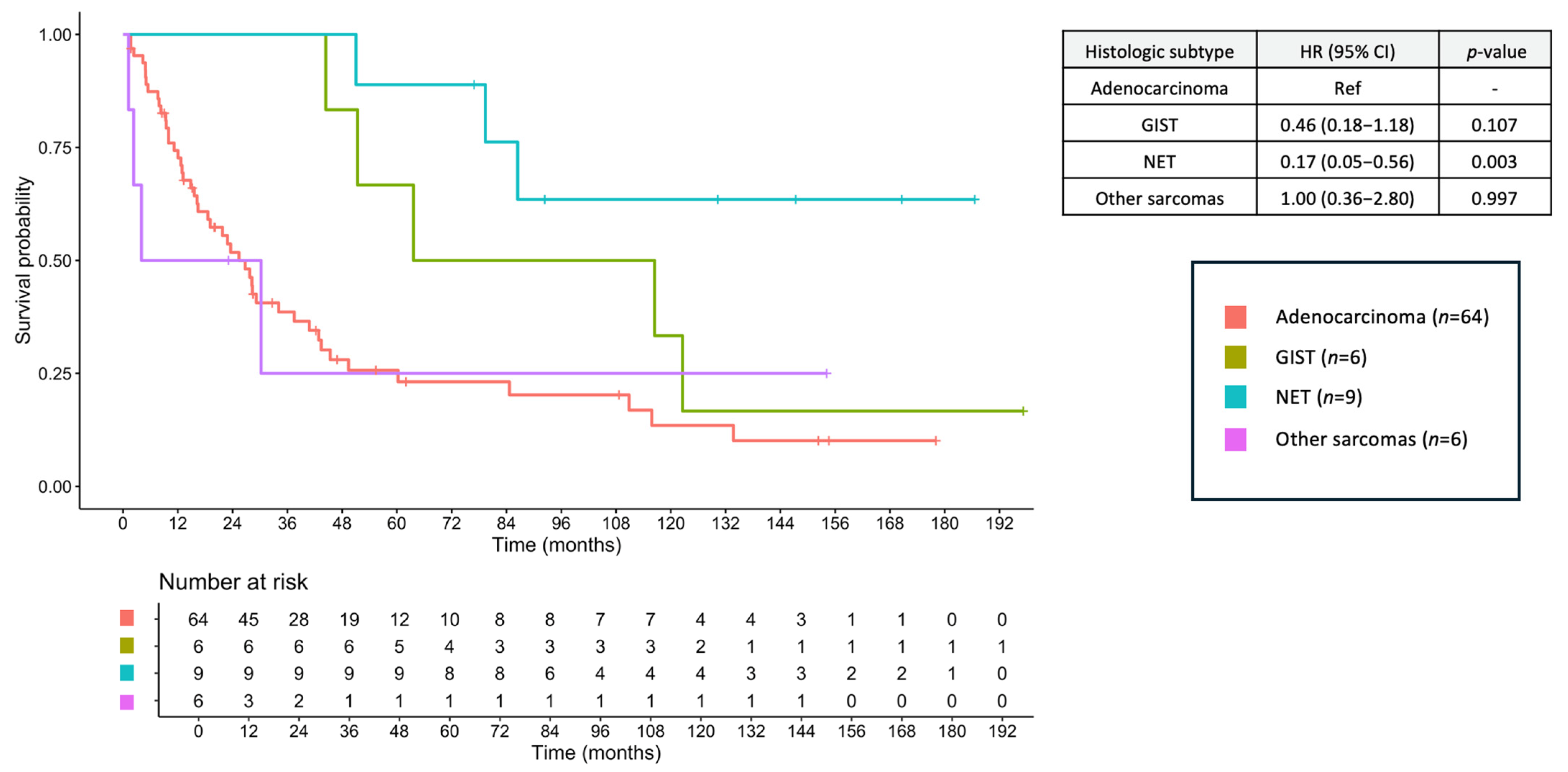
3.8. Survival Outcomes
3.9. Prognostic Factors for OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small Bowel Cancer in the United States: Changes in Epidemiology, Treatment, and Survival over the Last 20 Years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Azizmohammad Looha, M.; Akbari, M.E.; Zarean, E.; Khodakarim, S. Epidemiology of Small Intestine Cancer in Iran. Cancer Rep. 2022, 5, e1593. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; McWilliams, R.R.; Donohue, J.H.; Quevedo, J.F. A Single-Institution Experience with 491 Cases of Small Bowel Adenocarcinoma. Am. J. Surg. 2010, 199, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Bouvier, A.M.; Robaszkiewicz, M.; Jooste, V.; Cariou, M.; Drouillard, A.; Bouvier, V.; Nousbaum, J.B.; French Network of Cancer Registries (FRANCIM). Trends in Incidence of Small Bowel Cancer According to Histology: A Population-Based Study. J. Gastroenterol. 2020, 55, 181–188. [Google Scholar] [CrossRef]
- Aparicio, T.; Zaanan, A.; Svrcek, M.; Laurent-Puig, P.; Carrere, N.; Manfredi, S.; Locher, C.; Afchain, P. Small Bowel Adenocarcinoma: Epidemiology, Risk Factors, Diagnosis and Treatment. Dig. Liver Dis. 2014, 46, 97–104. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal Stromal Tumors: Pathology and Prognosis at Different Sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef]
- Howe, J.R.; Karnell, L.H.; Scott-Conner, C. Small Bowel Sarcoma: Analysis of Survival from the National Cancer Data Base. Ann. Surg. Oncol. 2001, 8, 496–508. [Google Scholar] [CrossRef]
- Khan, F.; Vogel, R.I.; Diep, G.K.; Tuttle, T.M.; Lou, E. Prognostic Factors for Survival in Advanced Appendiceal Cancers. Cancer Biomark. 2017, 17, 457–462. [Google Scholar] [CrossRef]
- Ocasio Quinones, G.A.; Khan Suheb, M.Z.; Woolf, A. Small Bowel Neoplasms. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK560725/ (accessed on 5 July 2025).
- Sakae, H.; Kanzaki, H.; Nasu, J.; Akimoto, Y.; Matsueda, K.; Yoshioka, M.; Nakagawa, M.; Hori, S.; Inoue, M.; Inaba, T.; et al. The Characteristics and Outcomes of Small Bowel Adenocarcinoma: A Multicentre Retrospective Observational Study. Br. J. Cancer 2017, 117, 1607–1613. [Google Scholar] [CrossRef]
