Predictive Value of Epicardial Adipose Tissue Parameters Measured by Cardiac Computed Tomography for Recurrence of Atrial Fibrillation After Pulmonary Vein Isolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Acquisition and Image Analysis
2.3. Procedure Characteristics and Clinical Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Procedural Characteristics and Ablation Outcomes
3.3. EAT in “Recurrence” and “Non-Recurrence” Groups
3.4. Univariate Logistic Regression
3.5. Multivariate Logistic Regression Analysis
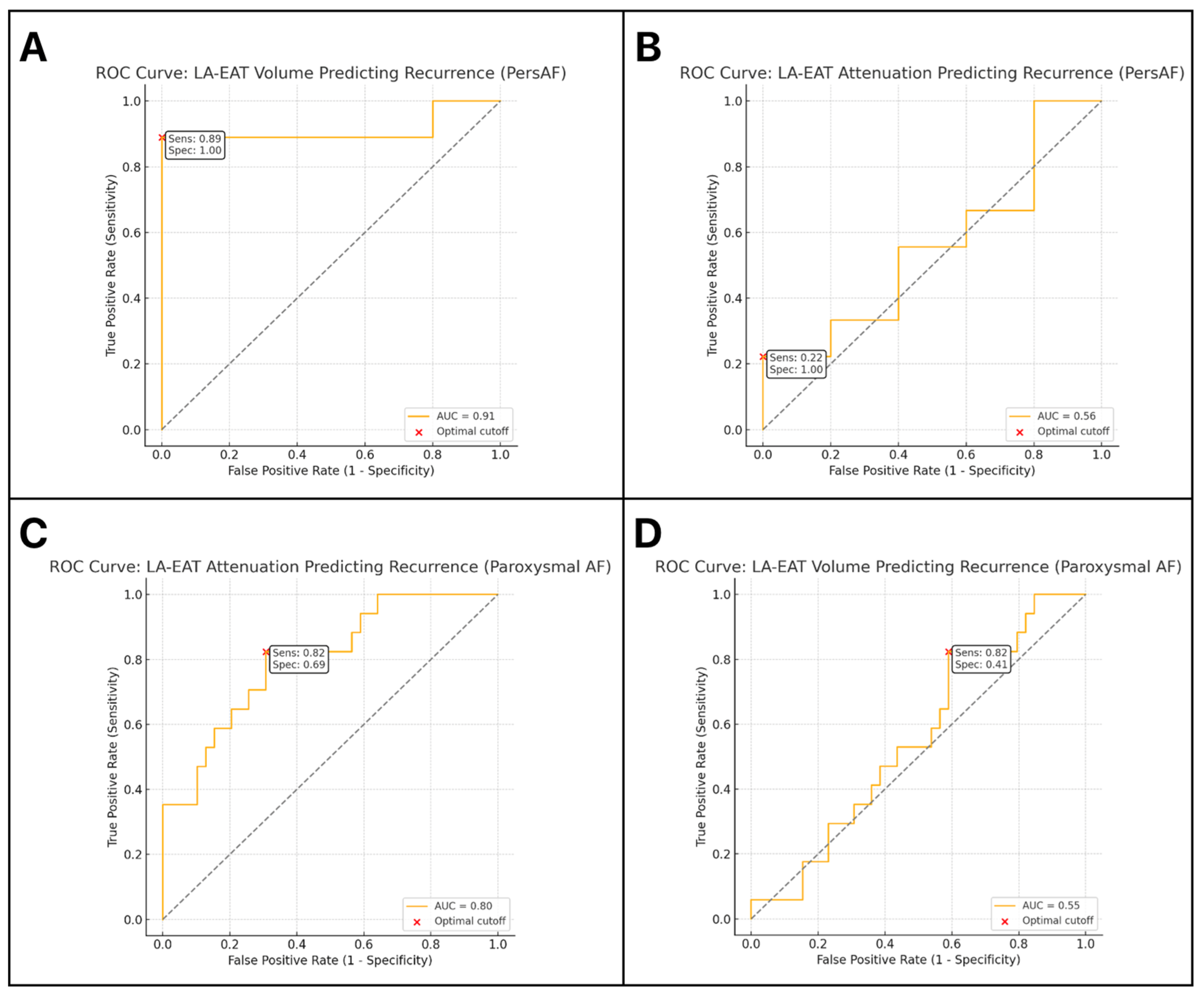
3.6. Analysis of ROC Curves for LA-EAT Parameters
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEI | Angiotensin-Converting Enzyme Inhibitor |
| AF | Atrial Fibrillation |
| AUC | Area Under the Curve |
| ARB | Angiotensin II Receptor Blocker |
| BB | Beta Blockers |
| BMI | Body Mass Index |
| CABG | Coronary Artery Bypass Grafting |
| CaB | Calcium Blockers |
| Ca-blocker | Calcium Channel Blocker |
| CAD | Coronary Artery Disease |
| CCS | Chronic Coronary Syndrome |
| cm3 | Cubic Centimetres |
| CBA | Cryoballoon Ablation |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| DPP4 | Dipeptidyl Peptidase-4 |
| EAT | Epicardial Adipose Tissue |
| ECG | Electrocardiogram |
| FDR | False Discovery Rate |
| GFR | Glomerular Filtration Rate |
| GIP | Glucose-Dependent Insulinotropic Polypeptide |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLP1ra | Glucagon-Like Peptide-1 Receptor Agonist |
| HF | Heart Failure |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| HU | Hounsfield Units |
| IQR, | Interquartile Range |
| LA | Left Atrium |
| LA-EAT | Left Atrium Epicardial Adipose Tissue |
| LAA | Left Atrial Appendage |
| LVEF | Left Ventricular Ejection Fraction |
| MI | Myocardial Infarction |
| MRA | Mineralocorticoid Receptor Antagonist |
| NOAC | Non-Vitamin K Antagonist Oral Anticoagulant |
| OBS | Obstructive Sleep Apnea |
| OR | Odds Ratio |
| OSA | Obstructive Sleep Apnea |
| PCI | Percutaneous Coronary Intervention |
| PET | Positron Emission Tomography |
| PersAF | Persistent Atrial Fibrillation |
| PFA | Pulsed Field Ablation |
| PVAT | Perivascular Adipose Tissue |
| PVI | Pulmonary Vein Isolation |
| RCT | Randomized Controlled Trial |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| SGLT2i | Sodium-Glucose Cotransporter-2 Inhibitor |
| T2D | Type 2 Diabetes |
| Total-EAT | Total Epicardial Adipose Tissue |
| TSH | Thyroid-Stimulating Hormone |
| VKA | Vitamin K Antagonist |
References
