NIR Indocyanine–White Light Overlay Visualization for Neuro-Oto-Vascular Preservation During Anterior Transpetrosal Approaches: A Technical Note
Abstract
1. Introduction
2. Materials and Methods
2.1. Anatomical Report
2.2. Clinical Case
2.3. Instrumentation
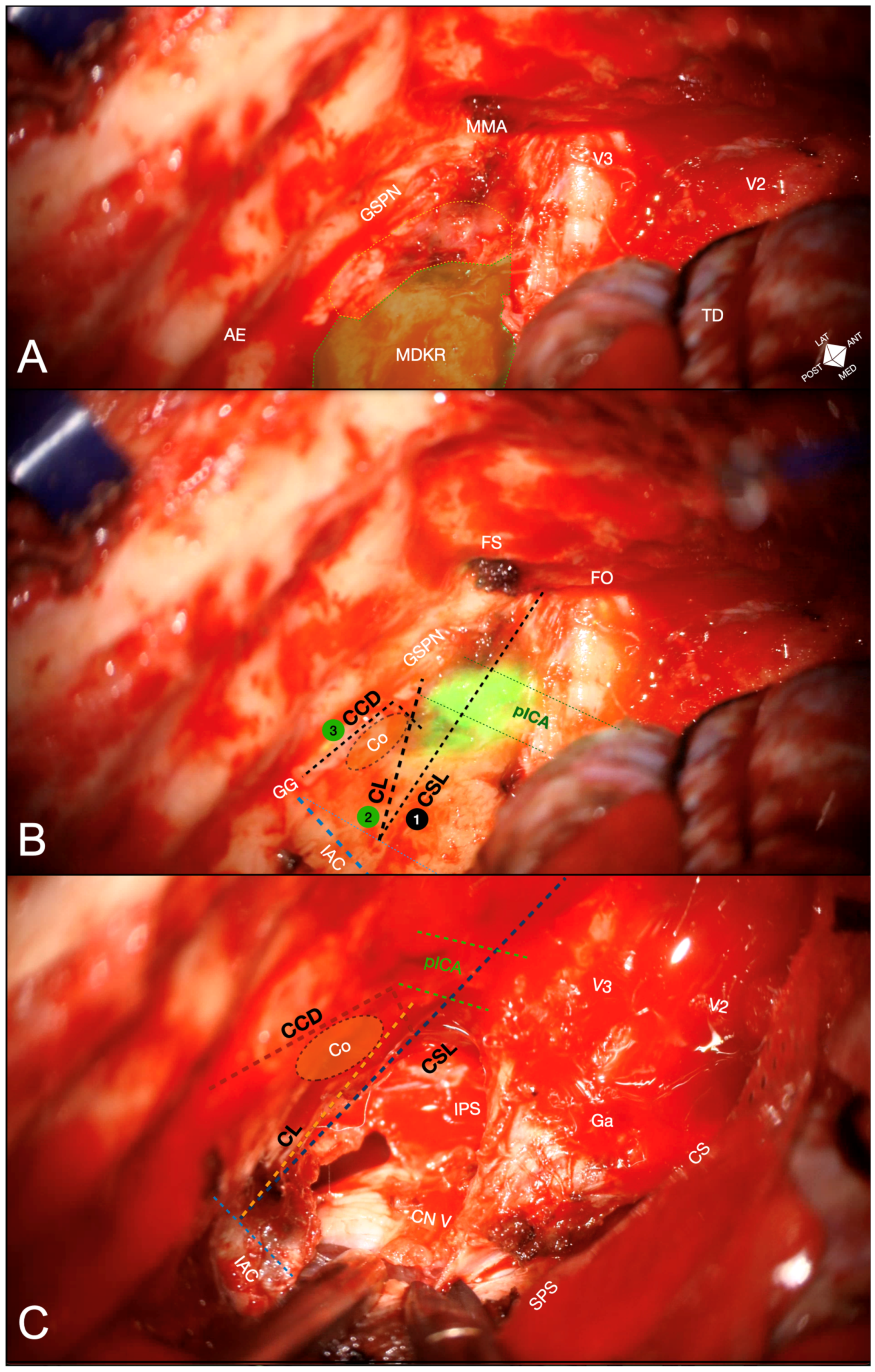
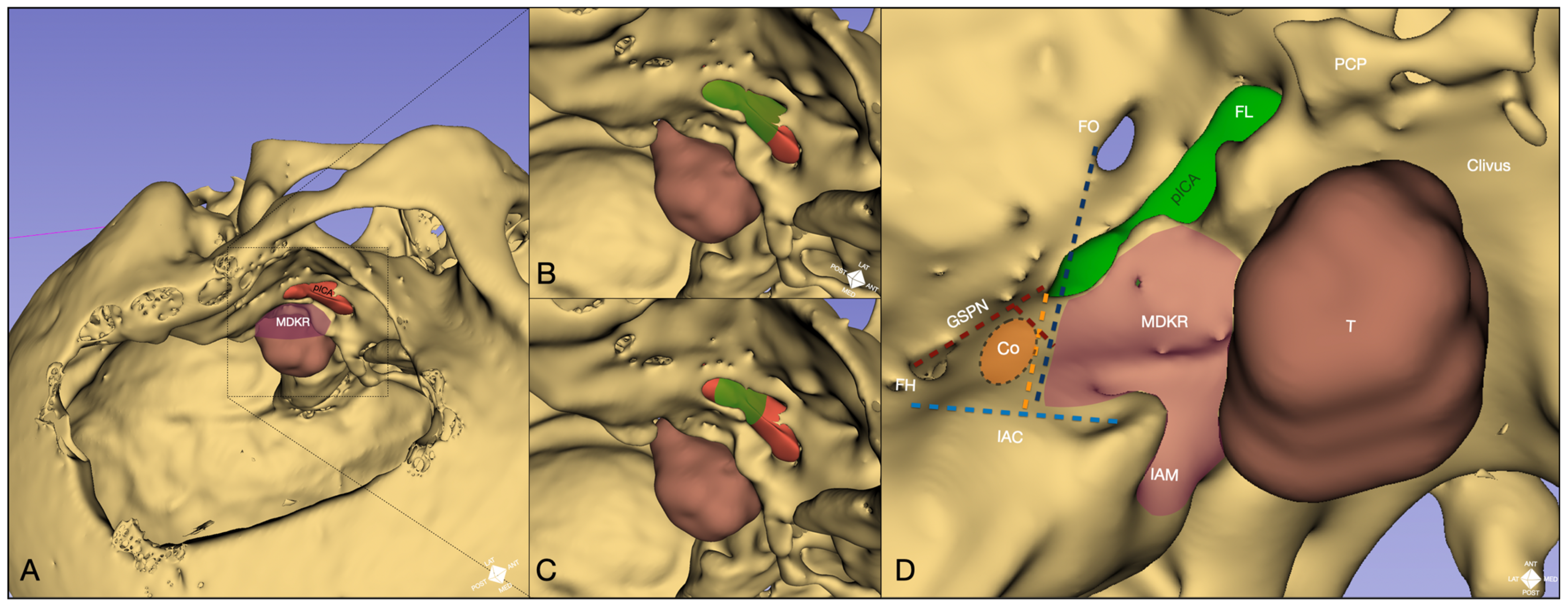
2.4. Anatomical Landmarks
- Cochlear safety line (CSL): Defined as the perpendicular line connecting the lateral rim of the foramen ovale to the transition fold between the roof and anterior wall of the internal acoustic canal (IAC). This line laterally delineates the cochlea [10].
- Petrous ICA (pICA): Identified using intraoperative ICGva.
- Cochlear line (CL): Projected perpendicularly from the petrous ICA–GSPN intersection (measured during ICGva) onto the IAC. This line marks the lateral position of the basal cochlear turn [11].
- Carotido-cochlear distance (CCD): Adapted from Dew et al., measured as the distance between the medial ICA wall (defined with ICGva) and the geniculate ganglion at the fallopian hiatus [12]. Probing through the hiatus or GSPN electrical stimulation with facial nerve EMG confirmation refined this measurement. A 7 mm distance from the geniculate ganglion crotch was designated the safest boundary for identifying the basal cochlear turn [12].