- Khosla, D.; Dey, T.; Madan, R.; Gupta, R.; Goyal, S.; Kumar, N.; Kapoor, R. Small Bowel Adenocarcinoma: An Overview. World J. Gastrointest. Oncol. 2022, 14, 413–422. [Google Scholar] [CrossRef]
- Barsouk, A.; Rawla, P.; Barsouk, A.; Thandra, K.C. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med. Sci. 2019, 7, 46. [Google Scholar] [CrossRef]
- Overman, M.J.; Hu, C.Y.; Kopetz, S.; Abbruzzese, J.L.; Wolff, R.A.; Chang, G.J. A Population-Based Comparison of Adenocarcinoma of the Large and Small Intestine: Insights into a Rare Disease. Ann. Surg. Oncol. 2012, 19, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Milione, M.; Parente, P.; Grillo, F.; Zamboni, G.; Mastracci, L.; Capella, C.; Fassan, M.; Vanoli, A. Neuroendocrine Neoplasms of the Duodenum, Ampullary Region, Jejunum and Ileum. Pathologica 2021, 113, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Qubaiah, O.; Devesa, S.S.; Platz, C.E.; Huycke, M.M.; Dores, G.M. Small Intestinal Cancer: A Population-Based Study of Incidence and Survival Patterns in the United States, 1992 to 2006. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Battaglin, F.; Lenz, H.-J. Management of Advanced Small Bowel Cancer. Curr. Treat. Options Oncol. 2018, 19, 69. [Google Scholar] [CrossRef]
- Hatzaras, I.; Palesty, J.A.; Abir, F.; Sullivan, P.; Kozol, R.A.; Dudrick, S.J.; Longo, W.E. Small-Bowel Tumors: Epidemiologic and Clinical Characteristics of 1260 Cases from the Connecticut Tumor Registry. Arch. Surg. 2007, 142, 229–235. [Google Scholar] [CrossRef]
- Gustafsson, B.I.; Siddique, L.; Chan, A.; Dong, M.; Drozdov, I.; Kidd, M.; Modlin, I.M. Uncommon Cancers of the Small Intestine, Appendix and Colon: An Analysis of SEER 1973–2004, and Current Diagnosis and Therapy. Int. J. Oncol. 2008, 33, 1121–1131. [Google Scholar]
- Lopes, L.F.; Bacchi, C.E. Imatinib Treatment for Gastrointestinal Stromal Tumour (GIST). J. Cell. Mol. Med. 2010, 14, 42–50. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Zhang, C.; Liao, Z.; Li, T.; Yang, T.; Zhang, G.; Yang, J. Pan-soft Tissue Sarcoma Analysis of the Incidence, Survival, and Metastasis: A Population-Based Study Focusing on Distant Metastasis and Lymph Node Metastasis. Front. Oncol. 2022, 12, 890040. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Francescutti, V.; Miller, A.; Satchidanand, Y.; Alvarez-Perez, A.; Dunn, K.B. Management of bowel obstruction in patients with stage IV cancer: Predictors of outcome after surgery. Ann. Surg. Oncol. 2013, 20, 707–714. [Google Scholar] [CrossRef]