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.; Lee, X.W.; Elwashahy, M.; Arumugam, P.; Yang, I.A.; Denman, R.; Haqqani, H.; Ranasinghe, I. Freedom from atrial arrhythmia and other clinical outcomes at 5 years and beyond after catheter ablation of atrial fibrillation: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, J.; Ruwald, M.H.; Pallisgaard, J.; Rasmussen, P.V.; Johannessen, A.; Hansen, J.; Worck, R.H.; Zörner, C.R.; Riis-Vestergaard, L.; Middelfart, C.; et al. Lower Recurrence Rates of Atrial Fibrillation and MACE Events After Early Compared to Late Ablation: A Danish Nationwide Register Study. J. Am. Heart Assoc. 2024, 13, e032722. [Google Scholar] [CrossRef]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef]
- Le Jemtel, T.H.; Samson, R.; Ayinapudi, K.; Singh, T.; Oparil, S. Epicardial Adipose Tissue and Cardiovascular Disease. Curr. Hypertens. Rep. 2019, 21, 36. [Google Scholar] [CrossRef]
- Iacobellis, G.; Barbaro, G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm. Metab. Res. 2008, 40, 442–445. [Google Scholar] [CrossRef]
- Ernault, A.C.; Meijborg, V.M.F.; Coronel, R. Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1730–1745. [Google Scholar] [CrossRef]
- Chen, J.; Mei, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zeng, R.; Liu, Q. Epicardial adipose tissue is associated with higher recurrence risk after catheter ablation in atrial fibrillation patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2022, 22, 264. [Google Scholar] [CrossRef]
- Anagnostopoulos, I.; Kousta, M.; Kossyvakis, C.; Paraskevaidis, N.T.; Vrachatis, D.; Deftereos, S.; Giannopoulos, G. Epicardial Adipose Tissue and Atrial Fibrillation Recurrence following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6369. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Kilicaslan, F.; Minor, S.; Schweikert, R.A.; Saliba, W.; Cummings, J.; Burkhardt, J.D.; et al. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: An independent predictor of procedural failure. J. Am. Coll. Cardiol. 2005, 45, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Teshima, Y.; Kondo, H.; Kaku, H.; Kira, S.; Ikebe, Y.; Saito, S.; Fukui, A.; Shinohara, T.; Yufu, K.; et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 2018, 15, 1717–1727. [Google Scholar] [CrossRef]
- La Fazia, V.M.; Pierucci, N.; Schiavone, M.; Compagnucci, P.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Horton, R.; Al-Ahmad, A.; Di Biase, L.; et al. Comparative effects of different power settings for achieving transmural isolation of the left atrial posterior wall with radiofrequency energy. Europace 2024, 26, euae265. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef]