3. Results
3.1. Cadaveric Simulation
3.2. Surgical Application
4. Discussion
4.1. Sequential Landmarks Adoption
4.2. Applications
4.3. Practical Considerations and Future Directions
4.4. Limitations
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Arcuate Eminence |
| ATPA | Anterior Transpetrosal Approach |
| ATSTA | Anterior Transpetrosal Subtemporal/Transcavernous Approach |
| CCD | Carotido-Cochlear Distance |
| CL | Cochlear Line |
| CS | Cavernous Sinus |
| CSL | Cochlear Safety Line |
| CT | Computed Tomography |
| EMG | Electromyography |
| FH | Fallopian Hiatus |
| FL | Foramen Lacerum |
| FO | Foramen Ovale |
| FR | Foramen Rotundum |
| FS | Foramen Spinosum |
| Ga | Gasserian (Trigeminal) Ganglion |
| GSPN | Greater Superficial Petrosal Nerve |
| IAC | Internal Acoustic Canal |
| IAM | Internal Acoustic Meatus |
| ICA | Internal Carotid Artery |
| ICG | Indocyanine Green |
| ICGva | Indocyanine Green Videoangiography |
| INFRARED 800 (IR800) | ZEISS near-infrared fluorescence mode |
| IP S | Inferior Petrosal Sinus |
| IRB | Institutional Review Board |
| LSC | Lateral Semicircular Canal |
| LV | Vein of Labbé |
| Ma | Malleus |
| MCF | Middle Cranial Fossa |
| MDK | Modified Dolenc–Kawase (approach) |
| MDKR | Modified Dolenc–Kawase Rhomboid |
| MMA | Middle Meningeal Artery |
| MRI | Magnetic Resonance Imaging |
| NIR | Near-Infrared |
| NIR-ICG | Near-Infrared Indocyanine Green |
| PCP | Posterior Clinoid Process |
| pICA | Petrous (Segment of the) Internal Carotid Artery |
| PSC | Posterior Semicircular Canal |
| SPS | Superior Petrosal Sinus |
| SSC | Superior Semicircular Canal |
| SS | Sigmoid Sinus |
| TD | Temporal Dura |
| UDTF | Upper Dural Transition Fold |
| V2 | Maxillary Division of the Trigeminal Nerve |
| V3 | Mandibular Division of the Trigeminal Nerve |
| WHO | World Health Organization |
References
- Kawase, T.; Bertalanffy, H.; Otani, M.; Shiobara, R.; Toya, S. Surgical Approaches for Vertebro-Basilar Trunk Aneurysms Located in the Midline. Acta Neurochir. 1996, 138, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Evins, A.I.; Atchley, T.J.; Surdell, D.L.; Thorell, W.E.; Nonaka, M.; Stieg, P.E.; Bernardo, A. The Complete Anterior Petrosectomy: An Expanded Extended-Middle Fossa Approach with Removal of the Infratrigeminal Petrous Apex and Drilling of the Lateral Clivus. J. Neurosurg. 2024, 141, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Dolenc, V.V. Frontotemporal Epidural Approach to Trigeminal Neurinomas. Acta Neurochir. 1994, 130, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Deo, R.C.; Suri, A.; Srivastav, V.; Baby, B.; Kumar, S.; Kalra, P.; Banerjee, S.; Prasad, S.; Paul, K.; et al. Quantitative Analysis of the Kawase versus the Modified Dolenc-Kawase Approach for Middle Cranial Fossa Lesions with Variable Anteroposterior Extension. J. Neurosurg. 2015, 123, 14–22. [Google Scholar] [CrossRef]
- Spiessberger, A.; Baumann, F.; Stauffer, A.; Marbacher, S.; Kothbauer, K.; Fandino, J.; Moriggl, B. Extended Exposure of the Petroclival Junction: The Combined Anterior Transpetrosal and Subtemporal/Transcavernous Approach. Surg. Neurol. Int. 2018, 9, 259. [Google Scholar] [CrossRef]