| Adenocarcinoma (n = 128) | GIST (n = 9) | NEC (n = 4) | NET (n = 9) | Other Sarcomas (n = 8) | |
|---|---|---|---|---|---|
| Sex, n (%) Female Male | 59 (46.1) 69 (53.9) | 3 (33.3) 6 (66.7) | 2 (50.0) 2 (50.0) | 3 (33.3) 6 (66.7) | 3 (37.5) 5 (62.5) |
| Age, years (SD) * Age ≥ 70 years, n (%) | 62.2 (12.6) 40 (31.2) | 50.3 (15.4) 1 (11.1) | 42.2 (15.3) 0 (0) | 57.3 (16.6) 2 (22.2) | 49.7 (13.4) 0 (0) |
| BMI, n (%) <18.5 kg/m2 18.5–22.9 kg/m2 ≥23.0 kg/m2 | 51 (39.8) 45 (35.2) 32 (25.0) | 4 (44.4) 3 (33.3) 2 (22.2) | 0 (0) 2 (50.0) 2 (50.0) | 2 (22.2) 4 (44.4) 3 (33.3) | 2 (25.0) 4 (50.0) 2 (25.0) |
| ECOG PS, n (%) * 0–1 ≥2 | 74 (57.8) 54 (42.2) | 8 (88.9) 1 (11.1) | 3 (75.0) 1 (25.0) | 9 (100) 0 (0) | 6 (75.0) 2 (25.0) |
| Tumor location, n (%) * Duodenum Jejunum Ileum | 106 (82.8) 14 (10.9) 8 (6.2) | 1 (11.1) 5 (55.6) 3 (33.3) | 2 (50.0) 1 (25.0) 1 (25.0) | 6 (66.7) 1 (11.1) 2 (22.2) | 4 (50.0) 1 (12.5) 3 (37.5) |
| Clinical presentation, n (%) # Bowel obstruction Bowel perforation Abdominal pain Abdominal distension Abdominal mass * Significant weight loss Melena Chronic diarrhea Jaundice Anemia Anorexia | 44 (34.4) 4 (3.1) 42 (32.8) 8 (6.2) 0 (0) 23 (18.0) 11 (8.6) 3 (2.3) 29 (22.7) 4 (3.1) 10 (7.8) | 0 (0) 0 (0) 4 (44.4) 1 (11.1) 2 (22.2) 0 (0) 1 (11.1) 0 (0) 0 (0) 1 (11.1) 0 (0) | 1 (25.0) 0 (0) 2 (50.0) 0 (0) 0 (0) 2 (50.0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) | 2 (22.2) 0 (0) 4 (44.4) 0 (0) 0 (0) 0 (0) 2 (22.2) 0 (0) 1 (11.1) 0 (0) 0 (0) | 2 (25.0) 2 (25.0) 2 (25.0) 0 (0) 3 (37.5) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 1 (12.5) |
| Lesion from imaging, n (%) Mass Bowel wall thickening Undetected lesion | 73 (57.0) 47 (36.7) 8 (6.2) | 8 (88.9) 1 (11.1) 0 (0) | 3 (75.0) 1 (25.0) 0 (0) | 6 (66.7) 1 (11.1) 2 (22.2) | 7 (87.5) 1 (12.5) 0 (0) |
| Tumor size Largest diameter of mass, cm (IQR) <5 cm, n (%) ≥5 cm, n (%) NA, n (%) | (n = 73) 4.4 (3.2, 5.6) 34 (46.6) 20 (27.4) 19 (26.0) | (n = 8) 7.5 (7.0, 13.0) 0 (0) 7 (87.5) 1 (12.5) | (n = 3) 11.9 (11.5, 12.4) 0 (0) 2 (66.7) 1 (33.3) | (n = 6) 3.8 (2.8, 6.2) 3 (50.0) 1 (16.7) 2 (33.3) | (n = 8) 8.0 (5.2, 9.8) 2 (25.0) 5 (62.5) 1 (12.5) |