- El Mahdiui, M.; Simon, J.; Smit, J.M.; Kuneman, J.H.; van Rosendael, A.R.; Steyerberg, E.W.; van der Geest, R.J.; Száraz, L.; Herczeg, S.; Szegedi, N.; et al. Posterior Left Atrial Adipose Tissue Attenuation Assessed by Computed Tomography and Recurrence of Atrial Fibrillation After Catheter Ablation. Circ. Arrhythm. Electrophysiol. 2021, 14, e009135. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Tuttolomondo, D.; Martini, C.; Nicolini, F.; Formica, F.; Pini, A.; Secchi, F.; Volpi, R.; De Filippo, M.; Gaibazzi, N. Perivascular Adipose Tissue Attenuation on Computed Tomography beyond the Coronary Arteries. A Systematic Review. Diagnostics 2021, 11, 1495. [Google Scholar] [CrossRef]
- Huber, A.T.; Fankhauser, S.; Wittmer, S.; Chollet, L.; Lam, A.; Maurhofer, J.; Madaffari, A.; Seiler, J.; Servatius, H.; Haeberlin, A.; et al. Epicardial adipose tissue dispersion at CT and recurrent atrial fibrillation after pulmonary vein isolation. Eur. Radiol. 2024, 34, 4928–4938. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, J.P.; Palombi, M.; Bonanni, M.; Matteucci, A.; Arcari, L.; Pierucci, N.; La Fazia, V.M.; Lavalle, C.; Mariani, M.V. The Role of Cardiac Magnetic Resonance in Characterizing Atrial Cardiomyopathy and Guiding Substrate Ablation in Atrial Fibrillation: A Narrative Review. J. Cardiovasc. Dev. Dis. 2025, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Momot, K.; Krauz, K.; Pruc, M.; Szarpak, L.; Rodkiewicz, D.; Mamcarz, A. Association Between Left Atrial Epicardial Adipose Tissue Attenuation Assessed by Cardiac Computed Tomography and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4771. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Bao, W.; Xu, Z.; Qin, L.; Zhang, N.; Yan, F.; Yang, W. Association between epicardial adipose tissue and recurrence of atrial fibrillation after ablation: A propensity score-matched analysis. Int. J. Cardiovasc. Imaging 2022, 38, 1865–1872. [Google Scholar] [CrossRef]
- Ciuffo, L.; Nguyen, H.; Marques, M.D.; Aronis, K.N.; Sivasambu, B.; de Vasconcelos, H.D.; Tao, S.; Spragg, D.D.; Marine, J.E.; Berger, R.D.; et al. Periatrial Fat Quality Predicts Atrial Fibrillation Ablation Outcome. Circ. Cardiovasc. Imaging 2019, 12, e008764. [Google Scholar] [CrossRef]
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide causes large and rapid epicardial fat reduction. Obesity 2017, 25, 311–316. [Google Scholar] [CrossRef]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev. Port. Cardiol. (Engl. Ed.) 2019, 38, 419–423. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Parisi, V.; Moschetta, D.; Valerio, V.; Conte, M.; Massaiu, I.; Bozzi, M.; Celeste, F.; Leosco, D.; Iaccarino, G.; et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 23. [Google Scholar] [CrossRef]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 2008, 16, 1693–1697. [Google Scholar] [CrossRef]