- Fernandez-Miranda, J.C. Extended Middle Fossa Approach With Anterior Petrosectomy and Anterior Clinoidectomy for Resection of Spheno-Cavernous-Tentorial Meningioma: The Hakuba–Kawase–Dolenc Approach: 3-Dimensional Operative Video. Oper. Neurosurg. 2017, 13, 281. [Google Scholar] [CrossRef]
- Kawase, T.; Jean, W.C. Anterior Transpetrosal Approach: 2-Dimensional Operative Video. Oper. Neurosurg. 2023, 25, e22. [Google Scholar] [CrossRef]
- Tomio, R.; Horiguchi, T.; Borghei-Razavi, H.; Tamura, R.; Yoshida, K.; Kawase, T. Anterior Transpetrosal Approach: Experiences in 274 Cases over 33 Years. Technical Variations, Operated Patients, and Approach-Related Complications. J. Neurosurg. 2022, 136, 413–421. [Google Scholar] [CrossRef]
- Mantica, G.; Leonardi, R.; Diaz, R.; Malinaric, R.; Parodi, S.; Tappero, S.; Paraboschi, I.; Álvarez-Maestro, M.; Yuen-Chun Teoh, J.; Garriboli, M.; et al. Reporting ChAracteristics of Cadaver Training and SUrgical Studies: The CACTUS Guidelines. Int. J. Surg. 2022, 101, 106619. [Google Scholar] [CrossRef]
- Guo, X.; Tabani, H.; Griswold, D.; Tayebi Meybodi, A.; Gonzalez Sanchez, J.J.; Lawton, M.T.; Benet, A. Hearing Preservation During Anterior Petrosectomy: The “Cochlear Safety Line”. World Neurosurg. 2017, 99, 618–622. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, H.Y.; Kim, H.K.; Zabramski, J.M. Cochlear Line: A Novel Landmark for Hearing Preservation Using the Anterior Petrosal Approach. J. Neurosurg. 2015, 123, 9–13. [Google Scholar] [CrossRef]
- Dew, L.A.; Shelton, C.; Harnsberger, H.R.; Thompson, B.G. Surgical Exposure of the Petrous Internal Carotid Artery: Practical Application for Skull Base Surgery. Laryngoscope 1997, 107, 967–976. [Google Scholar] [CrossRef]
- Day, J.D.; Fukushima, T.; Giannotta, S.L. Microanatomical Study of the Extradural Middle Fossa Approach to the Petroclival and Posterior Cavernous Sinus Region. Neurosurgery 1994, 34, 1009–10016. [Google Scholar] [CrossRef]
- Cokkeser, Y.; Aristegui, M.; Naguib, M.B.; Saleh, E.; Taibah, A.K.; Sanna, M. Identification of Internal Acoustic Canal in the Middle Cranial Fossa Approach: A Safe Technique. Otolaryngol.–Head Neck Surg. 2001, 124, 94–98. [Google Scholar] [CrossRef]
- Garcia-Ibanez, E.; Garcia-Ibanez, J.L. Middle Fossa Vestibular Neurectomy: A Report of 373 Cases. Otolaryngol.–Head Neck Surg. 1980, 88, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Shiobara, R.; Toya, S. Anterior Transpetrosal-Transtentorial Approach for Sphenopetroclival Meningiomas: Surgical Method and Results in 10 Patients. Neurosurgery 1991, 28, 869–876, discussion 875. [Google Scholar] [CrossRef] [PubMed]
- Suero Molina, E.; Bruneau, M.; Reuter, G.; Shahein, M.; Cavallo, L.M.; Daniel, R.T.; Kasper, E.M.; Froelich, S.; Jouanneau, E.; Manet, R.; et al. Fluorescence Guidance in Skull Base Surgery: Applications and Limitations—A Systematic Review. Brain Spine 2024, 4, 103328. [Google Scholar] [CrossRef] [PubMed]
- Hide, T.; Yano, S.; Shinojima, N.; Kuratsu, J. Usefulness of the Indocyanine Green Fluorescence Endoscope in Endonasal Transsphenoidal Surgery. J. Neurosurg. 2015, 122, 1185–1192. [Google Scholar] [CrossRef]