| Stage, n (%) * Non-metastatic disease Metastatic disease | 64 (50.0) 64 (50.0) | 6 (66.7) 3 (33.3) | 0 (0) 4 (100) | 9 (100) 0 (0) | 6 (75.0) 2 (25.0) |
| Organ metastasis, n (%) Peritoneum Lymph node Lung Liver Bone Pleura Adrenal Ovary Brain | 26 (20.3) 30 (23.4) 8 (6.2) 25 (19.5) 0 (0) 2 (1.6) 1 (0.8) 4 (3.1) 0 (0) | 1 (11.1) 0 (0) 0 (0) 2 (22.2) 0 (0) 0 (0) 1 (11.1) 0 (0) 0 (0) | 1 (25.0) 2 (50.0) 0 (0) 2 (50.0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) | - - - - - - - - - | 1 (12.5) 0 (0) 2 (25.0) 1 (12.5) 1 (12.5) 0 (0) 0 (0) 0 (0) 1 (12.5) |
| Number of metastases, n (%) 1 ≥2 | 38 (29.7) 26 (20.3) | 2 (22.2) 1 (11.1) | 3 (75.0) 1 (25.0) | - - | 0 (0) 2 (25.0) |
| Tumor differentiation, n (%) Well Moderately Poorly | 43 (33.6) 37 (28.9) 48 (37.5) | - - - | 0 (0) 0 (0) 4 (100) | 9 (100) 0 (0) 0 (0) | - - - |
| CEA level, n (%) <5 ng/mL ≥5 ng/mL | (n = 77) 43 (55.8) 34 (44.2) | (n = 4) 4 (100) 0 (0) | (n = 3) 3 (100) 0 (0) | (n = 1) 1 (100) 0 (0) | (n = 2) 2 (100) 0 (0) |
| CA19-9 level, n (%) <37 U/mL ≥37 U/mL | (n = 57) 32 (56.1) 25 (43.9) | (n = 3) 3 (100) 0 (0) | (n = 1) 1 (100) 0 (0) | (n = 1) 1 (100) 0 (0) | (n = 1) 1 (100) 0 (0) |
| Hemoglobin, n (%) <10 g/dL ≥10 g/dL | (n = 126) 63 (50.0) 63 (50.0) | (n = 7) 5 (71.4) 2 (28.6) | (n = 3) 2 (66.7) 1 (33.3) | (n = 6) 2 (33.3) 4 (66.7) | (n = 8) 3 (37.5) 5 (62.5) |
| Albumin, n (%) <3.5 g/dL ≥3.5 g/dL | (n = 126) 61 (48.4) 65 (51.6) | (n = 7) 1 (14.3) 6 (85.7) | (n = 3) 2 (66.7) 1 (33.3) | (n = 6) 3 (50.0) 3 (50.0) | (n = 8) 1 (12.5) 7 (87.5) |
| Treatment, n (%) Primary tumor resection Adjuvant systemic treatment Palliative systemic treatment | 59/128 (46.1) 29/64 (45.3) 19/64 (29.7) | 7/9 (77.8) 1/6 (16.7) 2/3 (66.7 | 1/4 (25.0) 0 (0) 3/4 (75.0) | 9/9 (100) 0 (0) 0 (0) | 5/8 (62.5) 1/6 (16.7) 2/2 (100) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age ≥ 70 years | 1.62 (1.11–2.36) | 0.013 | 1.23 (0.81–1.88) | 0.331 |
| Male vs. Female | 0.94 (0.67–1.33) | 0.739 | - | - |
| BMI <18.5 vs. ≥18.5 kg/m2 | 1.32 (0.92–1.88) | 0.131 | - | - |
| ECOG PS 0–1 vs. 2–3 | 0.17 (0.11–0.25) | <0.001 | 0.28 (0.17–0.44) | <0.001 |