- Christensen, R.H.; Wedell-Neergaard, A.S.; Lehrskov, L.L.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses From a Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 778–787. [Google Scholar] [CrossRef]
- Mine, T.; Shintaku, Y.; Terao, S.; Sugitani, M.; Kogame, T.; Ishihara, M. Sodium-glucose cotransporter 2 inhibitors are more effective than dipeptidyl peptidase 4 inhibitors in preventing mid-term recurrence after atrial fibrillation ablation. Europace 2025, 27 (Suppl. S1), euaf085-323. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-analysis. Adv Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Satti, D.I.; Karius, A.; Chan, J.S.K.; Isakadze, N.; Yadav, R.; Garg, K.; Aronis, K.N.; Marine, J.E.; Berger, R.; Calkins, H.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation. J Am Coll Cardiol EP. 2024, 10, 1848–1855. [Google Scholar] [CrossRef]
- Wu, J.Y.; Tseng, K.J.; Kao, C.L.; Hung, K.C.; Yu, T.; Lin, Y.M. Clinical effectiveness of tirzepatide for patients with atrial fibrillation and type 2 diabetes: A retrospective cohort study. Diabetes Res. Clin. Pract. 2025, 225, 112279. [Google Scholar] [CrossRef]
- Abdelhadi, N.A.; Ragab, K.M.; Elkholy, M.; Koneru, J.; Ellenbogen, K.A.; Pillai, A. Impact of Sodium-Glucose Co-Transporter 2 Inhibitors on Atrial Fibrillation Recurrence Post-Catheter Ablation Among Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2025, 36, 673–682. [Google Scholar] [CrossRef]
- Soliman, Y.; Abuelazm, M.; Amer, B.E.; Hukamdad, M.; Ellabban, M.H.; Hendi, N.I.; Mouffokes, A.; AbdelAzeem, B.; Hassaballa, H. Impact of SGLT2 Inhibitors on Atrial Fibrillation Recurrence after Catheter Ablation in Type 2 Diabetes Mellitus: A Meta-Analysis of Reconstructed Kaplan-Meier Curves with Trial Sequential Analysis. Am. J. Cardiovasc. Drugs 2024, 24, 629–640. [Google Scholar] [CrossRef]
- Mariani, M.V.; Manzi, G.; Pierucci, N.; Laviola, D.; Piro, A.; D’AMato, A.; Filomena, D.; Matteucci, A.; Severino, P.; Miraldi, F.; et al. SGLT2i effect on atrial fibrillation: A network meta-analysis of randomized controlled trials. J. Cardiovasc. Electrophysiol. 2024, 35, 1754–1765. [Google Scholar] [CrossRef]
- Peng, H.; Yang, Y.; Zhao, Y.; Xiao, H. The effect of statins on the recurrence rate of atrial fibrillation after catheter ablation: A meta-analysis. Pacing Clin. Electrophysiol. 2018, 41, 1420–1427. [Google Scholar] [CrossRef]
| Variable | Total Population (n = 70) | Non-Recurrence Group (n = 44) | Recurrence Group (n = 26) |
|---|---|---|---|
| Age, years, mean (SD) | 62.97 (9.54) | 61.82 (10.25) | 64.92 (8.00) |