- Jeon, J.W.; Cho, S.S.; Nag, S.; Buch, L.; Pierce, J.; Su, Y.S.; Adappa, N.D.; Palmer, J.N.; Newman, J.G.; Singhal, S.; et al. Near-Infrared Optical Contrast of Skull Base Tumors During Endoscopic Endonasal Surgery. Oper. Neurosurg. 2019, 17, 32–42. [Google Scholar] [CrossRef]
- Lee, J.Y.K.; Pierce, J.T.; Thawani, J.P.; Zeh, R.; Nie, S.; Martinez-Lage, M.; Singhal, S. Near-Infrared Fluorescent Image-Guided Surgery for Intracranial Meningioma. J. Neurosurg. 2018, 128, 380–390. [Google Scholar] [CrossRef]
- Borghei-Razavi, H.; Tomio, R.; Fereshtehnejad, S.-M.; Shibao, S.; Schick, U.; Toda, M.; Kawase, T.; Yoshida, K. Anterior Petrosal Approach: The Safety of Kawase Triangle as an Anatomical Landmark for Anterior Petrosectomy in Petroclival Meningiomas. Clin. Neurol. Neurosurg. 2015, 139, 282–287. [Google Scholar] [CrossRef]
- Anania, P.; Mirapeix-Lucas, R.; Zona, G.; Prior, A.; Cortes, C.A.; Muñoz Hernandez, F. Middle Cranial Fossa Approach: Anatomical Study on Skull Base Triangles as a Landmark for a Safe Anterior Petrosectomy. J. Neurol. Surg. B Skull Base 2021, 82, 202–207. [Google Scholar] [CrossRef]
- Maina, R.; Ducati, A.; Lanzino, G. The Middle Cranial Fossa: Morphometric Study and Surgical Considerations. Skull Base 2007, 17, 395–403. [Google Scholar] [CrossRef]
- Glasscock, M.E. Middle Fossa Approach to the Temporal Bone: An Otologic Frontier. Arch. Otolaryngol.-Head Neck Surg. 1969, 90, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, N.; Sanus, G.Z.; Ulu, M.O.; Tanriverdi, T.; Akar, Z.; Rubino, P.A.; Rhoton, A.L. Middle Fossa Approach: Microsurgical Anatomy and Surgical Technique from the Neurosurgical Perspective. Surg. Neurol. 2009, 71, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Osawa, S.; Rhoton, A.L.; Tanriover, N.; Shimizu, S.; Fujii, K. Microsurgical Anatomy and Surgical Exposure of the Petrous Segment of the Internal Carotid Artery. Oper. Neurosurg. 2008, 63, 210–239. [Google Scholar] [CrossRef] [PubMed]
- McCraney, S.A.; Shekhawat, D.; Cardona, J.J.; Chaiyamoon, A.; Reina, F.; Carrera, A.; Güngör, A.; Iwanaga, J.; Dumont, A.S.; Tubbs, R.S. Anatomy and Histology of the Petrous Carotid Membrane: Application to Skull Base Surgery. World Neurosurg. 2023, 175, e1360–e1363. [Google Scholar] [CrossRef]
- Hachem, L.D.; Mansouri, A.; Chen, J.; Pirouzmand, F. Feasibility of Real-Time Intraoperative Fluorescence Imaging of Dural Sinus Thrombosis. J. Clin. Neurosci. 2018, 52, 153–155. [Google Scholar] [CrossRef]
- Ueba, T.; Okawa, M.; Abe, H.; Nonaka, M.; Iwaasa, M.; Higashi, T.; Inoue, T.; Takano, K. Identification of Venous Sinus, Tumor Location, and Pial Supply during Meningioma Surgery by Transdural Indocyanine Green Videography. J. Neurosurg. 2013, 118, 632–636. [Google Scholar] [CrossRef]
- Acerbi, F.; Vetrano, I.G.; Sattin, T.; De Laurentis, C.; Bosio, L.; Rossini, Z.; Broggi, M.; Schiariti, M.; Ferroli, P. The Role of Indocyanine Green Videoangiography with FLOW 800 Analysis for the Surgical Management of Central Nervous System Tumors: An Update. Neurosurg. Focus 2018, 44, E6. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Jiang, F.; Yang, X.; Tan, X.; Chen, Z.; Liu, Y.; Zhu, Y.; Wang, Z.; Chen, G. Utility of Indocyanine Green Videoangiography with FLOW 800 Analysis in Brain Tumour Resection as a Venous Protection Technique. BMC Surg. 2022, 22, 126. [Google Scholar] [CrossRef]