| Hemoglobin ≥ 10 g/dL | 0.72 (0.51–1.02) | 0.066 | - | - |
| Albumin ≥ 3.5 g/dL | 0.57 (0.40–0.81) | 0.002 | 0.69 (0.46–1.02) | 0.064 |
| Location: Duodenum Jejunum Ileum | Ref 0.41 (0.23–0.73) 0.48 (0.27–0.85) | Ref 0.003 0.011 | Ref 0.45 (0.23–0.89) 0.46 (0.23–0.90) | Ref 0.021 0.024 |
| Metastatic stage | 5.10 (3.50–7.45) | <0.001 | 5.27 (3.33–8.34) | <0.001 |
| Bowel obstruction | 1.17 (0.81–1.71) | 0.406 | - | - |
| Bowel perforation | 2.04 (0.89–4.67) | 0.090 | - | - |
| Jaundice | 1.32 (0.86–2.03) | 0.211 | - | - |
| Lesion Mass Bowel wall thickening Undetected | Ref 1.47 (1.20–2.14) 0.80 (0.39–1.65) | Ref 0.048 0.545 | Ref 1.31 (0.88–1.97) 0.60 (0.28–1.29) | Ref 0.186 0.187 |
| Size ≥5 cm | 1.09 (0.72–1.65) | 0.68 | - | - |
| Histology Adenocarcinoma GIST NEC NET Other sarcomas | Ref 0.38 (0.18–0.79) 1.66 (0.61–4.54) 0.12 (0.04–0.39) 0.75 (0.33–1.72) | Ref 0.010 0.322 <0.001 0.501 | Ref 0.47 (0.18–1.27) 1.72 (0.50–5.96) 0.35 (0.11–1.18) 1.79 (0.72–4.44) | Ref 0.136 0.394 0.090 0.207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wonglhow, J.; Sunpaweravong, P.; Sathitruangsak, C.; Dechaphunkul, A.; Wetwittayakhlang, P. A 20-Year Real-World Study of Small Bowel Cancers: Histologic Subtypes, Clinical Features, and Survival Implications. J. Clin. Med. 2025, 14, 6962. https://doi.org/10.3390/jcm14196962
Wonglhow J, Sunpaweravong P, Sathitruangsak C, Dechaphunkul A, Wetwittayakhlang P. A 20-Year Real-World Study of Small Bowel Cancers: Histologic Subtypes, Clinical Features, and Survival Implications. Journal of Clinical Medicine. 2025; 14(19):6962. https://doi.org/10.3390/jcm14196962
Chicago/Turabian StyleWonglhow, Jirapat, Patrapim Sunpaweravong, Chirawadee Sathitruangsak, Arunee Dechaphunkul, and Panu Wetwittayakhlang. 2025. "A 20-Year Real-World Study of Small Bowel Cancers: Histologic Subtypes, Clinical Features, and Survival Implications" Journal of Clinical Medicine 14, no. 19: 6962. https://doi.org/10.3390/jcm14196962
APA StyleWonglhow, J., Sunpaweravong, P., Sathitruangsak, C., Dechaphunkul, A., & Wetwittayakhlang, P. (2025). A 20-Year Real-World Study of Small Bowel Cancers: Histologic Subtypes, Clinical Features, and Survival Implications. Journal of Clinical Medicine, 14(19), 6962. https://doi.org/10.3390/jcm14196962