| Female sex, n (%) | 20 (28.6%) | 14 (31.8%) | 6 (23%) |
| BMI, kg/m2, mean (SD) | 29.07 (4.13) | 28.02 (3.50) | 30.83 (4.57) |
| Echocardiography features | |||
| LVEF, %, mean (SD) | 57.41 (8.43) | 58.85 (7.36) | 55.08 (9.66) |
| LA diameter, millimeters, mean (SD) | 42.11 (4.80) | 40.50 (4.50) | 44.66 (4.20) |
| Comorbidities | |||
| Persistent AF, n (%) | 14 (20%) | 5 (11.4%) | 9 (34.6%) |
| Diabetes mellitus, n (%) | 14 (20%) | 8 (18.2%) | 6 (23.1%) |
| HFpEF, n (%) | 7 (10%) | 2 (4.5%) | 5 (19.2%) |
| HFrEF, n (%) | 5 (7.1%) | 3 (6.8%) | 2 (7.7%) |
| CAD, n (%) | 10 (14.3%) | 5 (11.4%) | 5 (19.2%) |
| Hypertension, n (%) | 44 (62.9%) | 24 (54.5%) | 20 (77%) |
| Thyroid disease, n (%) | 21 (30%) | 12 (27.3%) | 9 (33.3%) |
| Dyslipidemia, n (%) | 42 (60%) | 25 (56.8%) | 17 (65.4%0 |
| Obesity, n (%) | 26 (37.1%) | 12 (27.3%) | 14 (53.8%) |
| OSA, n (%) | 2 (2.8%) | 2 (4.5%) | 0 (0%) |
| COPD, n (%) | 1 (1.4%) | 0 (0%) | 1 (3.8%) |
| Asthma, n (%) | 3 (4.3%) | 1 (2.3%) | 2 (7.7%) |
| Smoking | 10 (14.3%) | 4 (9.1%) | 6 (23.1%) |
| Previous PCI/CABG, n (%) | 4 (5.7%) | 4 (9.1%) | 0 (0%) |
| Preprocedural laboratory variables | |||
| Creatinine, mg/L, mean (SD) | 82.80 (17.05) | 81.39 (15.97) | 85.19 (18.81) |
| CRP, mg/L, median (IQR) | 3.92 (9.93) | 2.36 (2.79) | 7.12 (16.68) |
| TSH, uIU/mL, median (IQR) | 1.45 (1.13, 2.23) | 1.69 (1.25, 2.37) | 1.43 (1.06, 2.2) |
| eGFR, mL/min/1.73, mean (SD) | 81.18 (15.50) | 82.76 (14.33) | 78.52 (17.28) |
| Medications | |||
| GLP1ra, n (%) | 3 (4.3%) | 0 (0%) | 3 (11.5%) |
| SGLT2i, n (%) | 11 (15.7%) | 5 (11.4%) | 6 (23.1%) |
| BB, n (%) | 51(72.8%) | 30 (68.2%) | 21(80.8%) |
| CaB, n (%) | 13 (18.6%) | 6 (13.6%) | 7 (26.9%) |
| ACEI/ARB, n (%) | 45 (64.3%) | 25 (56.9%) | 20 (77%) |
| Loop diuretics, n (%) | 9 (12.9%) | 5 (11.4%) | 4 (15.4%) |
| Thiazide diuretics, n (%) | 12 (17.1%) | 4 (9.1%) | 8 (30.8%) |
| MRA, n (%) | 16 (22.9%) | 11 (25%) | 5 (19.2%) |
| VKA, n (%) | 3 (4.3%) | 1 (2.3%) | 2 (7.7%) |
| NOAC, n (%) | 63 (90%) | 40 (91%) | 23 (88.5%) |
| Levothyroxine, n (%) | 14 (20%) | 8 (18.1%) | 6 (22.2%) |
| Metformin, n (%) | 9 (12.9%) | 5 (11.4%) | 4 (15.4%) |
| Insulin, n (%) | 2 (2.8%) | 1 (2.3%) | 1 (3.8%) |
| Antiarrhythmic drugs, n (%) | 15 (21.4%) | 7 (16%) | 8 (30.8%) |
| Statines, n (%) | 53 (75.7%) | 31 (70.5%) | 22 (84.6%) |
| Variable | Total Population (n = 70) | Non-Recurrence Group (n = 44) | Recurrence Group (n = 26) |
|---|---|---|---|
| Patients with three pulmonary veins, n (%) | 9 (12.8%) | 5 (11.4%) | 4 (15.4%) |
| Patients with four pulmonary veins, n (%) | 45 (64.3%) | 31 (70.4%) | 14 (53.8%) |