- Zhang, R.; Xue, Y.-Z.; Yang, X.-F. Biomedical Optical Properties of Color Light and Near-Infrared Fluorescence Separated-Merged Imager. J. Innov. Opt. Health Sci. 2019, 12, 1940001. [Google Scholar] [CrossRef]
- Pruimboom, T.; Van Kuijk, S.M.J.; Qiu, S.S.; Van Den Bos, J.; Wieringa, F.P.; Van Der Hulst, R.R.W.J.; Schols, R.M. Optimizing Indocyanine Green Fluorescence Angiography in Reconstructive Flap Surgery: A Systematic Review and Ex Vivo Experiments. Surg. Innov. 2020, 27, 103–119. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; Van Der Vorst, J.R.; Kuppen, P.J.K.; Löwik, C.W.G.M.; Frangioni, J.V.; Van De Velde, C.J.H.; Vahrmeijer, A.L. The Clinical Use of Indocyanine Green as a Near-infrared Fluorescent Contrast Agent for Image-guided Oncologic Surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef]
- Litvack, Z.N.; Zada, G.; Laws, E.R. Indocyanine Green Fluorescence Endoscopy for Visual Differentiation of Pituitary Tumor from Surrounding Structures. J. Neurosurg. 2012, 116, 935–941. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef]
- Meola, A.; Cutolo, F.; Carbone, M.; Cagnazzo, F.; Ferrari, M.; Ferrari, V. Augmented Reality in Neurosurgery: A Systematic Review. Neurosurg. Rev. 2017, 40, 537–548. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariciotti, L.; Rodas, A.; De Andrade, E., Jr.; Revuelta Barbero, J.M.; Zohdy, Y.M.; Soriano, R.; Vuncannon, J.R.; Maldonado, J.; Lohana, S.; DiMeco, F.; et al. NIR Indocyanine–White Light Overlay Visualization for Neuro-Oto-Vascular Preservation During Anterior Transpetrosal Approaches: A Technical Note. J. Clin. Med. 2025, 14, 6954. https://doi.org/10.3390/jcm14196954
Tariciotti L, Rodas A, De Andrade E Jr., Revuelta Barbero JM, Zohdy YM, Soriano R, Vuncannon JR, Maldonado J, Lohana S, DiMeco F, et al. NIR Indocyanine–White Light Overlay Visualization for Neuro-Oto-Vascular Preservation During Anterior Transpetrosal Approaches: A Technical Note. Journal of Clinical Medicine. 2025; 14(19):6954. https://doi.org/10.3390/jcm14196954
Chicago/Turabian StyleTariciotti, Leonardo, Alejandra Rodas, Erion De Andrade, Jr., Juan Manuel Revuelta Barbero, Youssef M. Zohdy, Roberto Soriano, Jackson R. Vuncannon, Justin Maldonado, Samir Lohana, Francesco DiMeco, and et al. 2025. "NIR Indocyanine–White Light Overlay Visualization for Neuro-Oto-Vascular Preservation During Anterior Transpetrosal Approaches: A Technical Note" Journal of Clinical Medicine 14, no. 19: 6954. https://doi.org/10.3390/jcm14196954
APA StyleTariciotti, L., Rodas, A., De Andrade, E., Jr., Revuelta Barbero, J. M., Zohdy, Y. M., Soriano, R., Vuncannon, J. R., Maldonado, J., Lohana, S., DiMeco, F., Garzon-Muvdi, T., Reyes, C., Solares, C. A., & Pradilla, G. (2025). NIR Indocyanine–White Light Overlay Visualization for Neuro-Oto-Vascular Preservation During Anterior Transpetrosal Approaches: A Technical Note. Journal of Clinical Medicine, 14(19), 6954. https://doi.org/10.3390/jcm14196954