| Patients with five pulmonary veins, n (%) | 12 (17.1%) | 6 (13.6%) | 6 (23%) |
| LA-EAT attenuation, HU, mean (SD) | −92.34 (4.49) | −93.91 (3.95) | −89.69 (4.14) |
| LA-EAT attenuation dispersion, mean (SD) | 34.37 (4.01) | 35.58 (3.61) | 32.33 (3.89) |
| LA-EAT volume, cm3, median (IQR) | 20.14 (12.59, 28.20) | 17.96 (11.98, 24.97) | 23.67 (14.53, 34.69) |
| Total-EAT attenuation, HU, median (IQR) | −89.98 (−91.83, −88.21) | −90.10 (−92.56, −88.70) | −89.29 (−91.17, −86.83) |
| Total-EAT attenuation dispersion, mean (SD) | 31.40 (3.87) | 32.36 (3.57) | 29.79 (3.87) |
| Total-EAT volume, cm3, median (IQR) | 80.87 (56.31, 104.44) | 72.95 (51.24, 93.23) | 99.98 (78.7, 171.74) |
| Variable | Odds Ratio (95% CI) | p-Value | OR per Clinical Step (95% CI) |
|---|---|---|---|
| LA-EAT attenuation, HU | 1.09 (1.02–1.17) | 0.01519 | 3.87 per + 5 HU (1.53–9.81) |
| Whole-EAT volume, cm3 | 2.41 (1.16–4.99) | 0.01773 | 1.45 per + 25 cm3 (0.96–2.19) |
| LA diameter, mm | 1.44 (1.04–2.00) | 0.02954 | 3.28 per + 5 mm (1.32–8.12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momot, K.; Pruc, M.; Rodkiewicz, D.; Koźluk, E.; Krauz, K.; Piątkowska, A.; Zalewska, Z.; Buksińska-Lisik, M.; Szarpak, L.; Mamcarz, A. Predictive Value of Epicardial Adipose Tissue Parameters Measured by Cardiac Computed Tomography for Recurrence of Atrial Fibrillation After Pulmonary Vein Isolation. J. Clin. Med. 2025, 14, 6963. https://doi.org/10.3390/jcm14196963
Momot K, Pruc M, Rodkiewicz D, Koźluk E, Krauz K, Piątkowska A, Zalewska Z, Buksińska-Lisik M, Szarpak L, Mamcarz A. Predictive Value of Epicardial Adipose Tissue Parameters Measured by Cardiac Computed Tomography for Recurrence of Atrial Fibrillation After Pulmonary Vein Isolation. Journal of Clinical Medicine. 2025; 14(19):6963. https://doi.org/10.3390/jcm14196963
Chicago/Turabian StyleMomot, Karol, Michal Pruc, Dariusz Rodkiewicz, Edward Koźluk, Kamil Krauz, Agnieszka Piątkowska, Zuzanna Zalewska, Małgorzata Buksińska-Lisik, Lukasz Szarpak, and Artur Mamcarz. 2025. "Predictive Value of Epicardial Adipose Tissue Parameters Measured by Cardiac Computed Tomography for Recurrence of Atrial Fibrillation After Pulmonary Vein Isolation" Journal of Clinical Medicine 14, no. 19: 6963. https://doi.org/10.3390/jcm14196963
APA StyleMomot, K., Pruc, M., Rodkiewicz, D., Koźluk, E., Krauz, K., Piątkowska, A., Zalewska, Z., Buksińska-Lisik, M., Szarpak, L., & Mamcarz, A. (2025). Predictive Value of Epicardial Adipose Tissue Parameters Measured by Cardiac Computed Tomography for Recurrence of Atrial Fibrillation After Pulmonary Vein Isolation. Journal of Clinical Medicine, 14(19), 6963. https://doi.org/10.3390/jcm14196963







